Sigambra magnuncus Paterson & Glover, 2000
|
publication ID |
https://doi.org/ 10.1093/zoolinnean/zlad069 |
|
publication LSID |
lsid:zoobank.org:pub:65B60DD3-64C9-4262-B7B2-74DA4D3D889F |
|
DOI |
https://doi.org/10.5281/zenodo.10497538 |
|
persistent identifier |
https://treatment.plazi.org/id/743EE917-FFFC-FF80-FF2A-25611881FB29 |
|
treatment provided by |
Plazi |
|
scientific name |
Sigambra magnuncus Paterson & Glover, 2000 |
| status |
|
Sigambra magnuncus Paterson & Glover, 2000 View in CoL
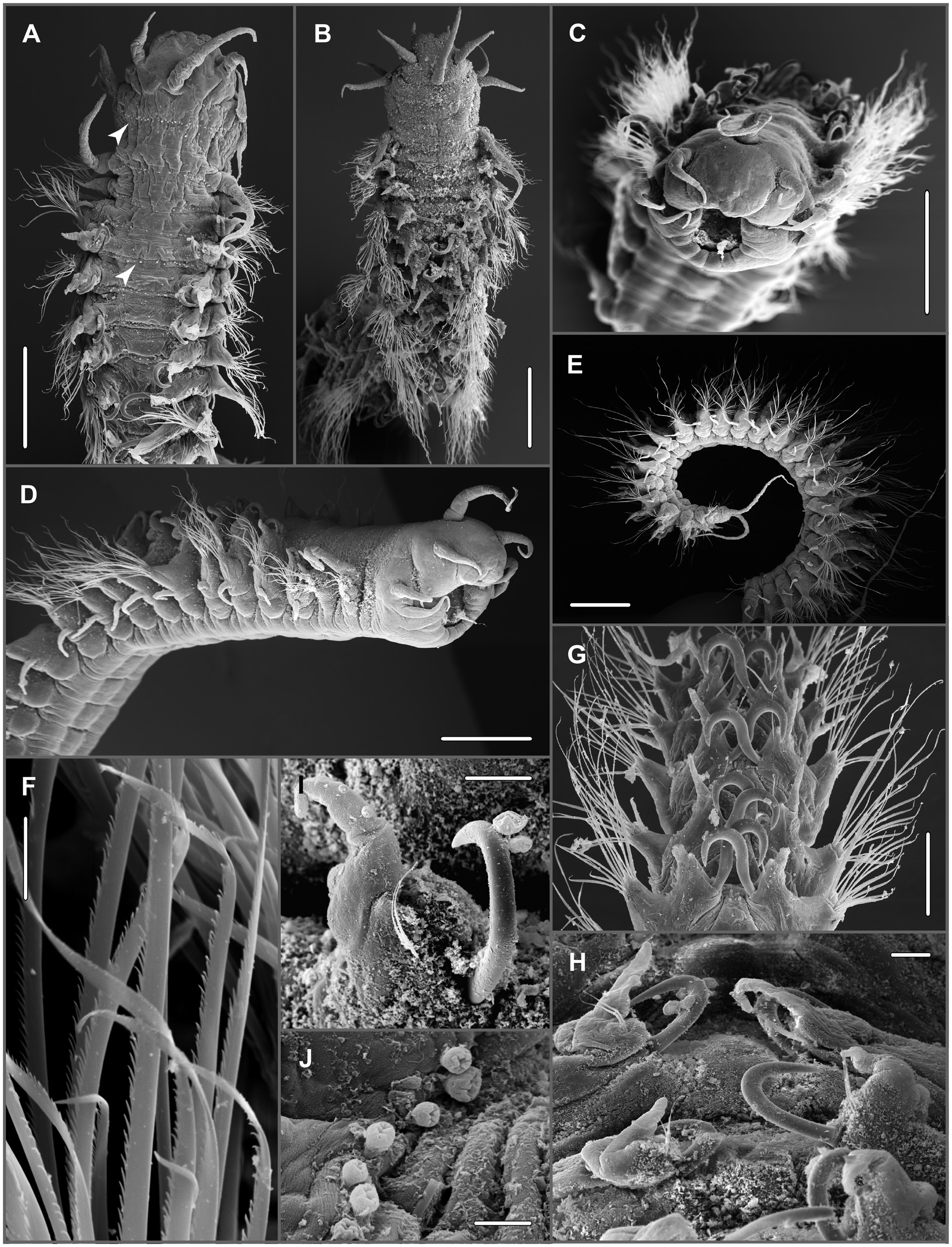
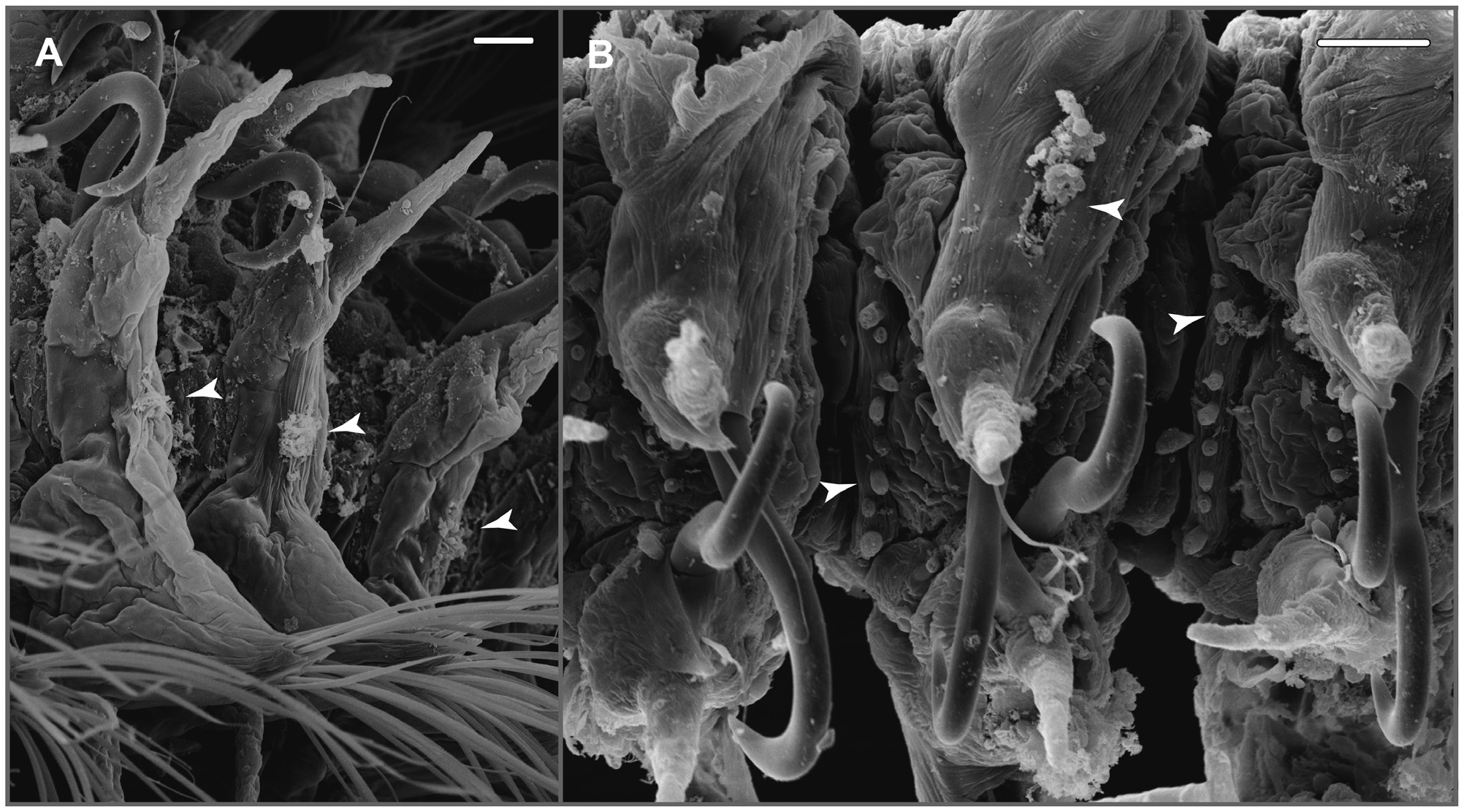
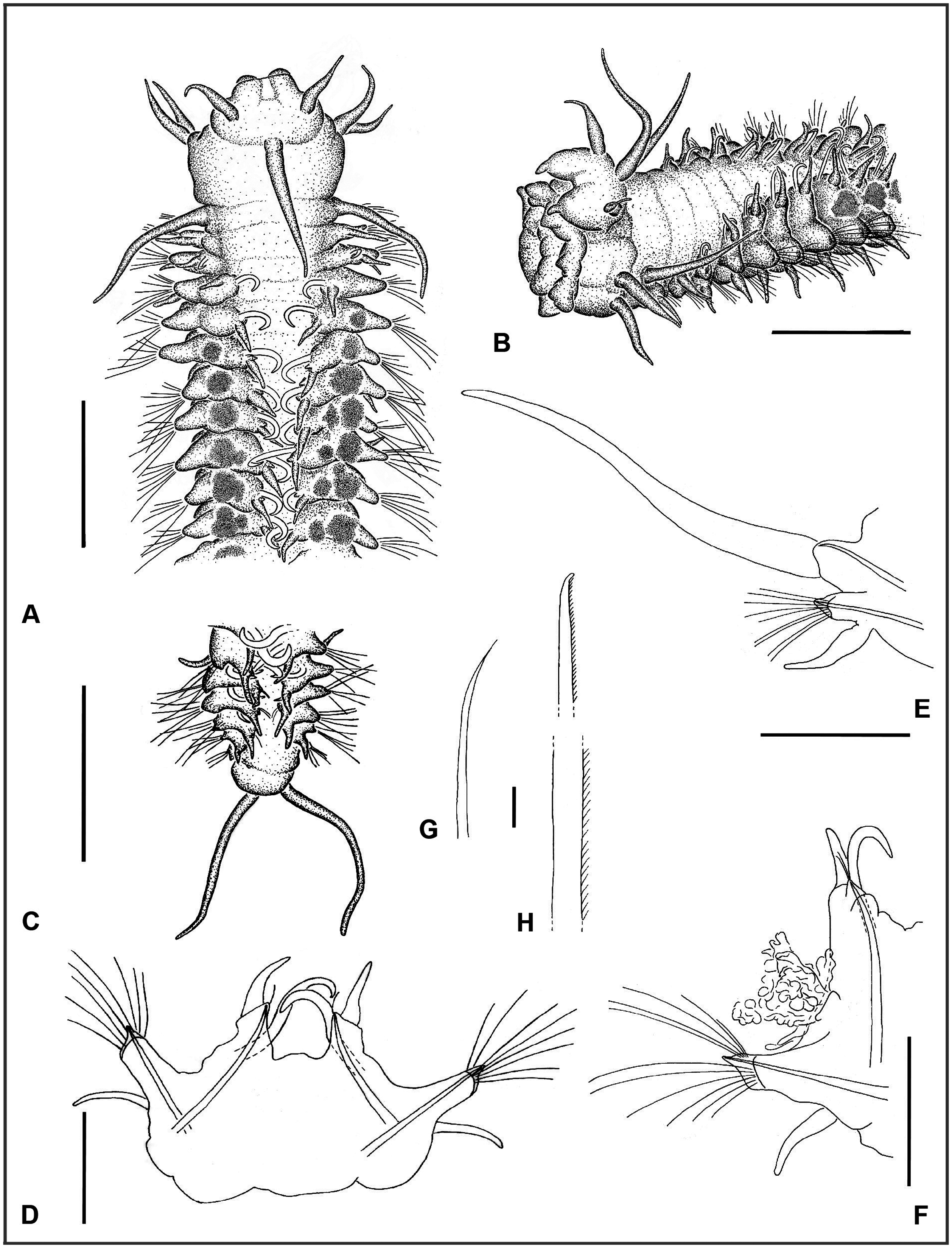
( Figs 6A–J View Figure 6 , 7A, B View Figure 7 , 8A–H View Figure 8 )
‘S igambra armata ’ Borowski, 1996: 116–121, figs 24A–D, 25A–D (manuscript name).
Sigambra magnuncus Paterson and Glover, 2000: 167–169 View in CoL , figs 1–5. – Nishi et al. 2007: 65 (in table 1). – Böggemann 2009: 376–378, figs 114A–F, 115A–K. – Fiege et al. 2010: appendix, tables 3 and 5 (name only). – Salazar-Vallejo et al. 2019: 46 (key only). – Bhowmik et al. 2021: 56 (in table 3), 62 (key).
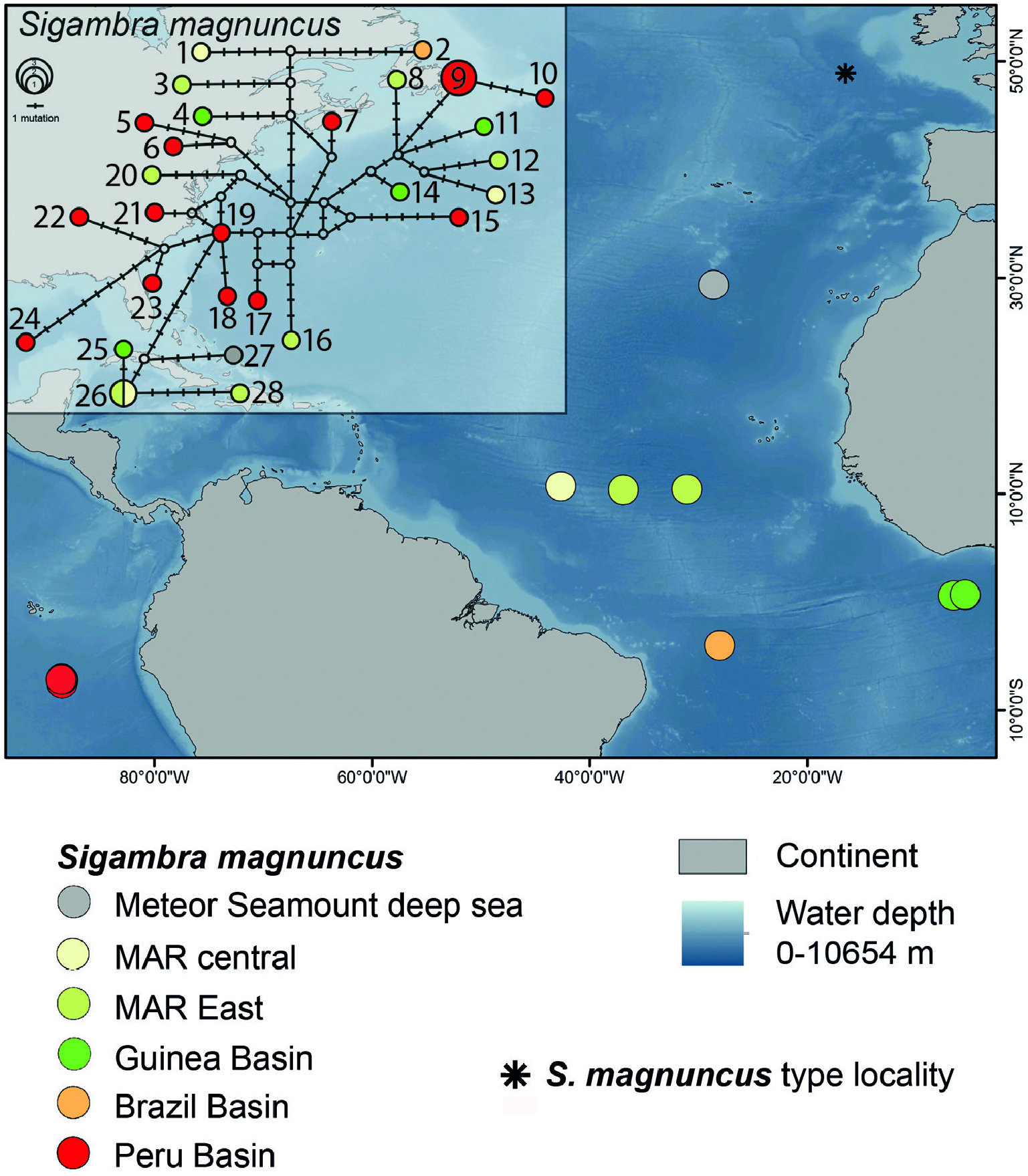
Material examined: Central Atlantic Ocean, Meteor Seamount deep sea, M 79-1 ( DIVA 3), station 636-1, 4339 m ( SMF 30563). Mid-Atlantic Ridge, Central, SO 237 (VEMA-Transit), station 8-4, 5176 m ( SMF 30564, ZMH-P 27949, ZMH-P 27950, ZMH-P 29710, ZMH-P 29711, ZMH-P 29712). Mid-Atlantic Ridge, East, SO 237 (VEMA-Transit), station 6-7, 5085 m ( ZMH-P 29707); station 6-8, 5119 m ( SMF 30562, + SEM 1321, ZMH-P 27947, ZMH-P 27948, ZMH-P 29708); station 4-8, 5735 m ( ZMH-P 27946, ZMH-P 29705, ZMH-P 29706); station 2-6, 5520 m ( ZMH-P 27943). SW Atlantic Ocean, Brazil Basin N, M 79-1 ( DIVA 3), station 605-1, 518 m, ( SMF 30585). SE Pacific Ocean, Peru Basin ( DISCOL area), SO 61 ( DISCOL 1), station 1264-1, 4133 m ( SMF 30534); station 1270-1, 4154 m ( SMF 30535); SO 64 ( DISCOL 2), station 1417-1, 4150 m ( SMF 30536, SMF 30538, + SEM 1322, SMF 30540); station 1421-1, 4175 m ( SMF 30541, ZMH-P 30445); station 1425- 1, 4162 m ( SMF 30537); SO 77 ( DISCOL 3), station 1456-1, 4165 m ( SMF 30543); station 1460-1, 4162 m ( ZMH-P 30444, SMF 30545); station 1464-1, 4154 m ( SMF 30546, SMF 30547); SO 242 ( JPIO-DISCOL 1), station 37-1, 4161 m ( SMF 30577, SMF 30578, SMF 30579, SMF 30580, SMF 30581, SMF 30582, SMF 30588); station 81-3, 4157 m ( SMF 30465, SMF 30466); station 85-4, 4147 m ( SMF 30468); station 93-5, 4185 m ( SMF 30571, SMF 30574, SMF 30575, SMF 30576); station 117-7, 4154 m ( SMF 30572, SMF 30573); station 122-8, 4078 m ( SMF 30569, SEM 1332 + 1333; SMF 30570); station 126-9, 4257 m ( SMF 30583, SMF 30584). – for details and additional specimens see the Supporting Information, Table S2 View Table 2 .
Measurements of largest specimen studied: central Atlantic (SMF 30562): length: 8.7 mm, width: 0.5 mm (excl. parapodia), 57 chaetigers.
Description: Body slightly dorsoventrally flaưened except anteriormost chaetigers, anterior chaetigers widest ( Figs 6A, B View Figure 6 , 8A, B View Figure 8 ). Prostomium wider than long, rounded anteriorly, slightly indented laterally at insertion of lateral antennae and peristomium ( Fig. 6A, C, D View Figure 6 ). One pair of palps and three antennae. Palps biarticulate, palpophores fused basally; palpostyles digitiform, pointing antero-ventrally ( Figs 6B View Figure 6 , 8B View Figure 8 ). Median antenna inserted near posterior margin of prostomium, ca. 1.5 to more than 2× longer than laterals, inserted medio-laterally. Nuchal organs not observed. Eyes absent. Pharynx with eight papillae of equal size ( Fig. 8B View Figure 8 ). Peristomium 2 to 3× longer than first chaetiger, two pairs of tentacular cirri inserted at anterior end, dorsal cirri slightly longer than ventral; longer than lateral antennae but shorter than median ( Figs 6A, B View Figure 6 , 8A, B View Figure 8 ). Transverse row of dense small papillae across dorsum (best visible in SEM). Some specimens with additional row along posterior margin of following segments but fewer papillae and less dense; papillae inverted pyriform, apically indented [ Fig. 6A View Figure 6 (arrow), B, J].
Parapodia biramous; parapodia in chaetigers 1–3 small, oriented laterally; middle and posterior parapodia larger (largest ca. above chaetigers 9–10) with notopodia shiħed dorsally resulting in wide gap between neuro- and notopodia; notopodia of both sides coming close together dorsally ( Figs 6G, H View Figure 6 , 8A–D View Figure 8 ). Numerous specimens with fibrous secretions from pores between parapodial rami in chaetigers from midbody to posterior (from chaetigers 4–10 in specimens from DISCOL 1–3) ( Figs 7A, B View Figure 7 , 8A, B, F View Figure 8 ). Notopodia of chaetigers 1–2 small, bluntly triangular; increasing in length from chaetiger 3. Dorsal cirrus of first chaetiger cirriform, longer than median antenna and 2× length of tentacular cirri; cirri of following chaetigers shorter than tentacular cirri ( Figs 6A, B, D View Figure 6 , 8A, B, E View Figure 8 ). Neuropodia triangular with wide base and conical tip with fascicle of chaetae. Ventral cirri from chaetiger 1 but absent in chaetiger 2, similar in shape to dorsal ones and subequal or slightly longer ( Fig. 6D View Figure 6 ). Notochaetae absent from chaetigers 1–2; single prominent dorsal hook with tip curved strongly inwards from chaetiger 3 to near posteriormost chaetigers; hooks of both sides crossing in midline resembling zipper in dorsal view. Hooks accompanied by short, thin capillaries, one in anterior chaetigers, two in posterior ones ( Figs 6D, G–I View Figure 6 , 8D, F, G View Figure 8 ). Neurochaetae flaưened capillaries of varying length tapering into fine tip, finely denticulate along one edge ( Fig. 6F View Figure 6 , 8H View Figure 8 ).
Pygidium rounded with two long anal cirri inserted ventrally, extending back to chaetigers 4–5 before last ( Figs 6E View Figure 6 , 8C View Figure 8 ).
Remarks: Specimens match well the descriptions of Sigambra magnuncus published by Paterson and Glover (2000) and Böggemann (2009), and main distinguishing characters listed for known species of Sigambra by Nishi et al. (2007: table 1) and Bhowmik et al. (2021: table 3). Revision of the specimens described by Borowski (1996) as ‘ Sigambra armata ’ showed that they belong to Sigambra magnuncus .
Among the 26 described Sigambra species currently recognized (Read and Fauchald 2022), Sigambra magnuncus is closest to S. qingdaoensis Licher & Westheide, 1997 , S. bidentata Britayev and Saphronova, 1981 , S. healyae Gagaev, 2008 , and S. papagayu Bamber in Muir and Bamber 2008, which all share eight pharyngeal papillae, the presence of notopodial capillaries, and dorsal cirri that are longer than ventral ones. However, S. magnuncus is the only species with large notopodial hooks always starting from chaetiger 3, and with ventral cirri subequal or slightly longer than dorsal ones. The median antenna in specimens of S. magnuncus is up to 2× longer than laterals but variable among specimens, making it a less reliable character for distinction among species. In S. qingdaoensis , notopodial hooks start from chaetigers 3–8, and are accompanied by a single capillary chaeta, which is also present in anteriormost chaetigers without notopodial hooks; the median antenna is twice the length of laterals, and anal cirri are relatively short, i.e. as long as lateral antennae. Sigambra bidentata shares with S. magnuncus dorsally-shiħed notopodia along most of the body and hooks crossing in the dorsal midline. It differs from the laưer by the presence of neurochaetae with bidentate tips (neurochaetae tapering to fine tip in S. magnuncus ) and median antenna being barely longer than lateral ones. In S. healyae , notopodial hooks start from chaetiger 4 and are accompanied by a single capillary starting from chaetiger 20; neurochaetae comprise two types, both serrated and with a bidentate tip; the median antenna is 1.5× longer than lateral ones; anal cirri extend back to chaetiger 7 or 8 before last (to chaetigers 4–5 before last in S. magnuncus ). Sigambra healyae is the only species among those listed above with a ventral cirrus present on chaetiger 2 and it shows reddish-brown eyespots which are absent in S. magnuncus . In S. papagayu notopodial hooks start from chaetigers 3–5, the median antenna is 1.75× length of laterals, neurochaetae are simple capillaries with a number of slender, curved, serrate capillaries located supraacicular with serrations low and rounded, and a pair of distinct pectinate chaetae located subacicular with 14–17 teeth each and a naked slender distal extension; two anal cirri extend back to chaetigers>5 before last (Bamber in Muir and Bamber 2008: fig. 1C). Sigambra papagayu is the only species among those listed above without notopodial capillaries. Sigambra magnuncus has been recorded only from abyssal depths between 3900 and 5700 m in the Atlantic and Pacific Ocean. Sigambra healyae was found at ca. 1800 m depth in the Arctic Ocean, S. bidentata between 510 and 2220 m in the Sea of Japan, S. quingdaoensis and S. papagayu in coastal waters in the Yellow Sea (near ºingdao, China) and South China Sea ( Hong Kong), respectively.
Rows of characteristically shaped papillae across the dorsum, as described for Sigambra magnuncus ( Figs 6A, B View Figure 6 , 7B View Figure 7 ), have also been noted for S. bassi (Hartman, 1947) , S. hanaokai ( Kitamori, 1960) , S. phuketensis Licher and Westheide, 1997 , S. tentaculata ( Treadwell, 1941) , S. parva (Day, 1963) , S. grubii , and S. hernandezi Salazar-Vallejo et al., 2019 ( Licher and Westheide 1997, Nishi et al. 2007, Moreira and Parapar 2002, Salazar-Vallejo et al. 2019). A sensory function for these papillae has been suggested by Paterson and Glover (2000).
In specimens of S. magnuncus collected in the Peru Basin (SE Pacific), Borowski (1996) described tuħs of fibrous matrix extruding from interramal pores as one to two amorphous strands fraying distally, sometimes doưed with singular cells. Pores start from chaetigers 4–10 and extend into the posterior region in most of the larger specimens, but are missing in small juveniles. Similar structures can also be seen in Paterson and Glover (2000: figs 1A and 2: whitish tuħs in mid-body chaetigers and chaetiger 4, leħ side). They were also observed in our more recently collected specimens from the Peru Basin (SE Pacific), as well as in specimens originating from the Angola Basin (SE Atlantic) ( Fig. 7A, B View Figure 7 ). These structures have been described as ‘hypertrophied gonopores’ by Salazar-Vallejo et al. (2019) for S. diazi Salazar-Vallejo et al., 2019 , S. hernandezi , S. olivai Salazar-Vallejo et al., 2019 , and S. grubii . Bhowmik et al. (2021) described and figured them as parapodial glands for S. sundarbanensis Bhowmik et al., 2021 between chaetigers 5 and 60, rudimentary in small and fully developed in larger specimens. Methyl green staining showed similarities to the chromophile glands in neuropodial pinnae of Tomopteridae but further histochemical and morphological studies are needed to clarify the function of these glands. Picture of a live specimen of S. hanaokai in Nishi et al. (2007: figs 2A, B) shows tuħs of greenish material possibly originating from such parapodial glands, which appear to be a quite common feature in species of Sigambra .
Sigambra magnuncus was originally described from the deep North Atlantic (Porcupine Basin to Cape Verde Islands) by Paterson and Glover (2000). The extended distribution of S. magnuncus indicated for the Atlantic by our samples from the Angola and Brazil Basins, and from the Guinea Basin studied by Böggemann (2009), as well as our samples from the Peru Basin initially studied by Borowski (1996), suggest that this species might not only have a continuous longitudinal distribution along the whole deep Atlantic east and west of the Mid-Atlantic Ridge but is also present in the SE Pacific. Böggemann (2009) did not find clear population paưerns in his molecular data, suggesting no restricted gene flow within the Guinea Basin. The presence of planktonic larvae among Pilargidae (Bhaud 1974, Achari 1975, Bhaud and Cazaux 1987) might provide an explanation for the wide and obviously even pan-oceanic distribution of this species.
Distribution: NE Atlantic: 4000–5085 m ( Paterson and Glover 2000); S Atlantic, Angola and Brazil Basins, 5179–5495 m (Böggemann 2009, this study); Central Atlantic, Guinea Basin and Vema Fracture Zone, 3945–5735 m (Böggemann 2009, this study); SE Pacific, Peru Basin, 4078–4257 m (this study). Molecular data confirm the distribution of Sigambra magnuncus for the Atlantic (Meteor Seamount deep sea, central Atlantic and Brazil and Guinea Basin) and the Pacific ( Peru Basin) ( Fig. 1 View Figure 1 ).
| SMF |
Forschungsinstitut und Natur-Museum Senckenberg |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Sigambra magnuncus Paterson & Glover, 2000
| Meissner, Karin, Schwentner, Martin, Göưing, Miriam & Fiege, Thomas Knebelsberger and Dieter 2023 |
Sigambra magnuncus
| Salazar-Vallejo SI & Rizzo AE & de Leon-Gonzalez JA 2019: 46 |
| Nishi E & Tanaka K & Fujioka Y 2007: 65 |
| Paterson GLJ & Glover AG 2000: 169 |