Achaeta paranensis Schmelz
|
publication ID |
https://doi.org/ 10.5281/zenodo.182758 |
|
DOI |
https://doi.org/10.5281/zenodo.6232935 |
|
persistent identifier |
https://treatment.plazi.org/id/506F87A9-FF87-C556-4BF3-87D1A153F816 |
|
treatment provided by |
Plazi |
|
scientific name |
Achaeta paranensis Schmelz |
| status |
sp. nov. |
Achaeta paranensis Schmelz View in CoL , sp. nov.
Figure 5 View FIGURE 5 , Table 4 View TABLE 4
Holotype: MZUSP #1367, mature spcm, stained whole mount. Cachoeira Natural Reserve, Paraná, Brazil, shrubland (intermediate successional stage from pasture to forest), soil type Cambisol; October 2004.
Paratypes: MZUSP #1368, one spcm from same locality and date as holotype; MZUSP #1369, four spms from urban parkland in Curitiba, close to entrance of Department of Soils and Agricultural Engineering, Federal University of Paraná ( UFPR), Section Agrosciences, March 2004 (3 spms), October 2004 (1 spcm). All stained whole mounts.
Further material: One specimen, ill-preserved.
Etymology: Named after the Brazilian state Paraná, where the species was found.
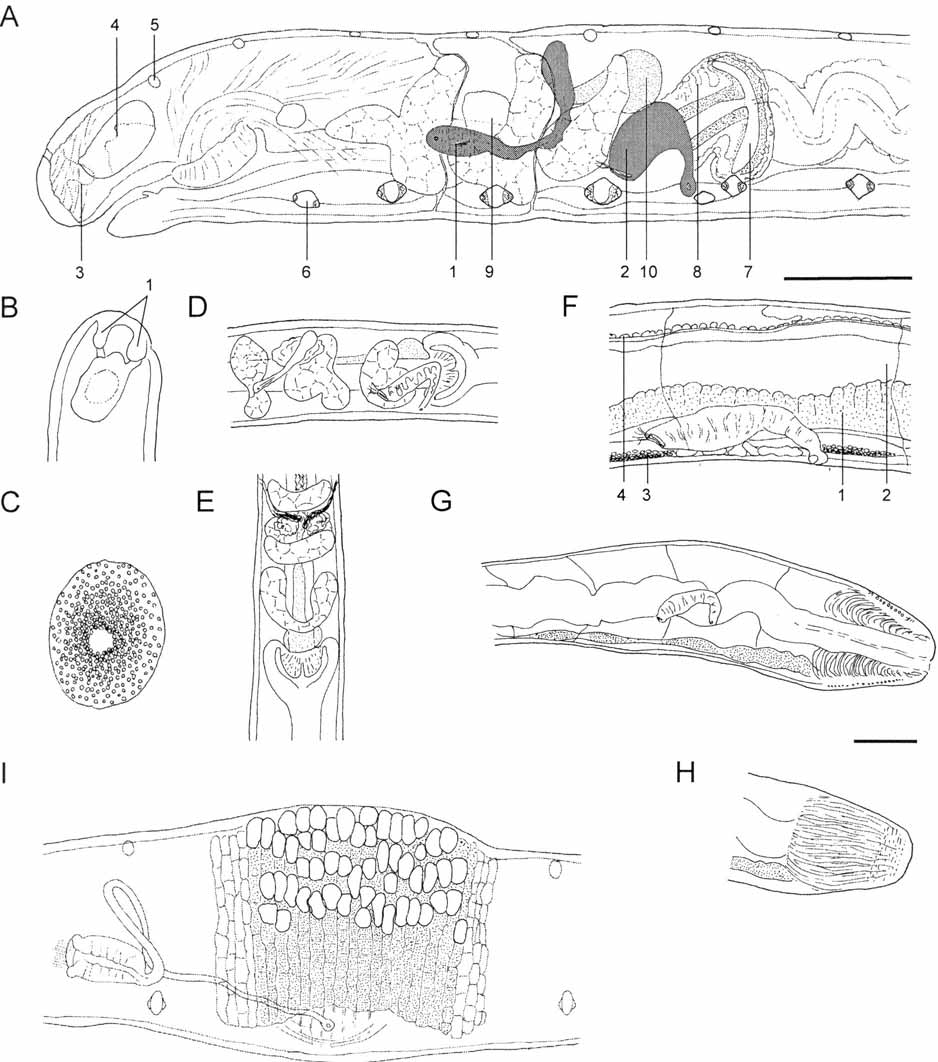
Description: Living specimens chequered whitish (not brilliant white) under top light. Slow body movements. Posterior end often thickened ( Fig. 5 View FIGURE 5 G). Region around male pores always slightly everted ( Fig. 5 View FIGURE 5 I). Body length 4-5 mm, diameter 0.15 mm (viv); fixed specimens 3.5–4 mm long and 0.13-0.17 mm wide (0.2 mm in XII/XIII in 1 spcm with mature egg). Segment number of fully mature specimens 28, 31 (N=2).
Pyriform glands absent. Six lentiform epidermal glands per segment ( Fig. 5 View FIGURE 5 A), in bilateral-symmetrical arrangement, three on each side of the body; two types present: Ventro-laterally, in posterior segment region right before septum, one segmental pair of glands, large (ca 30 µm: 20 μm, fix), roughly rhomboid, anterior and posterior ends hyaline, staining (fix), central part vesicular with nucleus in center (Type I glands, see A. hanagarthi ). Slighty more anteriorly but still in posterior fourth of a segment, four smaller hyaline epidermal gland cells (two on each side, diameter ca. 10 μm) in one transverse row, 2 dorsally, 2 laterally, the latter right above lateral line (Type II glands). Cells conspicuous only in preclitellar segments of well-preserved specimens, posteriorly perhaps absent.
Body wall thin, 3–5 µm, cuticle variable, almost as thick as rest of body wall, ca. 1.5–2 µm, or thinner. Ring muscles conspicuous, in ca. 23 segmental rows. Septa very thin, only those at 4/5 and 5/6 conspicuous.
Head pore at prostomial tip. Prostomial epithelium homogeneously thick ( Fig. 5 View FIGURE 5 A), thinner near upper lip. A pair of prostomial papillae present laterally and dorsally of prostomial nerves (prostomial ganglia?), in wide connection with frontal prostomial surface ( Fig. 5 View FIGURE 5 A,B). Prostomial musculature well-developed. Brain ( Fig. 5 View FIGURE 5 A,B) 1.5x as long as wide, 2x as long as thick, rounded posteriorly, anterior end with a small convex tip, sides parallel. Ventral nerve cord ( Fig. 5 View FIGURE 5 A,F) in segmental ganglia. Ganglia of II–IV fused into suboesophageal ganglion, with segmental parts remaining distinguishable. Subsequent ganglia with a hyaline zone (secretory cells?) in the middle, this trait increasingly less evident in posterior segments. Two pairs of postpharyngeal bulbs.
Oesophageal appendage ( Fig. 5 View FIGURE 5 A,D,E) in V, large, paired, lobed, with canal in IV. Pharyngeal glands ( Fig. 5 View FIGURE 5 A,D,E) in IV–VI, all united dorsally, dorsal connection wide in IV and V, narrow in VI, all with primary and without secondary ventral lobes; ventral lobes in IV and V with strong anterior and slight posterior projection; anterior projection in IV peculiar by its medial to sub-medial insertion (lateral view), not always present ( Fig. 5 View FIGURE 5 D). Chloragocytes from 1/ 2 VII, vesicles slightly larger than those of coelomocytes (viv); cells inconspicuous in fixed material, ca. 5 µm high. In VII an abrupt bulbiform intestinal dilatation ( Fig. 5 View FIGURE 5 A,D,E): posterior part (= beginning of intestine proper) superposed over anterior part (= end of oesophagus), creating a circumferal intestinal diverticulum, blind-ending anteriorly and open to intestine posteriorly. Mesodermal epithelium (chloragocytes or other unidentified tissue types) proliferated around diverticulum; blood sinus with wide lacunae here. Posterior intestine (in VII, VIII ff.) slightly narrower than bulbiform dilatation, wider than oesophagus, often meandering. Gut epithelium in VII and VIII not modified with respect to IX ff. Dorsal blood vessel ( Fig. 5 View FIGURE 5 A) from 1/ 2 VII, large, pulsating, anteriorly of intestinal dilatation. Pars tumida of midgut ( Fig. 5 View FIGURE 5 F) extending from XVII–XXIII and XVII–XXIV in the two mature specimens, respectively, cells distinguished from rest of intestinal epithelium also by brownish tint similar to gut contents, and by less intense paracarmine-staining of cytoplasm. Pygidium longer than wide, with strongly developed anal musculature ( Fig. 5 View FIGURE 5 G,H); hindmost segments including pygidium often wider than anterior segments.
One pair of preclitellar nephridia, at 6/7 ( Fig. 5 View FIGURE 5 A), sausage-shaped, rounded proximally and distally, 4x as long as wide, almost as long as worm body diameter and up to 1/4 as wide (e.g., 127 µm long and 34 µm wide, fix), tapering distad, no constriction at septum, the latter very thin, not always distinguished; anteseptale not longer than wide, nephrostome embedded obliquely in nephridial body, efferent duct not set off against postseptale, no terminal vesicle, ectal pore conspicuous. Postclitellar nephridia ( Fig. 5 View FIGURE 5 F) similar in shape as preclitellar nephridia, but extended, not bent, 5x as long as wide, and longer than worm body diameter; altogether only 1-5 nephridia in postclitellar segments, first at 14/15–18/19.
Coelomocytes ( Fig. 5 View FIGURE 5 C) numerous and of dark grey-brown coulour (viv), filled with refractile vesicles, vesicle concentration denser towards interior, nucleus visible as light spot in the center. Cells only slightly longer than wide or circular; lengths between 25 and 40 µm.
All reproductive organs in the usual position: Testis and sperm funnels in XI, ovary and male pores in XII.
Clitellum ( Fig. 5 View FIGURE 5 I) in ca. 24 separate rows (viv), or rows dense but distinct (fix). Cells absent mid-dorsally and mid-ventrally. Latero-dorsally mostly hyalocytes with a few interspersed granulocytes, hyalocytes not in longitudinal rows, or in disordered longitudinal rows dorsally or laterally, 2–5 hyalocytes per transverse row on each side. Latero-ventrally only granulocytes. Gland cells not projecting proximally into coelom.
Seminal vesicle absent, cysts free in XI. Spermatozoa not measured in vivo, heads 11 µm long (fix). Sperm funnel ( Fig. 5 View FIGURE 5 I) barrel-shaped, ca. 60 µm long (fix), 2x as long as wide, collar distinct, as wide as funnel body. Vas deferens in wide loops, 7 µm wide proximally (fix), ca. 5 µm at male pore. Male pores at XII, widely separate, on body surface. No bursa, no glandular bulbs, no accessory glands. Surrounding body wall region thickened, always slightly protruded or bulged outwards ( Fig. 5 View FIGURE 5 I).
Spermathecae ( Fig. 5 View FIGURE 5 A) confined to V or extending into VI, ectal pores lateral, ectal duct short, proximal part slightly thickened, with irregular outline, sperm not seen (viv), located ectally in slightly oblique position (fix); lumen of ental reservoirs not seen. One mature egg at a time, extending over two segments.
Remarks. This new species stands our by five peculiarities, (1) dark coelomocytes, (2) (sub-)medial insertion of anterior ventral pharyngeal gland lobe in IV, (3) intestinal fold at the transition oesophagus/intestine, (4) strongly muscular pygidium, (5) absence of glands around male pore. A. paranensis is the only species in the genus that possesses oesophageal appendages together with intestinal folds. Most Achaeta species have oesophageal appendages, but intestinal diverticula-like folds are described only for A. iridescens Christoffersen, 1979 , a species without oesophageal appendages. Intestinal folds are also present in our series of A. neotropica (comp. Fig. 4 G). In addition, one specimen of our A. neotropica series has a muscular pygidium typical of A. paranensis . The distinction between A. paranensis and A. neotropica is nonetheless clearcut, difference are listed in Table 4 View TABLE 4 . A iridescens is much larger than A. paranensis , for further differences see again Table 4 View TABLE 4 .
Coloured coelomocytes are not uncommon in Achaeta , see A. hanagarthi , but the intensely dark-grey tint in the cells of A. paranensis is so far unique in the genus. Numerous species in other genera share this trait, e.g. Marionina argentea Michaelsen, 1889 (b), Fridericia nix Rota, 1995 , Enchytraeus bulbosus Nielsen & Christensen, 1963 , Mesenchytraeus lusitanicus Collado et al., 1993 , to name a few. The colour appears to be caused entirely by the refractile properties of the numerous vesicles, it disappears in fixed material. The darkgrey colour increases from the cell periphery towards the nucleus in the center, which itself is uncoloured ( Fig. 5 View FIGURE 5 C). Coelomocyte refractions often obscure other structures in living specimens.
* A. becki included
** "absent or little developed" ( Christoffersen 1979: 157)
TABLE 4. Comparison of currently known and accepted neotropical species of Achaeta.
| Body length [mm, mature ind.] | A. piti Bittencourt, 1974 5 | A. hanagarthi sp. nov. 5–7 | A. iridescens Christoffers. 1979 13.5–14.5 |
|---|---|---|---|
| Segment number | 31–36 | (24)-32-43 | 55–60 |
| Pyriform glands | 6 per segment | none | none |
| Segmental epidermal glands | absent - | 2 (-6) per segment 2 types | ?**? |
| Brain posteriorly | rounded | rounded | rounded |
| Ganglia II-IV | fused | fused | fused |
| Oesophageal appendages | IV–V | IV–V | absent |
| Pharyngeal glands in VI dorsally | joint | joint | separate |
| Thickened preclitellar septa | 4/5–7/8 | 4/5–6/7 | 4/5, 5/6 |
| Preclitellar nephridia | 2 pairs, 7/8, 8/9 | 1 pair, 7/8 | 2 pairs, 6/7, 8/9 |
| Origin of dorsal blood vessel | V-1/2 VIII | VII | XI-1/2 XIII |
| Intestinal fold in VII | absent | absent | present |
| Pars tumida of midgut | circumferal | circumferal | ? |
| Coelomocytes | pale | red-brown | pale |
| Clitellum and male pores at | XI | XI | XII |
| Seminal vesicle | absent | absent | present |
| Male gland length | ca. 25 µm | ca. 50 µm | ca. 115 µm |
| Spermathecae extending into | VI, VII | VI, VII | VIII |
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
SubClass |
Oligochaeta |
|
Order |
|
|
Family |
|
|
Genus |