Marenzelleria sp.
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5081.3.3 |
|
publication LSID |
lsid:zoobank.org:pub:0173F43D-38AB-4010-8B2A-1DFD5F118A2C |
|
DOI |
https://doi.org/10.5281/zenodo.5776893 |
|
persistent identifier |
https://treatment.plazi.org/id/4633878E-FFAD-B04A-4B83-FF3063E56714 |
|
treatment provided by |
Plazi |
|
scientific name |
Marenzelleria sp. |
| status |
|
( Figs 2 View FIGURE 2 , 4–6 View FIGURE 4 View FIGURE 5 View FIGURE 6 )
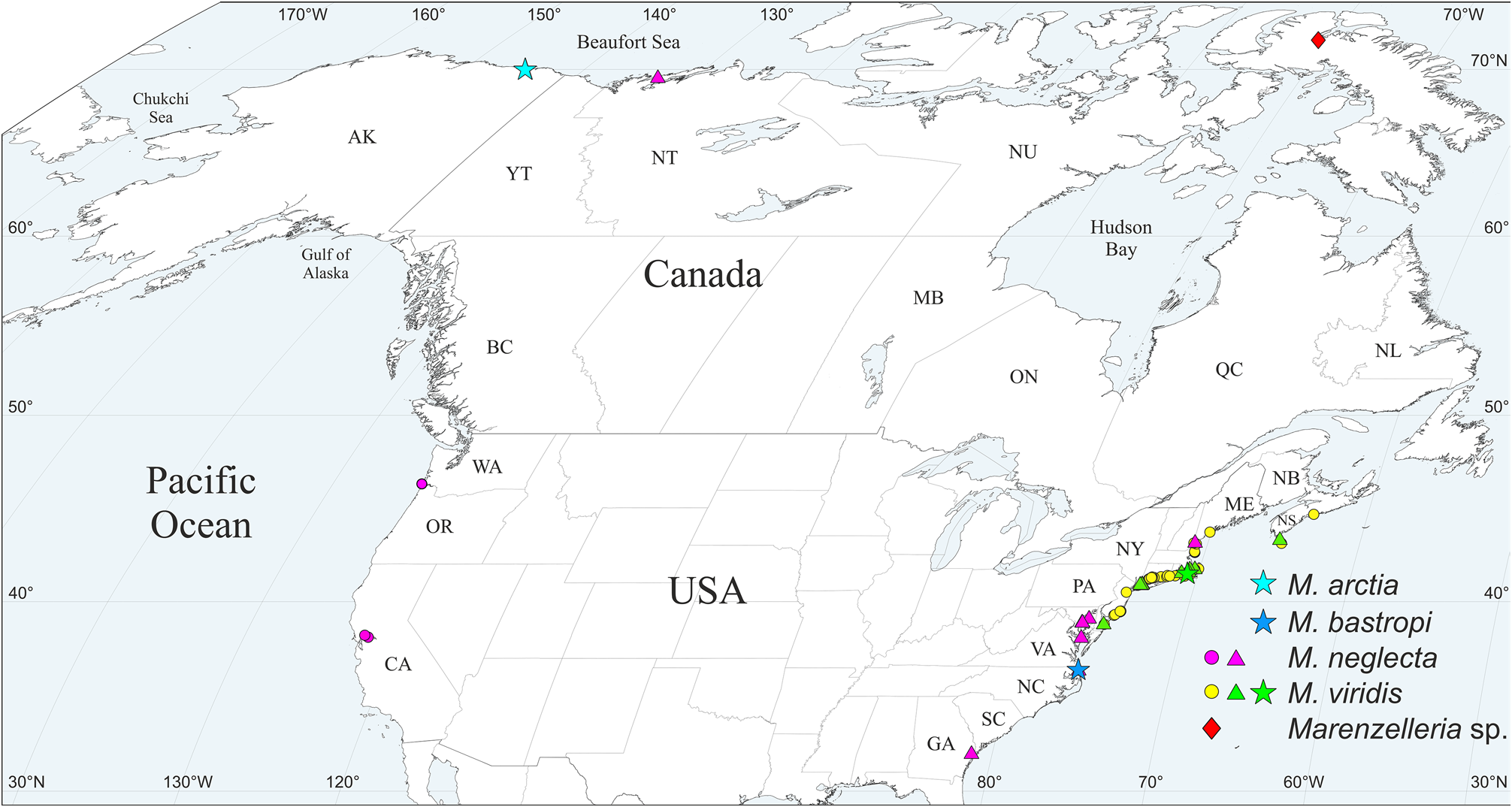
Material. Canada, Nunavut, Baffin Is., Koluktoo Bay , 3–21 m, 2018–2020, MIMB 40927 View Materials , 40928 View Materials , 42134–42138 View Materials (50 spec.). Complete information about this material is given in Table S 5 .
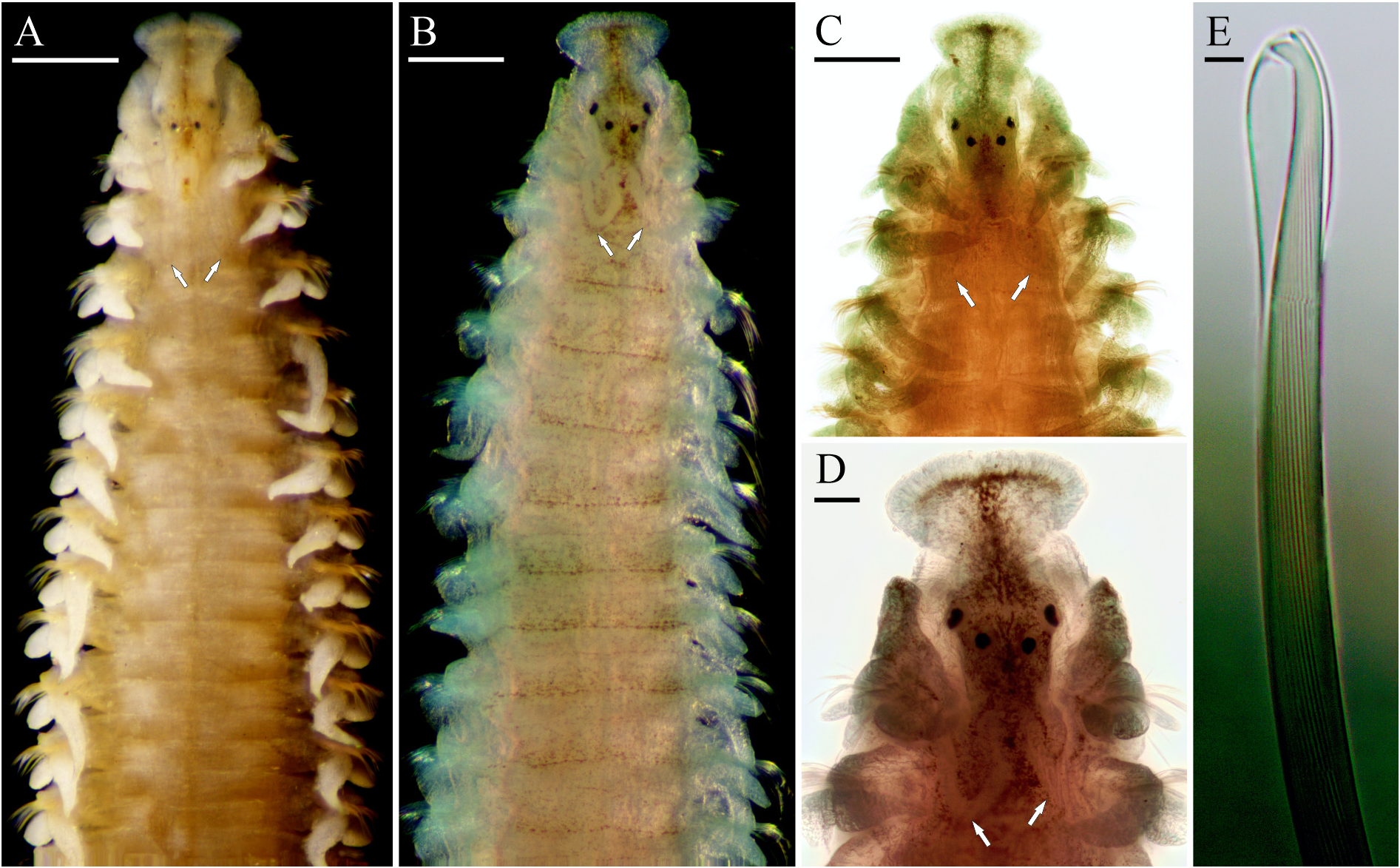
Description. Anterior fragments and complete juveniles 10–15 mm long, 0.3–0.8 mm wide for 57–88 chaetigers. Fine dark pigment usually scattered on dorsal side of prostomium and 1–2 anterior chaetigers ( Fig. 4A–D View FIGURE 4 ). Prostomium anteriorly broadly rounded, usually with small incision on frontal margin ( Fig. 4A–D View FIGURE 4 ), rarely entire. Two pairs of small red eyes arranged trapezoidally; lateral eyes situated anteriorly and set wider apart; occasionally eyes absent. Nuchal organs U-shaped ciliary bands, extending posteriorly to end of chaetiger 2, shorter in small individuals ( Figs 4A–D View FIGURE 4 , 5A View FIGURE 5 ). Chaetiger 1 well developed, with capillary chaetae and postchaetal lamellae in both rami; notochaetae as long as those on chaetiger 2, arranged in three distinct groups, comprising short anterior-row capillaries with wide limbation, slightly longer posterior-row capillaries with wide limbation, and long thin alimbate superior capillaries. Sabre chaetae in neuropodia from chaetiger 4 onwards in worms of all sizes ( Fig. 5E View FIGURE 5 ). Hooded hooks in notopodia from chaetigers 24–40, in neuropodia from chaetigers 21−31 onwards ( Fig. 5E, F View FIGURE 5 ); hooks bidentate with outer hood only and slightly curved shaft ( Fig. 4E View FIGURE 4 ). Branchiae from chaetiger 1 on anterior half of body ( Fig. 5C, D View FIGURE 5 ), fused to notopodial postchaetal lamellae at least basally on anterior chaetigers, with surfaces oriented perpendicular to body axis, with ciliation on inner and outer edges ( Fig. 4A View FIGURE 4 ). Pygidium with up to five pairs of cirri, comprising one pair of short thick ventral cirri, and four pairs of long thin dorsal cirri; dorsal cirri fewer in small individuals ( Fig. 5B View FIGURE 5 ).
Remarks. Marenzelleria species have no unique morphological features and can be distinguished only by the maximum values of morphometric characteristics, such as the length of the nuchal organs, the arrangement of the branchiae and chaetae. Unfortunately, the worms are long and fragile, and mostly anterior fragments are usually available for examination. Sikorski & Bick (2004) and Syomin et al. (2016) provided values of morphometric characteristics for different size classes of M. arctia , M. neglecta , M. viridis , and M. wireni , referring to the worm width at chaetiger 10. However, the values were highly variable and overlapped in individuals of different species less than 1.2 mm wide. Therefore, morphological identification keys have been suggested to be used for large specimens, more than 1.2 mm wide ( Sikorski & Bick 2004; Bick 2005). Only these mature individuals have nuchal organs of maximum length, and species-specific arrangement of branchiae and hooded hooks.
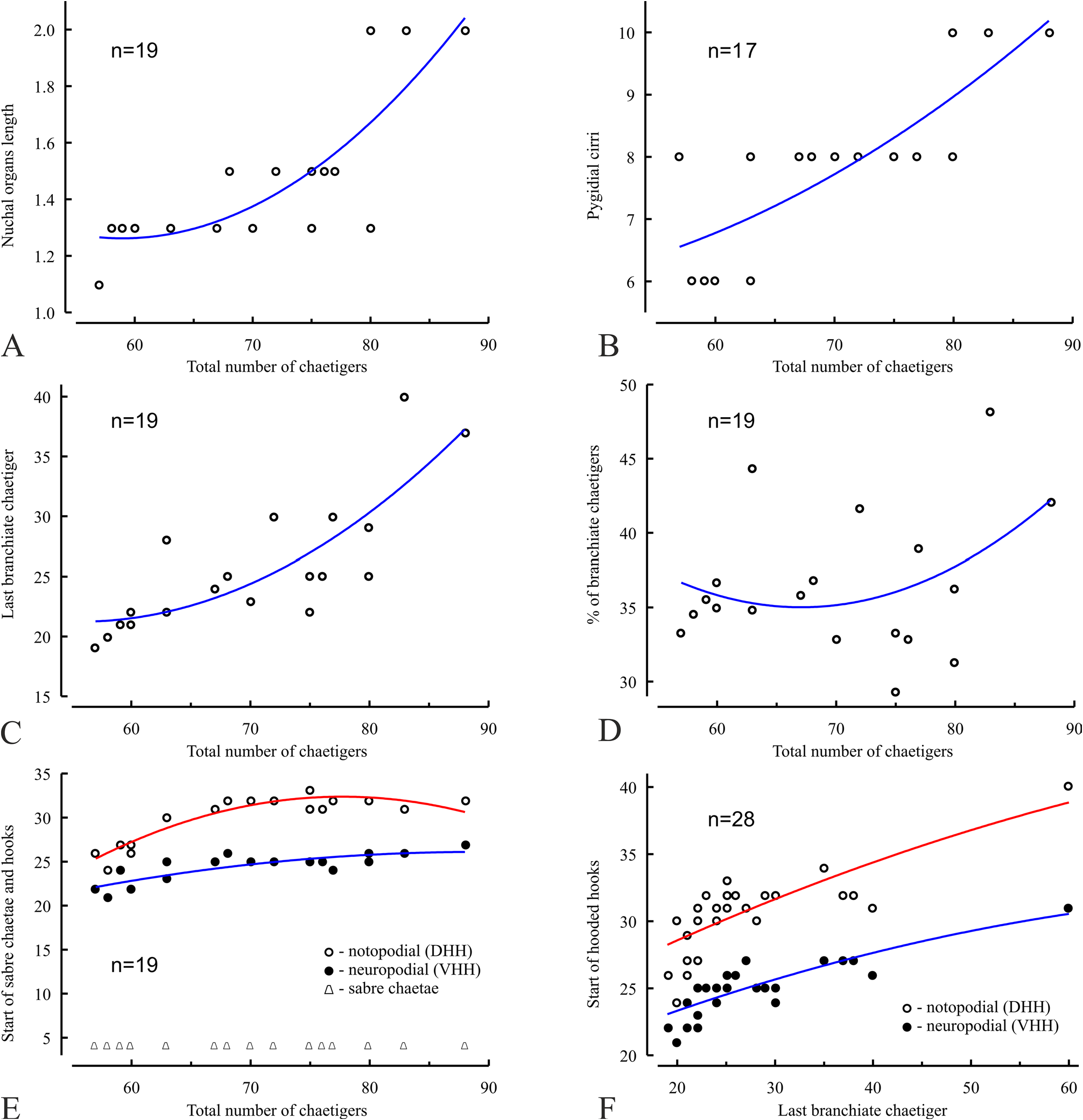
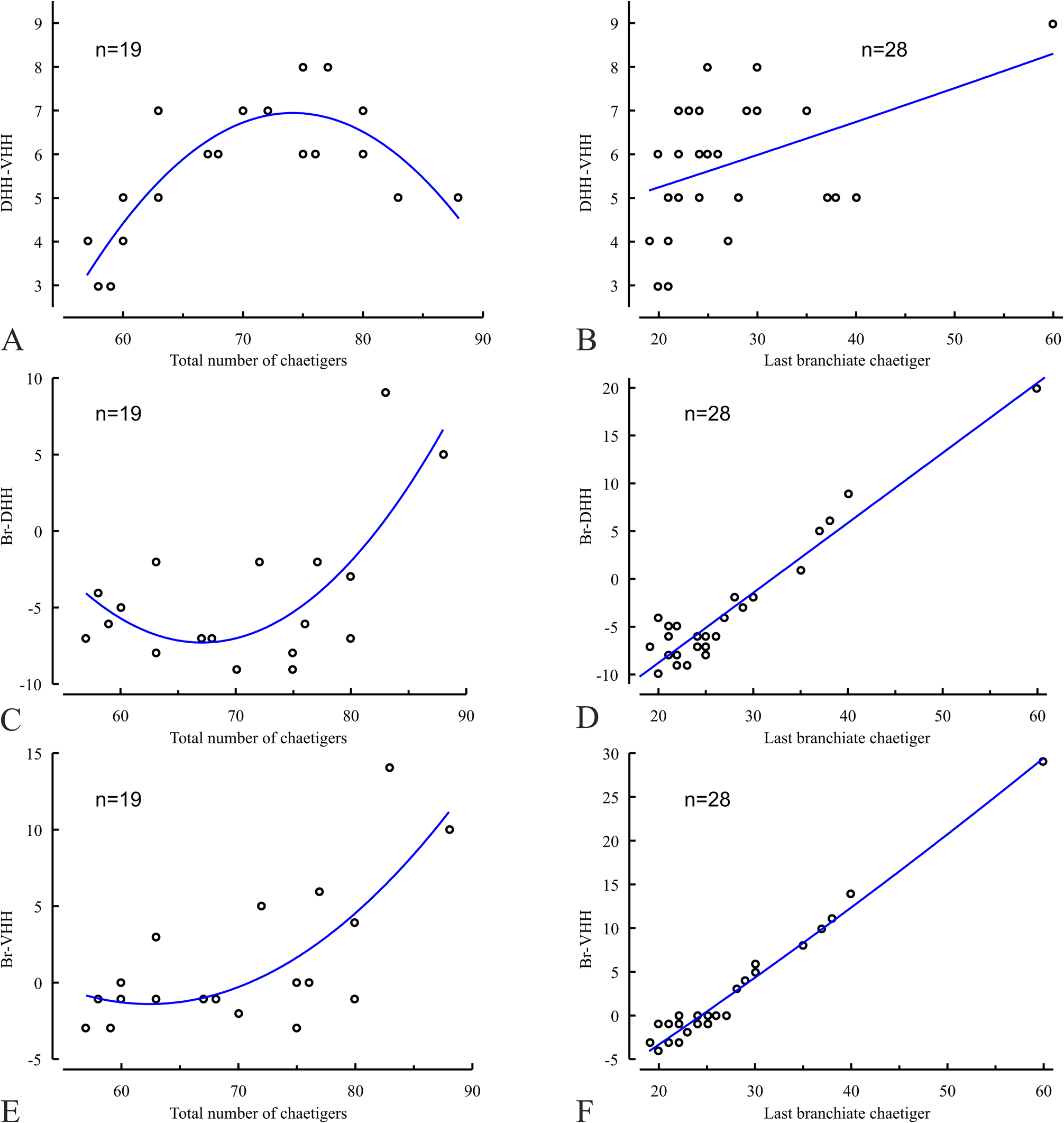
The Marenzelleria specimens collected in Nunavut ( Canada) in August–September 2018 -2020 are up to 0.8 mm wide and have up to 88 chaetigers. They have no gametes and are likely juveniles that cannot be reliably assigned to any particular species. However, the presence of complete specimens in the samples made it possible to describe a series of morphometric relationships between morphological features and the total number of chaetigers, as well as the number of branchiate chaetigers in individuals ( Figs 5 View FIGURE 5 , 6 View FIGURE 6 ). Analysis of these relationships shows that new chaetigers in the prepygidial growth zone develop faster than branchiae in individuals up to about 70 chaetigers, while after reaching the stage of about 80 chaetigers, new branchiae develop faster than new chaetigers ( Fig. 5C View FIGURE 5 ). This means that, in relation to the rate of development of new chaetigers, the development of new branchiae accelerates after about the 80-chaetiger stage. Such acceleration may be expected in order to increase gas exchange in a growing body (body surface is proportional to the square of its linear dimensions, while the volume is proportional to the cube). On the other hand, the rate of loosing of hooded hooks from noto- and neuropodia slows down with age, slightly faster in notopodia than in neuropodia ( Fig. 5E View FIGURE 5 ). These processes result in an age-related decrease in the number of chaetigers between the starts of hooded hooks in noto- and neuropodia ( Fig. 6A View FIGURE 6 ), as well as to an agerelated relationship between the arrangement of the branchiae and the hooded hooks ( Fig. 6C, D View FIGURE 6 ). It is noteworthy that the arithmetic differences between the start of the hooded hooks in noto- and neuropodia (DHH-VHH), as well as between the last branchiate chaetiger and the start of the hooded hooks in noto- (Br-DHH) and neuropodia (Br- VHH), are probably proportionally correlated with the number of the last branchiate chaetiger in individuals ( Fig. 6 View FIGURE 6 ,B, D, F). Although the presented analysis does not include fully developed large individuals, the nuchal organs reaching the end of chaetiger 2 and the high rate of branchial development ( Fig. 5C, D View FIGURE 5 ) in the examined worms lead us to assume that fully grown adults may have longer nuchal organs and greater number of branchiate chaetigers which are characteristic for M. wireni . This assumption must be confirmed by morphological examination of fully developed large individuals from Nunavut and by analysis of their genetic characteristics. Pending this confirmation, we tentatively refer Canadian specimens to as Marenzelleria sp.
Marenzelleria wireni was originally described by Augener (1913) based on specimens from the Barents and Kara Seas. Sikorski & Buzhinskaya (1998) and Sikorski & Bick (2004) re-described the species based on material from all the Eurasian Arctic seas, from the Barents Sea east to the Chukchi Sea. Bick (2005) described worms from Spitsbergen and noted that M. wireni differs from other species by the great number of branchiate chaetigers (branchiae on all chaetigers except the very last) and long nuchal organs (at least up to the end of chaetiger 3/beginning of chaetiger 4). The species has never been reported from North American Arctic region.
Distribution in North America. Baffin Is., Nunavut, Canada ( Fig. 2 View FIGURE 2 ).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.