Spiophanes pacificus, Meissner & Schwentner & Göưing & Fiege, 2023
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlad069 |
|
publication LSID |
lsid:zoobank.org:pub:65B60DD3-64C9-4262-B7B2-74DA4D3D889F |
|
DOI |
https://doi.org/10.5281/zenodo.10497562 |
|
persistent identifier |
https://treatment.plazi.org/id/743EE917-FFEE-FF95-FF9E-24D11961FBCC |
|
treatment provided by |
Plazi |
|
scientific name |
Spiophanes pacificus |
| status |
sp. nov. |
Spiophanes pacificus View in CoL sp. nov. Meissner, Schwentner and Fiege
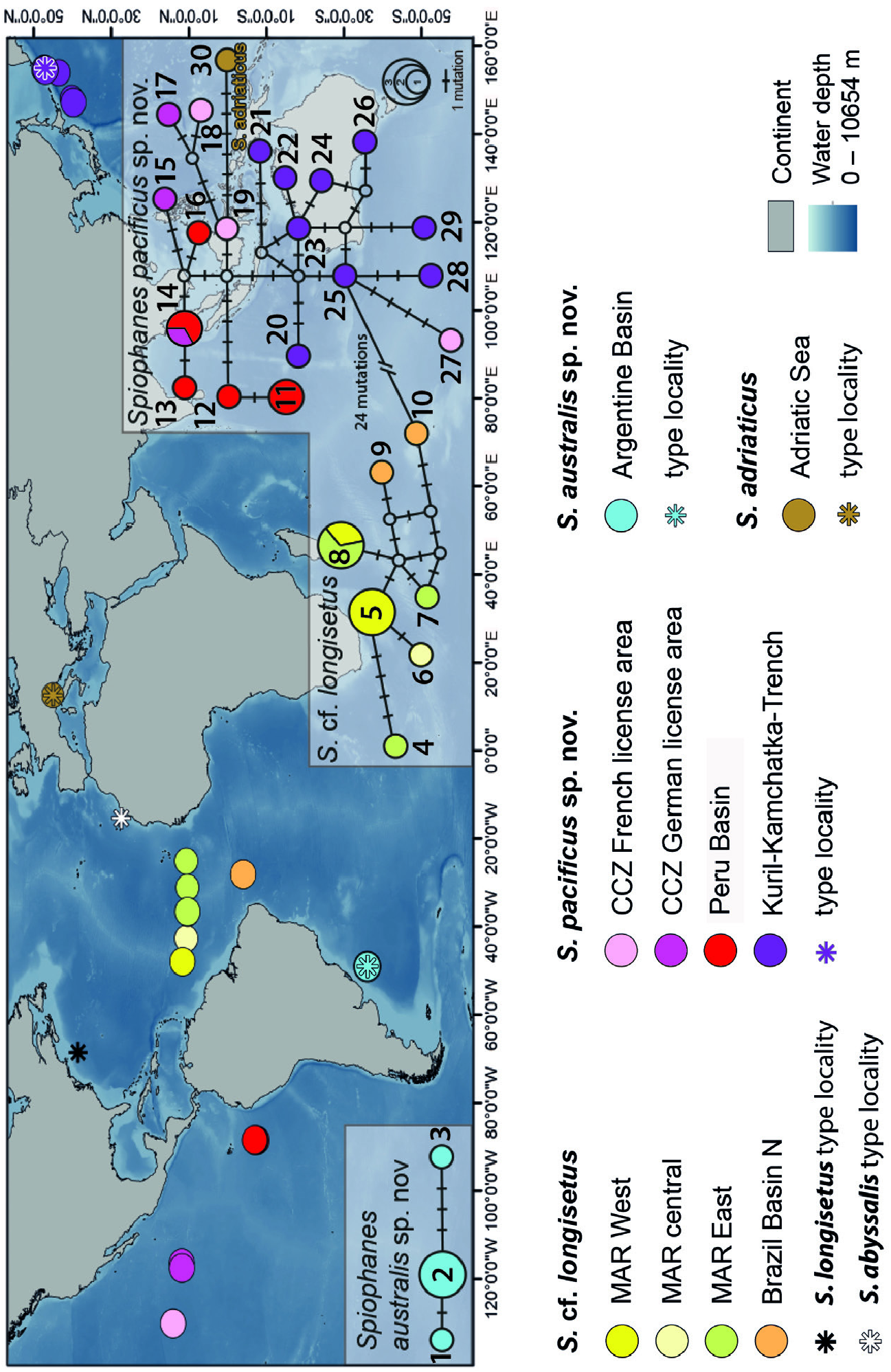
( Figs 13–15 View Figure 13 View Figure 14 View Figure 15 ; Table 6 View Table 6 )
Spiophanes sp. NHM_1897 Neal et al., 2022: 42–43, fig. 40a—d, table 1.
Type material: Holotype. NW Pacific Ocean, Kuril-Kamchatka trench, SO 223 (KuramBio), stn 4-5 BC, 5766 m, 7 Aug 2012, complete specimen (68 chaetigers, nearly 16 mm long, width 0.72 mm), now preserved in 70% EtOH ( ZMH-P 30460 ).
Paratypes: NW Pacific Ocean, Kuril-Kamchatka trench, SO 223 ( KuramBio ), stn 2-10 EBS Epi, 4859 m, 3 Aug 2012, one af ( ZMH-P 30453 ) ; stn 5-5 BC, 5379 m, 10 Aug 2012, four af ( SMF 32272 View Materials ) ; stn 5-10 EBS Epi, 5375 m, 11 Aug 2012, 1af ( SMF 30593 View Materials ) . SE Pacific Ocean, Peru Basin ( DISCOL Experimental Area , DEA), SO 242 ( JPIO-DISCOL 1 ) , stn 85-4 EBS, 4147 m, 14 Aug 2015, one af ( SMF 30610 View Materials ) .
Measurements for largest paratype from the Kuril-Kamchatka trench ( SMF 32272): af of about 21 chaetigers, length ~ 5.5 mm, width ~ 0.6 mm. Largest paratype from the Peru Basin ( SMF 30610): af of about 21 chaetigers, length 4.1 mm, width 0.9 mm (chaetae omiưed).
Non– type material: NW Pacific Ocean, Kuril-Kamchatka trench, SO 223 (KuramBio), stn 2-2, 5247.1 m ( ZMH-P 28455 ) ; stn 3-4, 4987.7 m ( ZMH-P28445 , ZMH-P28446 , ZMH-P28447 ) ; stn 4-4, 5773.6 m ( ZMH-P28451 ) ; stn 4-5, 5766 m ( SMF 32273 View Materials ) . SE Pacific Ocean, Peru Basin ( DISCOL area), SO 242 ( JPIO-DISCOL 1 ) , Reference area Hill NW, stn 117-7 EBS, 4154 m, 19 Aug 2015, one af ( SMF 30612 View Materials , SEM 1336 ) ; reference area S, stn 45-2 EBS, 4184 m, 4 Aug 2015, two af ( SMF 30616 View Materials , SEM 1324 ) . – for details and additional specimens see the Supporting Information, Table S2 View Table 2 .
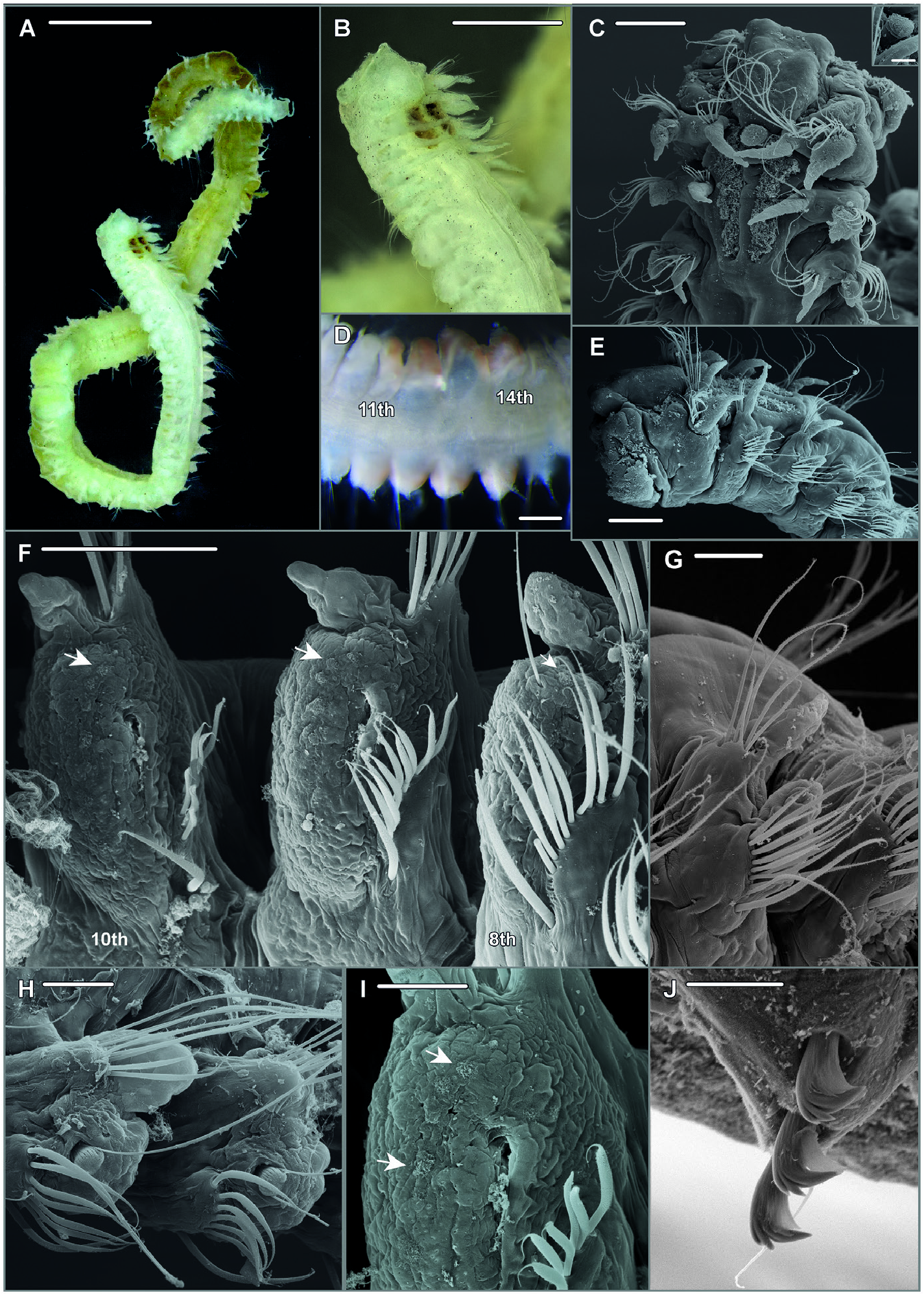
Description: Holotype complete specimen with 68 chaetigers, 0.72 mm wide and nearly 16 mm long ( Fig. 14A, B View Figure 14 ). Examined specimens between 0.3 and 0.9 mm in width, except for the holotype all anterior fragments. Body slender, subcylindrical.
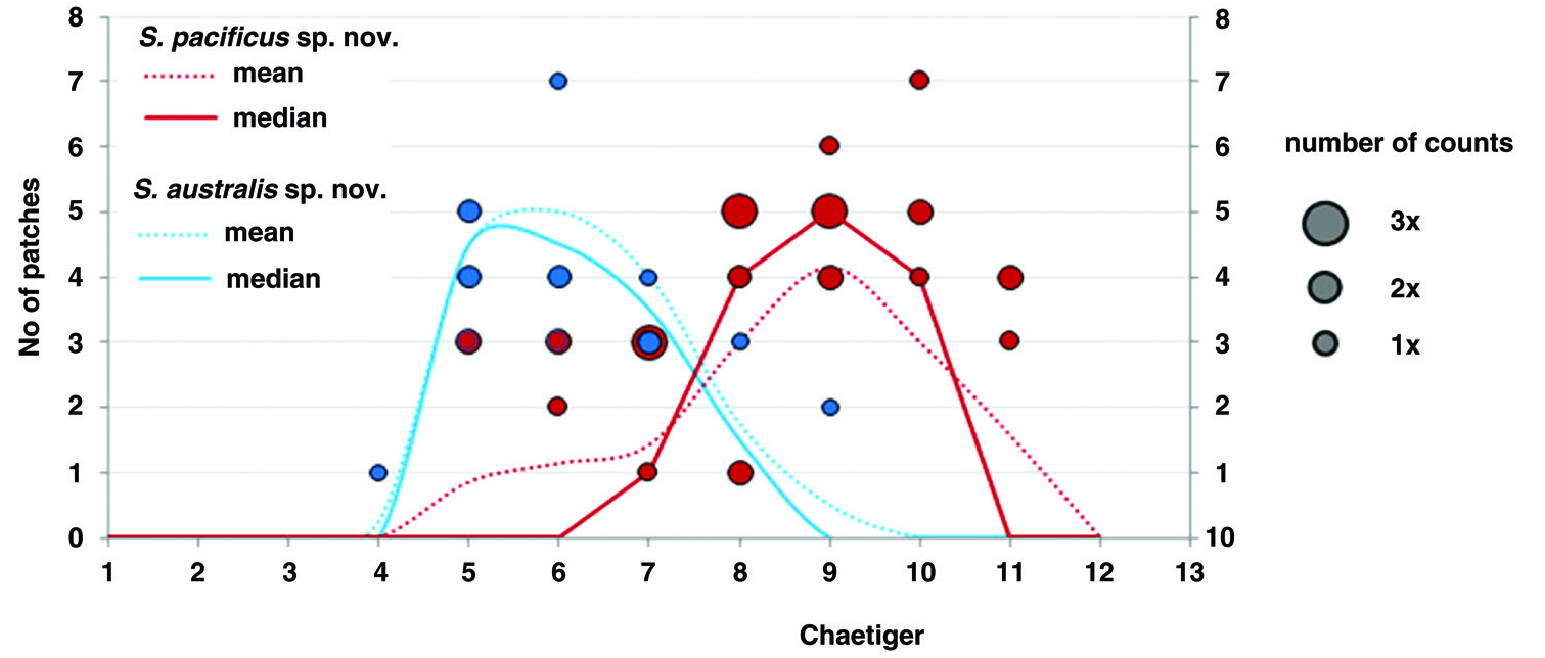
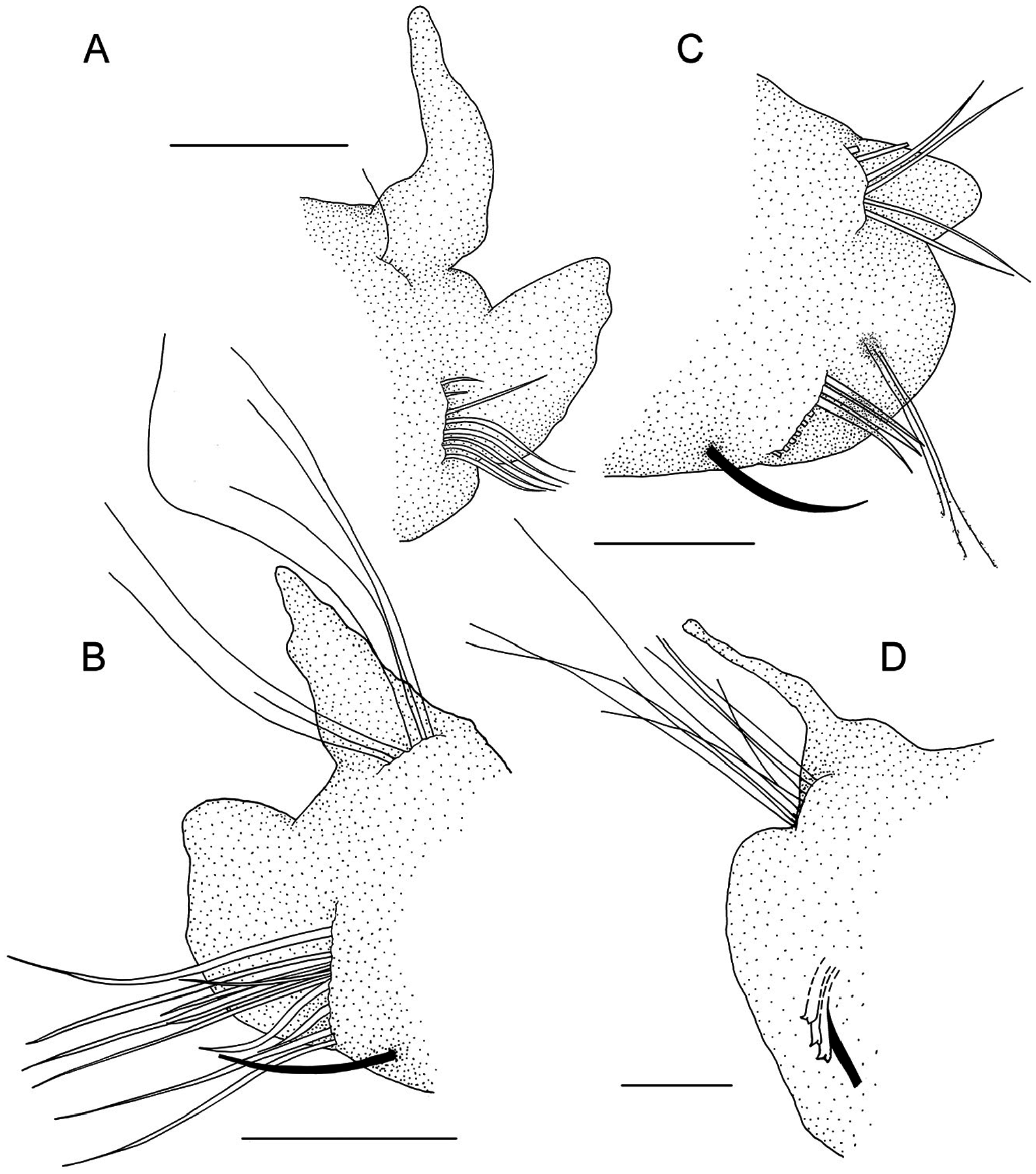
Prostomium bell-shaped with straight anterior margin extending into short anterolateral projections ( Fig. 14B, C View Figure 14 ), short stout cirriform occipital antenna with rounded tip present ( Fig. 14C, E View Figure 14 ). Dorsal ciliated organs (suggested to represent nuchal organs) as dorsal ciliated grooves posterior to the prostomium (ciliation detectable in SEM, Fig. 14C, E View Figure 14 ), if viewed with LM appearing as thick straight double lines of ochre or greenish colour reaching the end of the 2nd chaetiger ( Fig. 14A, B View Figure 14 ), sometimes outer margins of ciliated grooves demarcated resulting in a U-shaped appearance of the nuchal organ. Eyes absent. Peristomium moderately developed. First parapodium oriented dorsally, second dorsolaterally, thereaħer orientation of parapodia lateral ( Fig. 14E View Figure 14 ). Notopodial postchaetal lamellae of chaetigers 1–4 narrow subulate, longest in first three chaetigers, neuropodial postchaetal lamellae in first chaetiger also subulate with broad base and slender tip, only slightly shorter than in notopodium, in following chaetigers neuropodial lamellae much shorter than notopodial, and gradually changing into a subtriangular shape ( Fig. 14B, C, E, G View Figure 14 , 15A, B View Figure 15 ); chaetigers 5–8 with short, rounded notopodial and reduced neuropodial postchaetal lamella ( Figs 14H View Figure 14 , 15C View Figure 15 ); from chaetiger 9 notopodial lamella low with short acute tip, gradually base becoming more voluminous and the tip more slender ( Fig. 14F View Figure 14 ), neuropodial lamella reduced; from about chaetiger 14 throughout the end of the body notopodial lamella with broad base and cirriform tip, neuropodial lamella reduced ( Figs 14A View Figure 14 , 15D View Figure 15 ). Chaetal spreader of the ‘0 + 1 type’ with semicircular glandular opening developed in chaetigers 5–7, in chaetiger 8 chaetal spreader present but with small hole-like opening allowing the passage of very few or even only single bacillary chaeta ( Fig. 14F, H View Figure 14 ), glandular organ of chaetigers 9–14 opens as a lateral vertical slit, without chaetal spreader ( Fig. 14I View Figure 14 ). Ciliated patches might be observed laterally on neuropodia of chaetigers 5–11, with the highest number of patches in chaetigers 8–10 and up to seven patches arranged in a regular row ( Fig. 14F, I View Figure 14 ; see discussion above regarding this character for Spiophanes ). Ventrolateral intersegmental genital pouches absent. Dorsal membranous transverse crests very poorly developed from about chaetiger 18.
Chaetiger 1 bearing one or two stout, crook-like chaetae in neuropodium. Other chaetae in chaetigers 1–4 neuropodial of ciliated patches at chaetigers 9 and 10, and single patch at chaetiger 8 next to hole-like opening of glandular organ, arrows indicate upper patches. G, leħ parapodium of chaetiger 4; note long sabre chaeta in inferiormost position; anterolateral view. H, parapodia of chaetigers 5–6, lateral view; note semi-circular openings of glandular organs and exposed bacillary chaetae. I, right neuropodium of chaetiger 10, lateral view; note ciliated patches and opening of parapodial glandular organ as vertical slit with fibre-wool protruding from the gland, arrow heads indicate upper- and inferior-most patches. J, neuropodial quadridentate hooks with half-hood in chaetiger 18; note three hooks are arranged in a row together with thin accompanying capillary in inferior position, sabre chaeta not shown.—A, B, ZMH-P 30460 , holotype, NW Pacific; C, E, F, G, SMF 30612 View Materials ( SEM stub 1336); D, SMF 30610 View Materials , paratype, Peru Basin ; H, SMF 30616 View Materials ( SEM stub 1324), Peru Basin; I, SMF 30612 View Materials ( SEM stub 1336), Peru Basin; J, ZMH-P 28455 , NW Pacific. Scale Bars: A, B, 1 mm; C, E, 100 µm; D, 200µm; F, G, 50 µm; H, I, 30 µm; J, 10µm. capillaries arranged in two rows, chaetae in anterior row shorter and appearing granulated viewed with light microscopy, chaetae in second row smooth; notochaetae smooth capillaries, arranged in a tuħ ( Figs 14C, G View Figure 14 , 15B View Figure 15 ). Chaetigers 5–14 with granulated neurochaetae with narrow sheaths, arranged in one row ( Fig. 14F, H, I View Figure 14 , 15C View Figure 15 ); notochaetae both granulated and smooth capillaries arranged in two to three irregular rows ( Fig. 14F, H View Figure 14 ), longest chaetae in superior position. Posterior region starting at chaetiger 15 with first presence of neuropodial quadridentate hooks with main fang surmounted by single tooth and two smaller teeth in parallel position in uppermost position, hooks with half-hood from the tip of the main fang to the shaħ, usually three rarely four hooks arranged in one row; thin accompanying capillary in inferior position next to sabre chaeta present ( Fig. 14J View Figure 14 ); notopodia with slightly granulated capillaries arranged in a tuħ, among those few strikingly long capillaries,longest chaetae observed in posteriormost notopodia of the few complete specimens and in fragmented specimens (af) with many chaetigers.
Bacillary chaetae as thin hirsute bristles can be exposed on chaetigers 5–8 though never protruding much in chaetiger 8 compared to chaetigers 5, 6, and 7 ( Figs 14F, H View Figure 14 , 15C View Figure 15 ). Ventral sabre chaetae from chaetiger 4, very long in anterior and middle chaetigers ( Figs 14F–H View Figure 14 , 15B, C View Figure 15 ); appearing granulated near the tip under light microscope.
Pygidium with one pair of anal cirri in terminolateral position, both cirri about equal in length, thin cirriform ( Fig. 14A View Figure 14 ).
Pigmentation: Neuropodia of chaetigers 10–13(14) with faint yellowish to light brown pigment ( Fig. 14D View Figure 14 ); pigmentation only observed in few specimens originally preserved in formalin.
Methyl green staining pattern: Chaetigers of the anterior middle body region, and particularly chaetiger 8, most intensely and moreover most persistently stained compared to other parts of the body.
Biology: None of the studied specimens was observed bearing gametes.
Remarks: The species is morphologically very similar to other congeners from the deep-sea also discussed in this paper: S. australis sp. nov. (found in the SW Atlantic and adjacent Antarctic waters) and S. cf. longisetus from the Atlantic Ocean. These species are best distinguished by the distribution of lateral neuropodial ciliated patches along the middle body region and the number of neuropodial hooks ( Table 6 View Table 6 ; Figs 13 View Figure 13 , 14F, I View Figure 14 ). See introductory paragraph on Spiophanes above for details of morphological distinction. The species can also be distinguished based on information from molecular markers ( COI).
Searching GenBank for nucleotide sequences close to the ones gained in our study we came across a COI sequence deposited for Spiophanes adriaticus . The close genetic similarity between Spiophanes pacificus sp. nov. and S. adriaticus is surprising and the genetic distance is low enough to imply that these could be conspecific. However, ecologically it seems highly unlikely that these are the same species, one occurs in the deep sea of the Pacific, the other in shallow Mediterranean waters, Adriatic Sea (<30 m depth). This would be a truly remarkable vertical and horizontal distribution for a single species and even for sister taxa such different depth preferences would be surprising. The S. adriaticus sequences came from D’Alessandro et al. (2019) who reported to have sequenced three specimens, which are also depicted in their phylogenetic tree. However, only one sequence has been deposited in GenBank. Unfortunately, no information (e.g. collection details) was provided for the numerous other Spiophanes species that they studied and also none of these sequences appears to have been deposited in GenBank. This makes it impossible to verify their species identifications and raises the question whether the correct sequence has been deposited for S. adriaticus . Unfortunately, the authors have only very recently responded to our repeated aưempts to contact them but eventually new information could not be provided and thus the problem is not resolved. As already argued by Jourde et al. (2020), who studied the morphology of Spiophanes spp. from French coasts of the NE Atlantic and the Mediterranean Sea, we here too question the validity of S. adriaticus .
Etymology: The specific name pacificus refers to the known distribution of the species in soħ boưom habitats of the deep Pacific Ocean.
Distribution: The species has a wide distribution in the Pacific Ocean. Records came from the central (Clarion Clipperton Fracture Zone CCZ) and SE Pacific ( Peru Basin), and from the NW Pacific (Kuril–Kamchatka trench) ( Fig. 5 View Figure 5 ; Supporting Information, Table S2 View Table 2 ). Water depths were between 4900 and 5800 m in the NW Pacific, 4200–5100 m in the CCZ, and 4100– 4200 m in the Peru Basin.
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
| COI |
University of Coimbra Botany Department |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Spiophanes pacificus
| Meissner, Karin, Schwentner, Martin, Göưing, Miriam & Fiege, Thomas Knebelsberger and Dieter 2023 |
Spiophanes sp.
| Neal L & Wiklund H & Rabone M 2022: 42 |