Marphysa madrasi, Hutchings & Lavesque & Priscilla & Daffe & Malathi & Glasby, 2020
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4852.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:35AD6AD7-E477-4451-BA32-1617E04B8F44 |
|
DOI |
https://doi.org/10.5281/zenodo.4409879 |
|
persistent identifier |
https://treatment.plazi.org/id/8F338796-FFE6-FFEC-FF1D-11CC1540EE8E |
|
treatment provided by |
Plazi |
|
scientific name |
Marphysa madrasi |
| status |
sp. nov. |
Marphysa madrasi View in CoL n. sp.
Figs 1–5 View FIGURE 1 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 , 7 View FIGURE 7 , Tables 2, 3
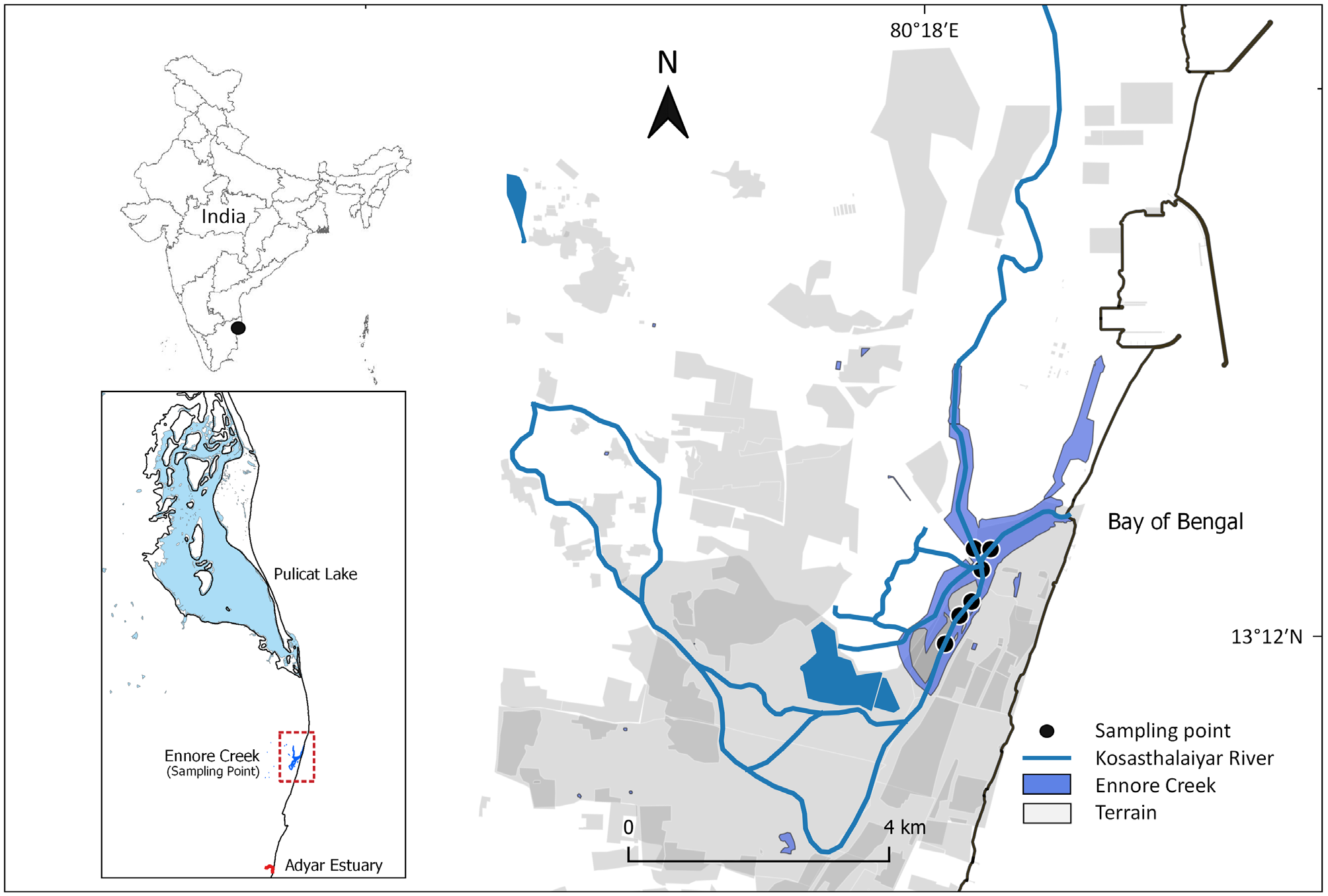
Material examined. Holotype: NL-ENNORE_01 ( ZSI), complete, Chennai , Ennore Creek, Bay of Bengal, India, 13°12’05.8”N 80°18’23.8”E ,, collected from intertidal mud flats at low tide, 15 October 2019, by LP (see Fig. 1 View FIGURE 1 ), parapodia 3, 81, 159, 237, 315 and far posterior removed for SEM and tissue sample removed for molecular studies. GoogleMaps
Paratypes ZSI-HQ / GNC/ An 6072/1, 4 specimens, collected October 2019, paratype ( ZSI) collected by Les Safarik from same location as holotype, collected June 2019, parapodia 3, 60, 110, 180, 240 removed for SEM and tissue sample removed from paratype 1 ( ZSI); 2 specimens collected by GoogleMaps LP same site as holotype, parapodia 3,72, 132, 200, 280, 340 removed for SEM ( ZSI) .
All material preserved in 95% alcohol.
Non-type material examined. 1 specimen ( NTM W25489), Pulicat Lake , India, reported in Malathi et al. (2011) as Marphysa gravelyi .
Description. Based on holotype, with variation in parentheses for paratypes, unless otherwise noted.
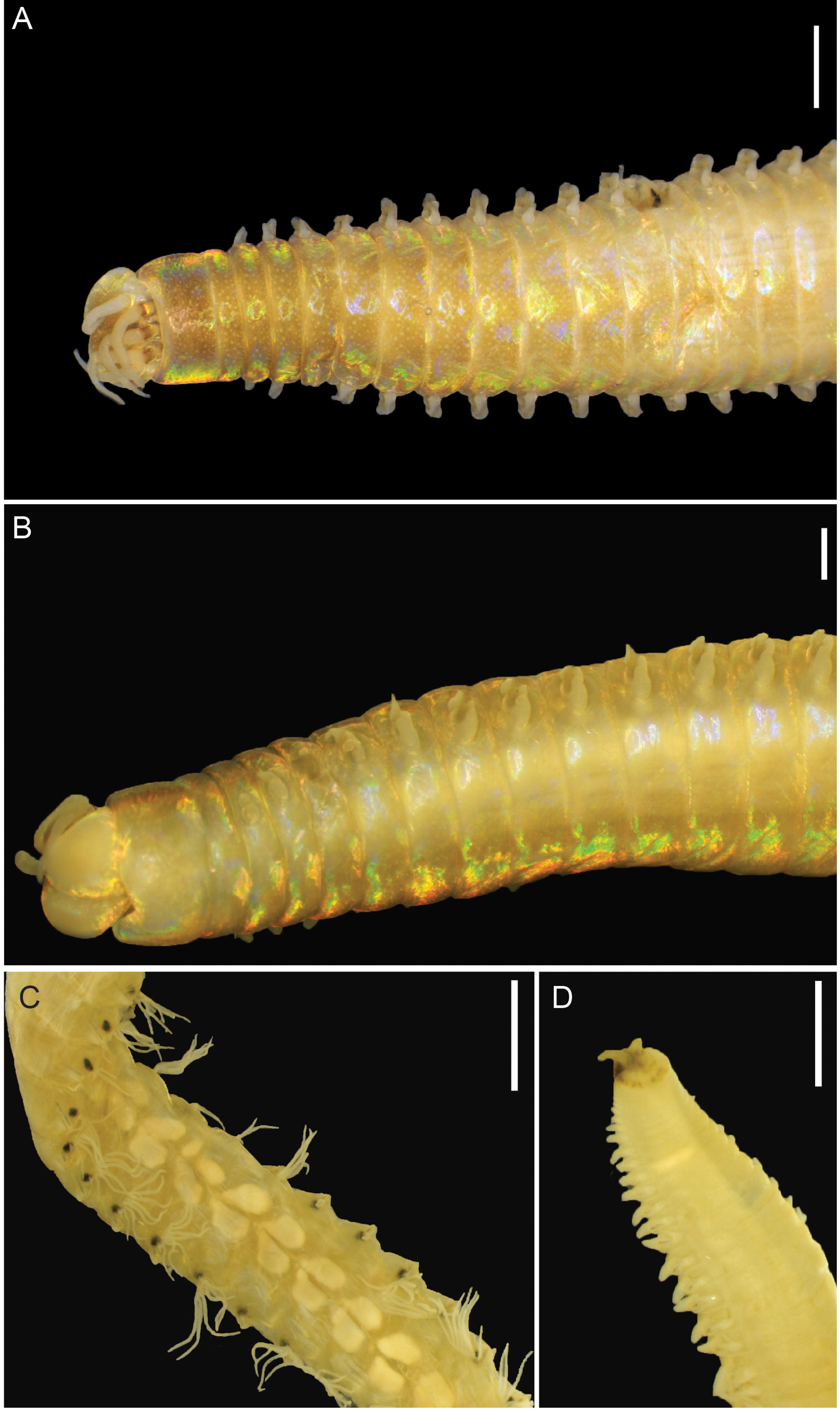
Preserved specimens 395 (220–488+) chaetigers, 286 mm (134–311+) long, 6 mm (4.0–9.0) in length to chaetiger 10, 2.5 mm (2.0–3.9) in width at chaetiger 10, excluding parapodia. Body elongate and tapering distally at both ends ( Fig. 2 View FIGURE 2 A–B, D). Colour of preserved material whitish and iridescent, head-end dorsally brownish with white spots except for white palps and antennae, parapodia of mid body with paired dark spots ( Fig. 2C View FIGURE 2 ); live material blood red.
Prostomium rounded anteriorly with two dorsoventrally flattened buccal lips and an anterior notch between them ( Fig. 2A, B View FIGURE 2 ). Two palps and three antennae slender and tapering, each with short palpophores and ceratophores arranged in an arc on posterior margin of prostomium.Antennae smooth, of equal length, slightly longer than palps, about 2x longer than prostomium ( Fig. 2A View FIGURE 2 ). Small pair of eyes present, very faint, located at posterior base between palps and lateral antennae (only visible on left side of holotype). First peristomial ring about 2.5–3.0x longer than second one, with rounded notch on anterior margin, ventrally ( Fig. 2B View FIGURE 2 ).
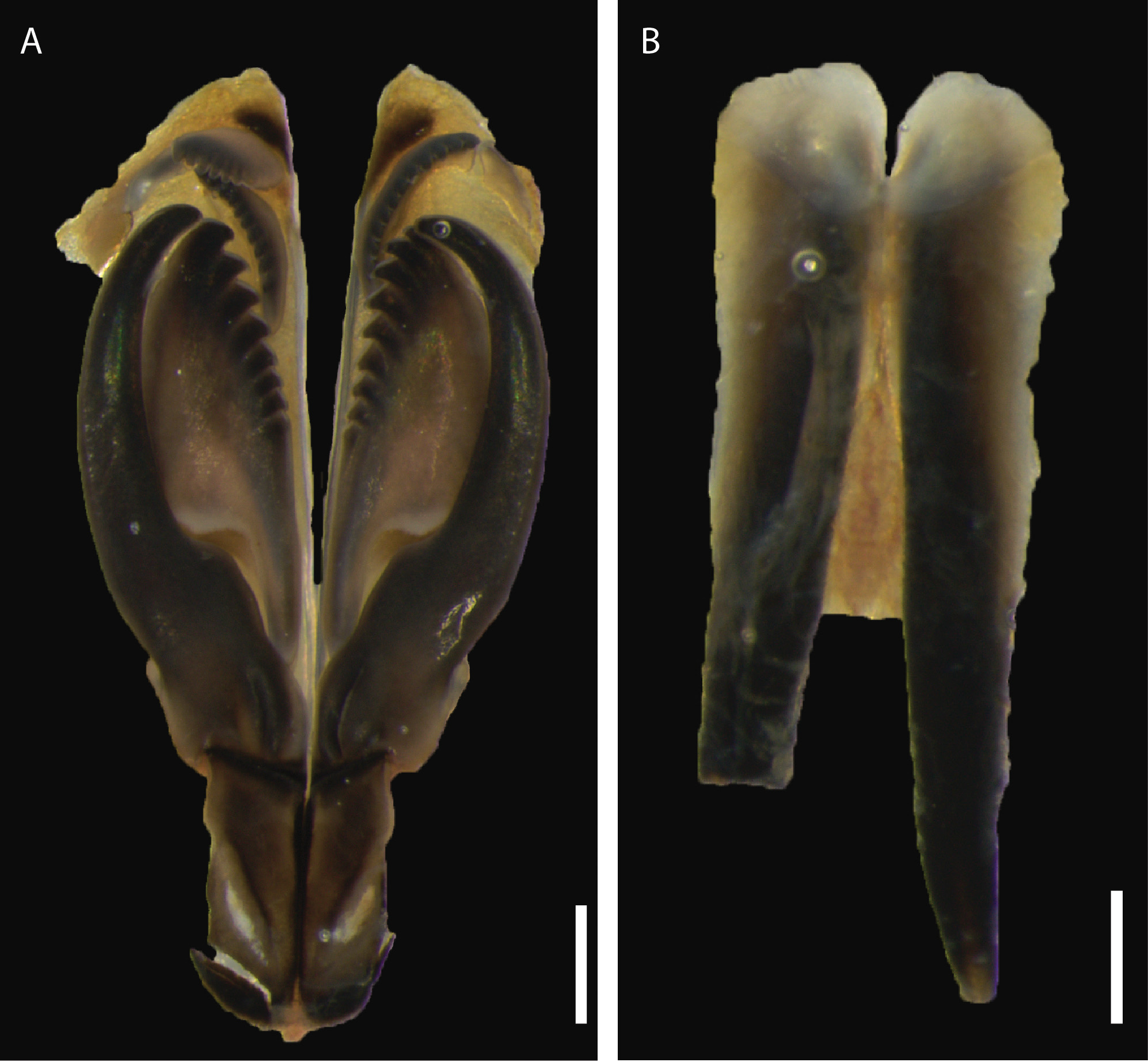
Maxillary apparatus dissected out from paratype (ZSI-HQ/GNC/An6072/1). Maxillae (M) with carriers and four paired elements and one single one, formula as follows: MF = 1+1, 8+9, 10+0, 7+11, 1+1 ( Fig. 4A View FIGURE 4 ). MI approximately two times longer than maxillary carrier. Carriers rectangular, with rounded tips posteriorly. MI forcepslike, without attachment lamellae; well-developed, sub-right-angle falchal arch. Closing system approximately 4.5 times shorter than MI. Ligament between MI and MII rectangular, dark. MII wide, without attachment lamella, teeth triangular, recurved, and distributed in less than half of plate length. Ligament between MII and MIII absent (or not sclerotized). MIII, single, slightly shorter than right MIV, curved forming distal arc; with equal-sized triangular teeth; absence of attachment lamella. Left MIV short (half the size of right MIV) with wide, rounded base, left two teeth longer than right-most one; attachment lamella dark, semi-circular. Right MIV with teeth triangular, decreasing in size posteriorly; attachment lamella dark, semi-circular, wide. MV, paired, rectangular (as long as wide), with a broad cutting edge, and no clearly-defined teeth (but following tradition to score as 1+1). Mandibles ( Fig. 4B View FIGURE 4 ) dark, with fine longitudinal growth stripes; slightly shorter than MI plus carriers; cutting plates whitish, without distinct growth rings.
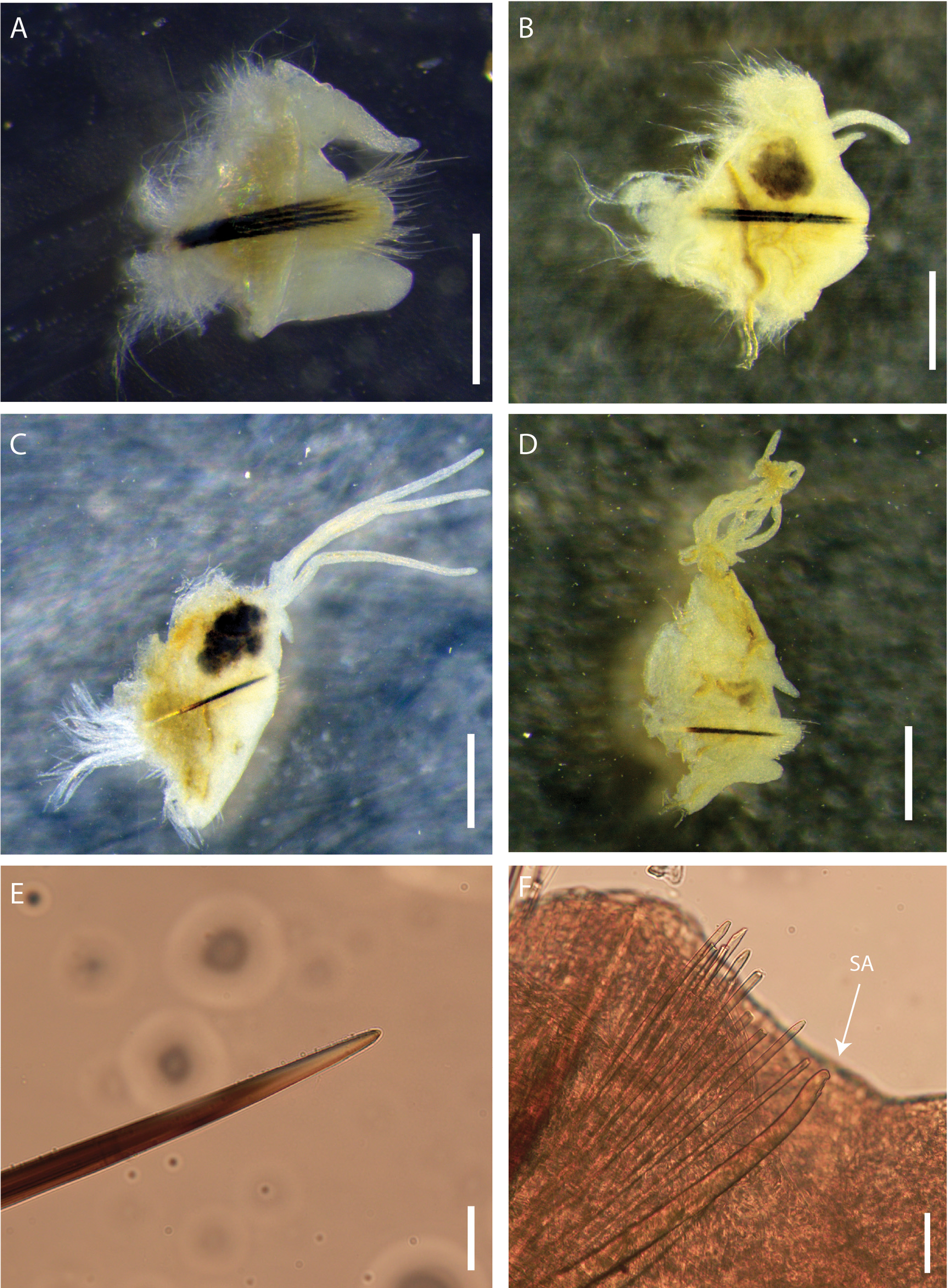
First two parapodia located below middle line of body wall, but gradually positioned dorsally to about midline in subsequent segments ( Fig. 2A, B View FIGURE 2 ). Notopodial dorsal cirri slender, tapering, extending laterally slightly beyond both post-chaetal lobe and ventral cirri in anterior body ( Fig. 3A View FIGURE 3 ); shorter than chaetal lobes in mid-body chaetigers ( Fig. 3B View FIGURE 3 ); and similar size as chaetal lobes in posterior chaetigers ( Fig. 3C View FIGURE 3 ), except for posterior-most ( Fig. 3D View FIGURE 3 ). Chaetal lobes comprising a low pre-chaetal lip and a tongue-like post-chaetal lobe ( Fig. 3A View FIGURE 3 ). Ventral cirri bluntly conical, bases slightly expanded, first few exceeding lengths of post-chaetal lobes, thereafter about same length as post-chaetal lobes ( Fig. 3A, D View FIGURE 3 ). Branchiae pectinate, commencing from chaetiger 48 (22–50), best developed from chaetigers 44–75 to mid-posterior body, and continuing to within 10 chaetigers from pygidium; number of filaments increasing from 1–2 anteriorly to 5–9 in mid-body ( Figs 2C View FIGURE 2 ; 3 View FIGURE 3 B–C), decreasing to 4–5 (6) ( Fig. 3D View FIGURE 3 ) in last several chaetigers. Branchiae wrinkled and vascularised at base where best developed.
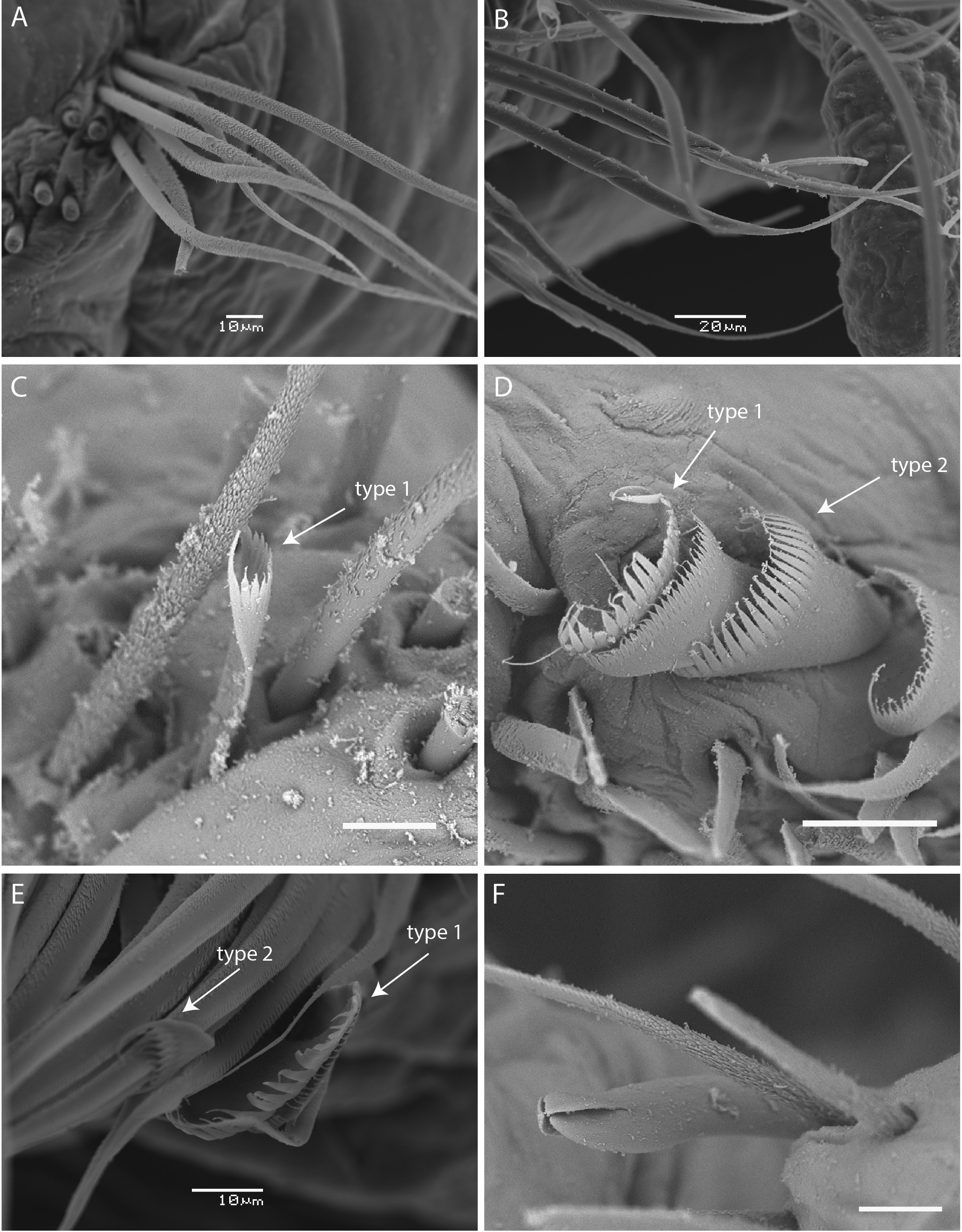
Aciculae black with paler blunt tips ( Fig. 3E View FIGURE 3 ), approximately four per parapodium in anterior chaetigers, two per parapodium in middle chaetigers, and one per parapodium in posterior chaetigers ( Fig. 3 View FIGURE 3 A–D). Single subacicular hook, with two guards covering bidentate tip ( Figs 3F View FIGURE 3 ; 5F View FIGURE 5 ) beginning from chaetiger 72 (33–66). Anterior parapodia with simple limbate capillaries, above and below acicula, with finely serrated surfaces ( Fig. 5A, C View FIGURE 5 ), many chaetae broken. Compound spinigers in subacicular position only, from chaetiger 83 with finely pointed tips ( Fig. 5B View FIGURE 5 ), continue to at least chaetiger 159 (chaetae on subsequent parapodia mostly broken so unknown if continue beyond chaetiger 159). Two types of pectinate chaetae present in supra-acicular position from chaetiger 3: thick shaft, symmetrical isodont with about 12–18 teeth, type 1 ( Fig. 5 View FIGURE 5 C–E) and thick symmetrical, curved isodont with a large number of teeth, type 2 ( Fig. 5 View FIGURE 5 D–E) with up to four in a fascicle. Pectinate chaetae continue to far posterior chaetigers.
Pygidium round, crenulated, dorsally positioned, with one pair of short pygidial cirri attached at ventral edge ( Fig. 2D View FIGURE 2 ).
Morphological variation. Variations in various characters for the paratypes is given in parentheses above. As all the material examined was fixed in 95% alcohol, some soft bodied structures may have been distorted, probably shrunken. Recently, Martin et al. (2020) suggested that the swelling of parapodial lobes and their bases may be useful specific characters; this could not be confirmed on this type material, possibly because of the fixation method.
Etymology. The species epithet “ madrasi ” refers to the old name (Madras) of the city of Chennai in which the species was collected. Although the origins of the old city name are unclear, it may have been named after a male fisherman.
Type locality. Ennore Creek , Chennai, India ( Fig. 1 View FIGURE 1 ); known for certain only from type locality. Possibly also in nearby waterways of Pulicat and Adyar estuary (see below). Ennore Creek is the name given to the lower Kosasthalaiyar River .
Habitat. Intertidal, mudflat.
Ecology. The new species occurs in Ennore Creek, Chennai, Tamil Nadu, East coast of India, in brackish water with salinity ranging from 28 to 34 ppt. Details of the developmental biology are provided by Malathi et al. (2011).
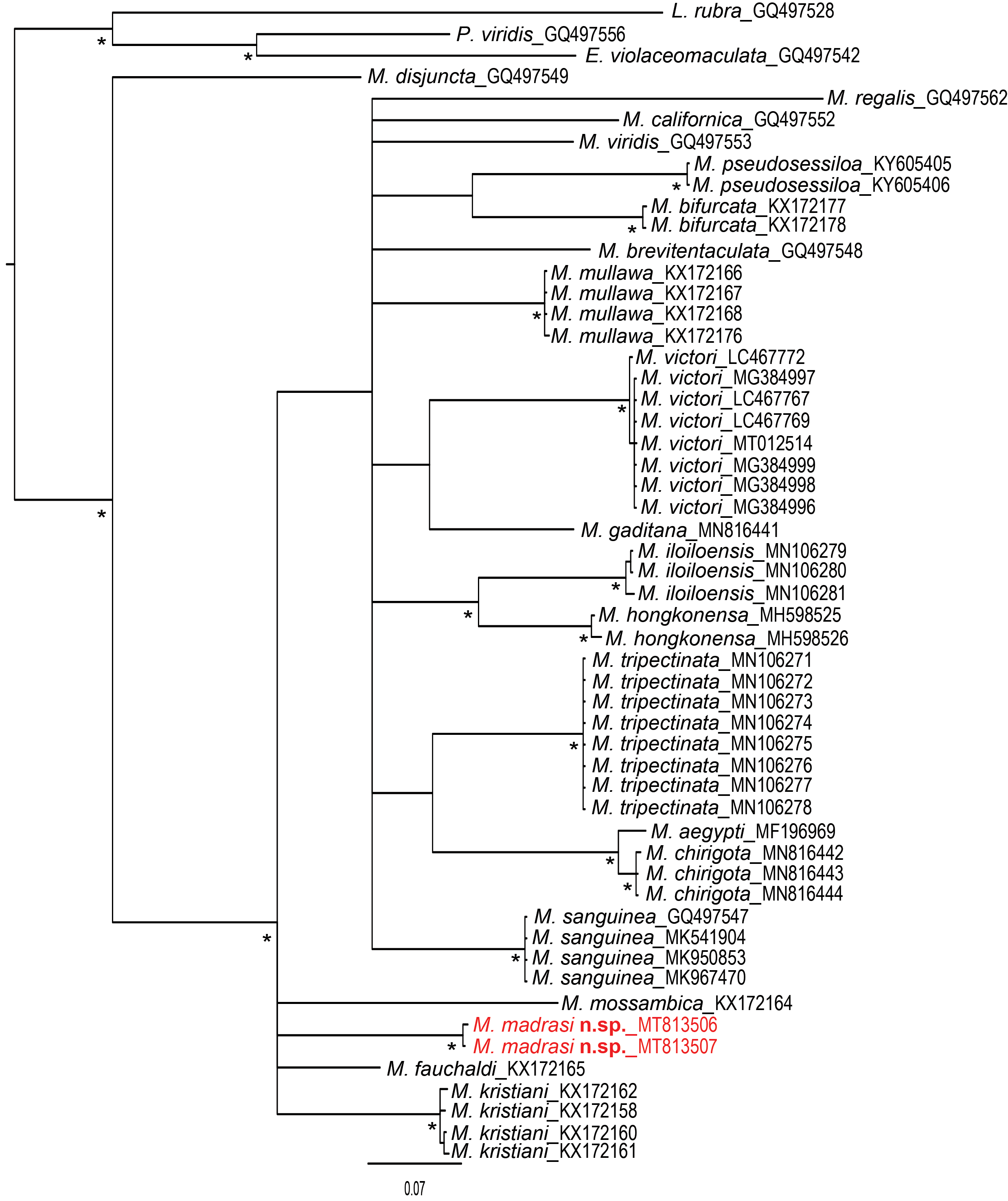
Remarks. Marphysa madrasi n. sp. can be distinguished from the only other described species from India, M. gravelyi Southern, 1921 , by having antennae about 2x longer than prostomium (extending only slightly beyond prostomium in M. gravelyi ), maxillae with a greater number of teeth (Mx II with 8+9 cf. 5+6; Mx IV with 7+11 cf. 4+8), and pectinate chaetae beginning from the first few anterior chaetigers ( M. gravelyi has pectinate chaetae beginning from about chaetiger 10–20). This information is based on a paratype of M. gravelyi (BMNH 1938.5.7.55) examined by Glasby & Hutchings (2010, Table 2). Among the species having molecular data, the new species is closest to M. fauchaldi Glasby & Hutchings, 2010 (K2P = 19%) and M. kristiani Zanol, da Silva & Hutchings, 2016 from Australia (K2P = 19.4%), and M. mossambica ( Peters, 1854) (K2P = 18.3%) from Iloilo, Philippines ( Fig. 7 View FIGURE 7 ). The most significant difference between the new species and M. fauchaldi is the definite presence of subacicular limbates (non-compound) in posterior chaetigers in M. fauchaldi (thought to be absent in the new species), and the narrower chaetiger range for the compound spinigers (chaetigers 13–55), which extend to at least chaetiger 159 in the new species. The close similarity between the M. madrasi n. sp. and M. fauchaldi is also evident in their body colouration and reproductive biology, which involves the nurturing of larvae in jelly cocoons (see also below).
Other diagnostic characters for species of the Mossambica- and Teretiuscula-groups reported from the coasts of India and the broader Indian Ocean are given in Table 3, which clearly shows the differences of M. madrasi n. sp. with all those other species which have been recorded from the region.
Comments on reproductive biology. Malathi et al. (2011) studied a species of Marphysa from Pulicat Lake, close to the type locality of M. madrasi n. sp., which they identified as M. gravelyi . The identification was tentatively confirmed in 2010 by CJG based on morphological examination of a damaged specimen (NTM W25489) from that lake. We suggest that the Pulicat Lake specimens are sufficiently different morphologically from M. gravelyi , as noted above, and probably represent the new species M. madrasi ; unfortunately, genetic confirmation has not been forthcoming probably because of degraded DNA. Malathi et al. (2011) studied these jelly masses (= jelly cocoons) where development of lecithotropic larvae occurs. The larvae settle on the sediment and the entire larval development takes 8–10 days and there is synchrony in the development of larvae inside the egg mass as well as synchrony within all the jelly masses collected within a particular area at a particular time. Egg masses are present throughout the entire year. While Pulicat Lake and Ennore Creek are linked, water flow only occurs during floods with the last observed flow in 2015. Unlike Adyar Creek (below), Ennore Creek never completely drains (see Fig. 1 View FIGURE 1 ).
Earlier, studies on an unnamed species of Marphysa from the Adyar estuary, (see Fig. 1 View FIGURE 1 ) just south of Chennai, SE India, by Aiyar (1931) provided detailed information on the development of gametes and the deposition of eggs in large cylindrical masses of jelly about 23 cm in length and 4 cm in width. He noted that the reproductive season begins immediately after the onset of the rainy season (October), and 12 chaetigerous juveniles are released from the jelly masses with no larval stage detected ( Aiyar 1931). He suggests that his material differs from that of Southern (1921) but he only identifies his species to the genus. We suggest that this material is also M. madrasi n. sp., as it was collected only about 25 km from the type locality of the species. Later studies by Krishnamoorthi (1951, 1962, 1966) also working in the same region, investigated the ability of the eggs and larvae to osmoregulate, and we also suggest these studies were based on M. madrasi n. sp. and not M. gravelyi as cited, although it is unlikely that any voucher material was deposited to confirm the species identity.
| LP |
Laboratory of Palaeontology |
| NTM |
Northern Territory Museum of Arts and Sciences |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.