Pseudopotamilla reniformis ( Bruguiére, 1789 ), Bruguiere, 1789
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4254.2.3 |
|
publication LSID |
lsid:zoobank.org:pub:88B33DE9-BCF2-4AE4-A1B4-F0D39DCDF5C3 |
|
DOI |
https://doi.org/10.5281/zenodo.6040988 |
|
persistent identifier |
https://treatment.plazi.org/id/747D7A68-FFDD-0614-FF41-FD79FD7CF72B |
|
treatment provided by |
Plazi |
|
scientific name |
Pseudopotamilla reniformis ( Bruguiére, 1789 ) |
| status |
|
Pseudopotamilla reniformis ( Bruguiére, 1789) View in CoL
( Figs 1–3 View FIGURE 1 View FIGURE 2 View FIGURE 3 )
Die nieren- formige Amphitrite Müller, 1771: 194 –200, pl. 16, figs 1–3, Iceland.
Amphitrite reniformis Bruguiére, 1789: 57 –58.
Sabella reniformis Leuckart, 1849: 183 View in CoL –189, Iceland.— Sars 1862: 123 –124, northern Norway, Iceland, Greenland and north America.
? Sabella oculifera Leidy, 1855: 145 View in CoL , pl. 11, figs. 55–61, Rhode Island, USA.
Sabella oculata Krøyer, 1856: 22 View in CoL ( ZMUC – POL 00424) and Krøyer’s unpublished notes and drawings ( RLC, New Royal collection, 322): Finmark, northern Norway.
Potamilla reniformis View in CoL .— Malmgren , 1867: 114, pl. 13, fig. 77A–D, Iceland, Greenland, Finmark and? North America.— Lukasch 1910: 34 ( ZISP 64 View Materials /13056) Pula Inlet, Kola Fjord, Barents Sea.
Other taxa incorrectly identified
Potamilla reniformis View in CoL .— Rioja 1917: 63–64, fig. 19 (MNCN 16.01/515), Gijon, north Spain.— Rioja 1923: 27–29, figs 21–22, Santander, north Spain (= Pseudopotamilla saxicava (Quatrefages)) View in CoL .
Potamilla reniformis View in CoL .— Fauvel 1927: 309 –310, fig. 10a–l, in part, English Channel, France, Spain and Mediterranean (= Pseudopotamilla saxicava (Quatrefages)) View in CoL .
Potamilla reniformis View in CoL .— Annenkova 1938: 212 –213 (ZISP 15/33740), Japan Sea (= Pseudopotamilla polymorpha ( Johnson, 1901) , see Discussion below).
Potamilla reniformis View in CoL sub sp. oligophthalmus Grube, 1878.— Annenkova 1938: 213 (ZISP 2/33774), north Japan Sea (= Pseudopotamilla myriops (Marenzeller, 1885)) View in CoL .
Pseudopotamilla reniformis View in CoL .— Knight-Jones 1981: 185, figs 13–17, 48–51, 58–60, 63; 1983: 254, fig. 3A–C; Chughtai 1984, 1986: 168, figs 9–14; Chughtai & Knight-Jones 1988: 231 –236, figs 1, 3, 5–6, 10A; Knight-Jones 1990: 280, fig. 6.22; Knight-Jones et al. 1991: 852 (= Pseudopotamilla saxicava (Quatrefages)) View in CoL .
Material examined. Type material: Neotype, Sandgerði, Iceland, stn 23 (64° 01.8′ N, 022° 42.8′ W), kelp holdfast, low shore ( NMW.Z.2001.042.0001), 26.06.2001 GoogleMaps . Paraneotypes , Sandgerði, Iceland, stn 23 (64° 01.8′ N, 022° 42.8′ W), kelp holdfast, low shore, 5 specimens ( NMW.Z.2001.042.0002), 26.06.2001 GoogleMaps . Additional material: Other reviewed specimens from Britain , Nova Scotia and Newfoundland are detailed in Materials and Methods section. Godhavn, Greenland ( ZMUC, Krøyer) ; Nova Scotia, Canada (det. MEP); Ermolinskaya Inlet , Marine Biological Station, White Sea ( ZMMSU, det. Kupriyanova in 1989).
Diagnosis. Collar with high, well-developed, rounded lappets dorsally; dorsal margins V-shaped; peristomium well exposed dorsal and laterally above collar margins; companion chaetae with handles slightly longer than handles of uncini.
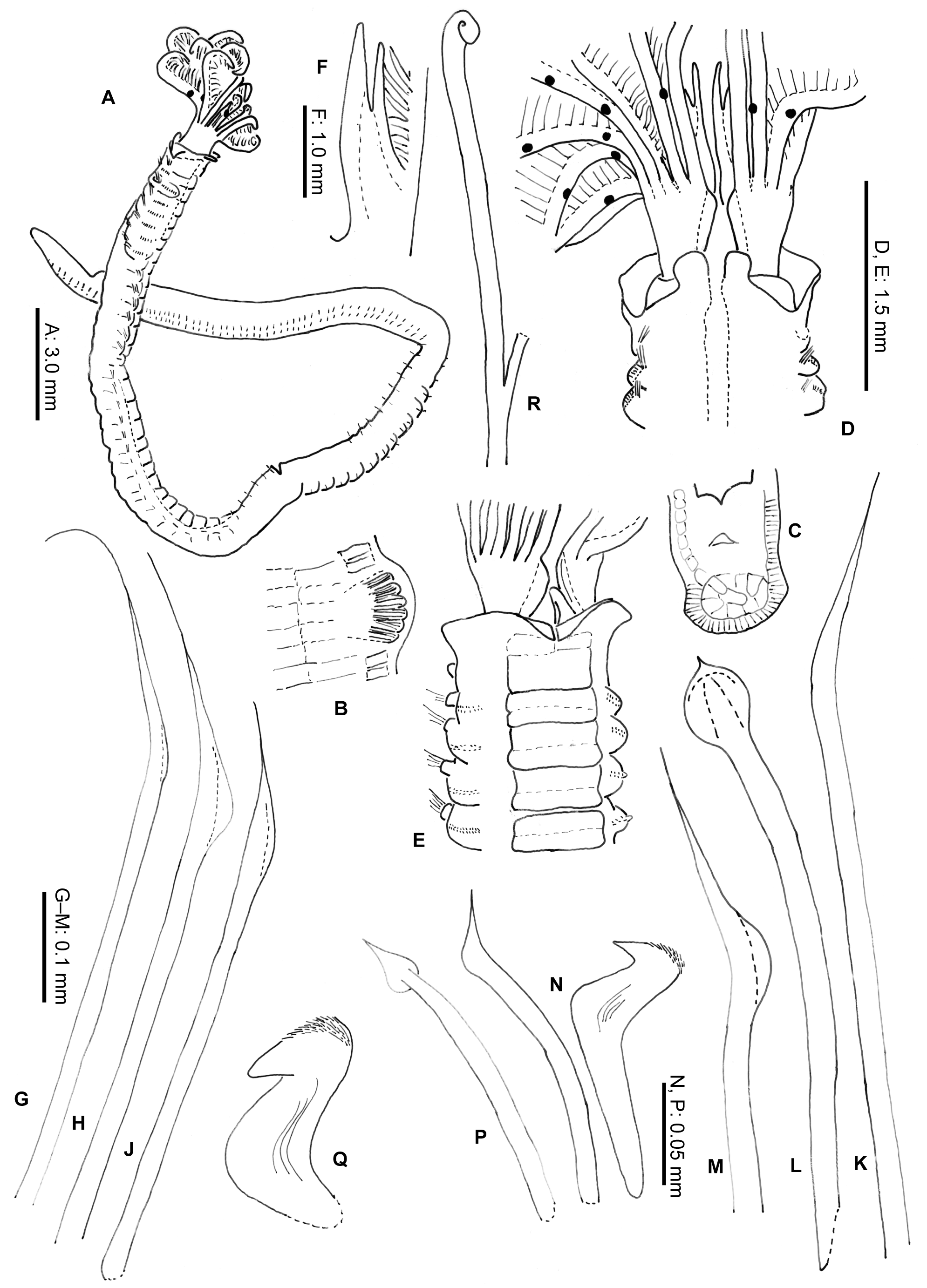
Description. Neotype complete in two parts. Body without radiolar crown about 38 mm long, 1.5 mm wide. Radiolar crown 4 mm long. Thorax with 8 segments and abdomen with about 120 ( Fig. 1 View FIGURE 1 A). Radiolar crown asymmetrical with radioles (24) shorter ventrally, tips short and blunt. Dorso-lateral radioles with one or two (can be up to four) unpaired, dark compound eyes, except the dorsal most pair ( Fig. 1 View FIGURE 1 B; R x2121 xx11xxx, L x 11221211 xxx). Interradiolar web absent. Radiolar lobes about 7.5 mm long, with dorsal and ventral basal flanges ( Fig. 1 View FIGURE 1 D), ventral ones more oblique to accommodate ventral sacs ( Fig. 1 View FIGURE 1 E). Long dorsal lips about half length of radioles with a bifid appearance, each being supported by both radiolar appendage (mid-rib) and enlarged pinnule from base of adjacent radiole ( Fig. 1 View FIGURE 1 F). Dorsal collar ( Fig. 2 View FIGURE 2 A) with two high, rounded lappets extending wellabove junction of crown and thorax; mid-dorsal margins of dorsal lappets fused to sides of midline faecal groove; dorsal collar lappets with lateral margins in line with body axis anteriorly and oblique posteriorly, part of a wide, Vshaped notch showing peristomium ( Fig. 1 View FIGURE 1 D). Lateral collar ( Fig. 2 View FIGURE 2 C) oblique anteriorly, highest ventral part close to midline incision and in front of ventral sacs. First ventral shield ( Fig. 2 View FIGURE 2 B) twice as long as following ones, anterior margin indented medially, following thoracic shields rectangular, each divided transversely, but indistinctly, into two halves, grooves separating shields well defined ( Fig. 1 View FIGURE 1 E). Collar chaetae with two rows of broadly hooded chaetae, hoods as wide as or narrower than handles ( Figs 1 View FIGURE 1 G-H, J). Superior notochaetae of following thoracic fascicles narrowly hooded ( Fig. 1 View FIGURE 1 K), few, slender and longer than inferior chaetae, each inferior paleate chaeta (about ten) with small distal mucro ( Fig. 1 View FIGURE 1 L). Abdominal neurochaetae of one kind, elongate broadly hooded, each with hood twice width of handle ( Fig. 1 View FIGURE 1 M). Thoracic tori short with gaps between ventral ends and lateral margins of shields ( Fig. 1 View FIGURE 1 E), each uncinus avicular, with numerous fine crest teeth covering half of main fang, distance between end of handle and breast nearly twice as long as that between breast and tip of main fang, each companion chaeta with longer handle than that of uncini ( Fig. 1 View FIGURE 1 N) and distal angled, tear-drop blade ( Fig. 1 View FIGURE 1 P). Abdominal uncini smaller than thoracic and with shorter handles ( Fig. 1 View FIGURE 1 Q). Pygidium bilobed. Tube, enrolled distally ( Fig. 1 View FIGURE 1 R), of thin semi-hardened mucus with outer layer of embedded detritus including fragments of black and white shell in emergent areas, sometimes found with posterior branch made by departed offspring.
Colour. Live specimens with liver-coloured pigments in broad bands around radiolar crown, on outside of crown base, on collar, surrounding 1st and 2nd parapodia and forming oval patches each side of 3rd and 4th parapodia. Paler tint flanking dorsal faecal groove and covering 2nd and 3rd ventral shields; rest of body pale and most ventral shields paler. Fixed specimens pale.
Variation. The observed variability is correlated to the presence of asexual reproduction. A large clump of 34 specimens of Pseudopotamilla reniformis from Hvalfjordur (collected 8.10.1994) was examined to see the frequency of scissiparity. One tube contained an ‘adult’, thorax and abdomen 37 mm long with a recently healed posterior (no pygidium) and a developing radiolar crown (two semi-circles of finger-like proto-radioles). Posterior to the parent were seven fragments of abdomen (largest 5 mm long) all with buds at both ends, only one with a distinct pygidium. One of these, a), with 7 segments, is 8 mm long, 3 mm wide, with crown an additional 1.5 mm long. The first and second segments have new parapodia with very small tori on the ventral sides of fascicles; segments 3–6 have both new thoracic and old abdominal parapodia, the latter with tori on the dorsal sides of fascicles.
After fission, some original abdominal parapodia degenerate ( Gross & Huxley 1935; Kolbasova et al. 2014), as new ones develop fascicles and tori arranged as in the thorax. The new complement of thoracic chaetigers will often be variable in number. The remaining abdominal segments retain their parapodia, the most posterior segment producing a new pygidium and new abdominal segments anterior to the pygidium. Another fragment, b), has a body 5 mm long, 1.7 mm wide, with a developing thorax of seven segments each with both new and old parapodia. The abdomen is left with nine original segments followed by new, short, narrow segments anterior to a new pygidium ( Fig. 3 View FIGURE 3 A–B). Many of the other thirty-three parents also lacked fully developed crowns. If the radioles were well developed, the pinnules were often under-developed and only three had compound eyes. Five specimens have eight thoracic segments, five specimens have nine segments, sixteen have ten segments, and five have eleven segments. These specimens produced 19 scissiparous offspring between them, ten with buds at one end, nine with buds at both ends and only two with a developing crown and posterior abdomen.
Fourteen collections of Pseudopotamilla reniformis from Nova Scotia and Newfoundland showed patchy distribution, but at stations 1, 2 and 9 (July and October, see Materials and Methods) they were found in considerable aggregations typical of asexual reproduction. The contents of nine tubes from station 2, Digby Neck (largest 69 mm long and 2 mm wide and the smallest 8 mm long and 0.7 mm wide) were examined (MEP). Four of the smaller tubes (8 to 50 mm long) had each branched from a larger parent tube, 25 mm or more from the tube aperture; two specimens in these tubes (now labelled 5 and 7) contained nearly entire small adults, but only one with a radiolar ocellus. One is 17 mm long with about 65 chaetigers and the other 11.3 mm long with 24. The former lacked offspring, but the latter had two. The largest adult in these four tubes was 23.9 mm long, with 28 chaetigers and a nearly complete crown (radioles without ocelli). It had dehisced posteriorly to produce three abdominal fragments ( Fig. 3 View FIGURE 3 C): fragment a) 4 mm long with 12 segments and developing radioles; fragment b) 2.5 mm long, 9 chaetigers and no radiolar buds and fragment c) 1.4 mm with 5.5 chaetigers, also without radiolar buds. Of the adults in the five larger non-branching tubes, four had radiolar crowns and three had 1–2 dehisced abdominal fragments. Only one out of the ten fragments examined showed any sign of re-organisation of fascicles and tori, from the abdominal to the thoracic arrangement.
The only noticeable difference between samples collected in July and August from eastern Canada and in June from Iceland is that thirty-one of thirty-four Icelandic adult specimens had re-developing radiolar crowns. Crowns can be lost by predation, but it seems unlikely that so many adults within the Hvalfjordur clumps could have lost their crowns in such a way, as their speed of withdrawal into tubes is so very fast. Such parents were fairly large (maximum body width 3 mm) so it is a puzzle as to why the development of their crowns had been delayed. This contrasts with material collected from Sandgerði in July 2001, most of which had fully formed crowns. Six of the nine specimens from Nova Scotia also had crowns. The uniformity of development of scissiparous offspring in all three collections and particularly the sparseness of chaetal re-organisation in fragments examined, suggests their asexual development had been more or less synchronous.
Remarks. Müller’s description of Amphitrite die nieren-formige ( Pseudopotamilla reniformis ) did not report features of the collar, but Leuckart (1849 as Sabella reniformis ), who had also studied Icelandic material, noted that the dorso-lateral collar margins were provided with a deep curved cut-out each side. Sars (1862, as Sabella reniformis ), studying material from the north Norwegian coast, also noted that the collar margins had “lateraliter profound incism” (deep lateral incision).
In synonymising Sabella oculifera Leidy (1885, type not available) with reniformis, Sars extended the distribution of P. reniformis to Norway and North America, which agrees with our studies. Malmgren (1867: 222, pl. 14) added to Sars’ synonymy, but wrongly included Sabella aspersa Krøyer , which is also found in Greenland. Malmgren’s figures (77 and 77A) show collar margins with distinct dorso-lateral notches, but that plate does not show the lengths of the handles of companion chaetae and uncini so it cannot be confirmed whether his species is P. reniformis or P. aspersa . Rioja’s (1923, fig. 21) and Fauvel’s (1927) schematic figure of P. reniformis (107b) resembles that of Malmgren, but his figure 107a is typical of the southern form Pseudopotamilla saxicava (Quatrefages) . Iroso, (1921, pl. 3) described three species from the Gulf of Naples, Potamilla troncatula , Potamilla obscura and Potamilla oligophthalma , all of which Hartman (1959) regarded as P. reniformis . Hartman was probably right in regarding them as one species (there is no type material), but they are more likely to be P. saxicava (see below).
Material from the Japan Sea identified by Annenkova (1938) as Potamilla reniformis is Pseudopotamilla polymorpha ( Johnson, 1901, relationship between Pseudopotamilla and Eudistylia is discussed below) and that identified by her as Potamilla reniformis sub sp. oligophthalmus Grube is Pseudopotamilla myriops ( Marenzeller, 1885 = Potamilla chiachouensis Kao et al. 1959 = Eudistylia catherinae Banse, 1979 ). The lack of P. reniformis in these waters agrees with Klebovich (1961) material from the Kurile Islands and that of Buzhinskaya (1985) from Sakhalin Island. More northerly material of P. reniformis from Kamchatka, donated by AVR (KJC), seems to be closer to P. aspersa , but differences suggest a new species Pseudopotamilla sp. A (PKJ). ‘ Potamilla’ reniformis from the Kommandor Islands ( Annenkova 1934) may well be Pseudopotamilla sp. A (PKJ).
Pettibone’s figure (1954, fig. 38o–u as Potamilla ) of material from Point Barrow (Alaska) agrees well with P. reniformis and her synonymy with Pseudopotamilla intermedia Moore, 1905 is probably correct, according to Moore’s description and Hartman’s (1938: pl. 2) figure 8 of the dorsal collar (type material). Some of Pettibone’s more southerly synonyms, however, are wrong (see above) and all others need confirming, including a detailed revision of specimens recorded as P. reniformis from Alaska, particularly with respect to the dorsal lappets of the collar, thoracic uncini and companion chaetae.
Habitat. Pseudopotamilla reniformis from Iceland is locally abundant in sheltered niches amongst encrusting fauna and kelp holdfasts. The more widespread sampling of Nova Scotian and Newfoundland material showed a patchy distribution, but at stations 1, 2, and 9 (see Materials and Methods) they were found in the typical aggregations where reproduction is asexual. It was interesting to find that the odd sample had penetrated the surface of shells amongst the Sabellaria flats at station 6.
Distribution. Fauvel’s (1927) list of synonyms in a book, which was used by many polychaetologists, gave the impression that the distribution of Pseudopotamilla reniformis was very wide spread, from the Arctic Seas to the Mediterranean. In fact P. reniformis seems to be restricted to cold northern waters. With the removal of records of P. reniformis that have been re-identified as Pseudopotamilla saxicava (Quatrefages) , Pseudopotamilla myriops and Pseudopotamilla polymorpha , the distribution of P. reniformis is reduced to Iceland, northern Norway and eastern Canada (Nova Scotia & Newfoundland).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Pseudopotamilla reniformis ( Bruguiére, 1789 )
| Knight-Jones, Phyllis, Darbyshire, Teresa, Petersen, Mary E. & Tovar-Hernández, María Ana 2017 |
Pseudopotamilla reniformis
| Knight-Jones 1991: 852 |
| Knight-Jones 1990: 280 |
| Chughtai 1988: 231 |
| Chughtai 1986: 168 |
| Knight-Jones 1981: 185 |
Potamilla reniformis
| Annenkova 1938: 212 |
Potamilla reniformis
| Annenkova 1938: 213 |
Potamilla reniformis
| Fauvel 1927: 309 |
Sabella reniformis
| Sars 1862: 123 |
Sabella oculata Krøyer, 1856 : 22
| Kroyer 1856: 22 |
Sabella oculifera
| Leidy 1855: 145 |
Amphitrite reniformis Bruguiére, 1789 : 57
| Bruguiere 1789: 57 |