Milnesium alpigenum Ehrenberg, 1853
Fig. 1
View FIGURE 1
, Table 3
View TABLE 3
M. tardigradum Marcus (1928)
Material examined: The neotype series consisting of the neotype and 37 neoparatypes (see Table 1
View TABLE 1
and “Type repositories” below for details).
Integrative redescription. Females (morphometrics in Table 3
View TABLE 3
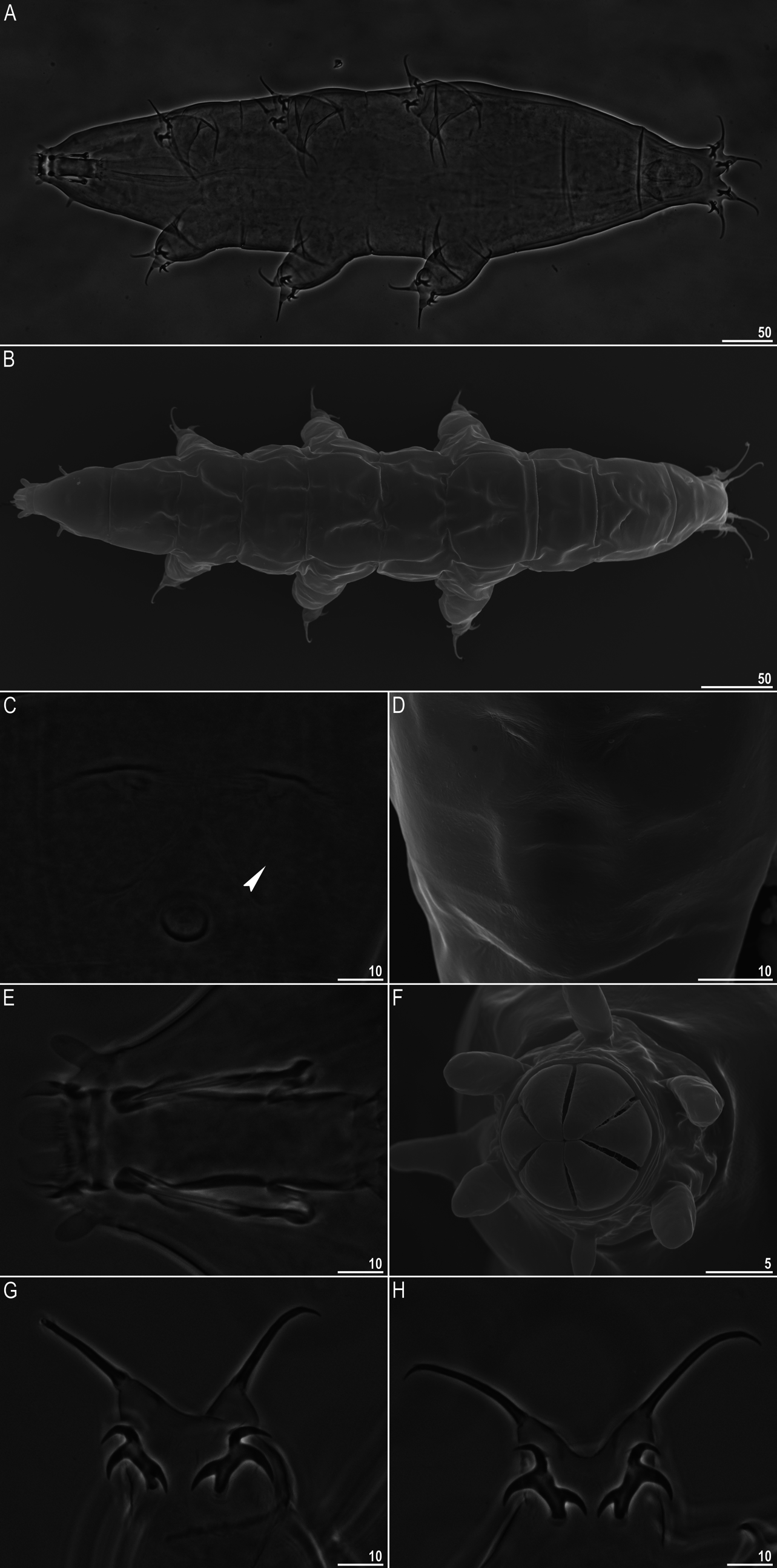
): Body slightly yellowish, rather slender as for a
Milnesium
( Fig. 1A
View FIGURE 1
). Eyes present in live specimens, quickly dissolving after fixation in Hoyer’s medium. Cuticle smooth in SEM and with minute pseudopores visible on the caudo-dorsal part only under high quality PCM ( Fig. 1C
View FIGURE 1
). Weakly outlined pseudoplates on the caudo-dorsal cuticle visible in some specimens only under SEM ( Fig. 1B and D
View FIGURE 1
). Six peribuccal papillae present, with the ventral being the smallest. Six triangular peribuccal lamellae of unequal size; the two lateral being slightly smaller than the dorsal and ventral lamellae, i.e. with the 4+2 configuration (identifiable only in SEM; Fig. 1F
View FIGURE 1
). Two lateral papillae present. Buccal tube funnel-shaped ( Fig. 1E
View FIGURE 1
). Claws slender, primary branches with tiny accessory points, more visible on claws IV. All secondary branches with three points, i.e. with the [3-3]-[3-3] CC ( Fig. 1
View FIGURE 1
G–H). Spurs on secondary branches long and slender, especially on internal and anterior claws. Cuticular bars under claws I–III present.
Males: No males were found in the sample or culture, confirming that the neotype population is parthenogenetic (at least facultatively).
Juveniles: Morphologically identical to adults, except for the lack of pseudopores.
Hatchlings: Morphologically identical to adults, except for the lack of pseudopores and the absence of cuticular bars under claws I–III in the majority of examined specimens (7/ 8 specimens = 88%).
Ontogenetic variability: No developmental variability in the CC. Pseudopores visible only in adults. Cuticular bars under claws I–III mostly absent in hatchlings but always present in juveniles and adults.
Eggs: Oval, yellow, smooth and laid in exuviae. In the culture, up to 12 eggs were recorded in a single clutch.
DNA markers: All sequences were of a very good quality and every marker was represented by a single haplotype: 18S rRNA (1054 bp,
MG996146
View Materials
); 28S rRNA (809 bp,
MH000384
View Materials
); ITS-2 (530 bp,
MH000382
View Materials
); and COI (560 bp,
MH000380
View Materials
). Sequences are provided in Appendix 1.
Neotype locality: 45°58'13''N, 07°57'07''E; 1370 m asl: Italy,
Monte Rosa
massif, lower chair-lift station of Macugnaga; moss on roof.
GoogleMaps
Etymology: Ehrenberg (1853) did not explain the choice of the species name; however, it seems reasonable to assume that Christian Ehrenberg named the species after the Alps, the mountain chain in which the type locality, the Monte Rosa massif, is located.
Type repositories: The neotype series consist of the neotype (slide IT.057.17) and 37 “neoparatypes” (IT.057.01–16; 18–38). The neotype and 15 neoparatypes (IT.057.01–12; 45–47) are preserved at the Institute of Zoology and Biomedical Research, Jagiellonian University, Gronostajowa 9, 30-387 Kraków, Poland),
further 14 neoparatypes ( IT.057.13–16; 18–26) are deposited in
Department of Animal Taxonomy
and Ecology,
Adam Mickiewicz University in Poznań
, Umultowska 89, 61-614 Poznań, Poland
,
10 neoparatypes ( IT.057.27–38) are stored in the Marine Biology & Ecology Research Centre, University of Plymouth,
Drake Circus
, Plymouth,
PL4
8AA, United Kingdom
,
one paratype ( IT.057.47) is deposited in Natural History Museum, Cromwell Road, London SW 7 5BD, United Kingdom
,
and the remaining 6 neoparatypes ( IT.057.39-44) are deposited in the collection of Binda & Pilato, Museum of the
Department of Biological
,
Geological
and
Environmental Sciences
, Section of Animal Biology “Marcello La Greca”, University of Catania, Italy
.
Phenotypic differential diagnosis:
Milnesium alpigenum
has the [3-3]-[3-3] CC and “smooth” cuticle ( i.e. cuticle smooth in SEM and with minute pseudopores visible only under high quality PCM, but with no sculpturing, such as reticulation, on cuticle surface). This places it in the largest group of
Milnesium
species that share these characteristics (20 species; Morek et al. 2016a; Pilato & Lisi 2016; Young et al. 2016; Pilato et al. 2016; Pilato et al. 2017; Schlabach et al. 2018). Nevertheless,
M. alpigenum
differs specifically from:
•
M. antarcticum Tumanov, 2006
, only recorded from the Antarctic ( Smykla et al. 2012), by the maximal length of the buccal tube (Ẽ 68 µm in
M. alpigenum
vs > 68 µm in
M. antarcticum
), a smaller buccal tube standard width ( 8.6–23.6 µm in
M. alpigenum
vs 25.9–31.8 µm in
M. antarcticum
), and by a statistically lower pt of the stylet support insertion point ( 61.1–70.3, on average 64.9 in
M. alpigenum
vs 70.0–73.7, on average 71.5 in
M. antarcticum
; t 38 = 16.708, p<0.001).
•
M. argentinum Roszkowska, Ostrowska & Kaczmarek, 2015
, reported from Argentina, by the appearance of cuticle (faint pseudopores visible only with a high quality PCM on the caudal part of the dorsal cuticle in
M. alpigenum
vs well-visible pseudopores in
M. argentinum
on the entire dorsum with a standard PCM), and by the lower pt of the primary branches IV ( 46.9–63.2 in
M. alpigenum
vs 28.4–36.4 in
M. argentinum
).
•
M. asiaticum Tumanov, 2006
, recorded from Kirghizstan ( type locality), China ( Beasley & Miller 2007), Estonia ( Zawierucha et al. 2014) and the Svalbard archipelago ( Kaczmarek et al. 2012), by a statistically lower pt of primary branches III ( 39.7–51.7, on average 45.8 in
M. alpigenum
vs 51.5–58.3, on average 55.3 in
M. asiaticum
; t 36 = 15.385, p<0.001) and by a lower pt of primary branches IV ( 46.9–63.2 in
M. alpigenum
vs 63.9–76.0 in
M. asiaticum
).
•
M. barbadosense Meyer & Hinton, 2012
, only reported from the type locality in Barbados, by a lower pt of the stylet support insertion point ( 61.1–70.3 in
M. alpigenum
vs 71.6–82.1 in
M. barbadosense
) and by a higher pt of the primary branches IV ( 46.9–63.2 in
M. alpigenum
vs 28.4–42.2 in
M. barbadosense
).
•
M. beatae Roszkowska, Ostrowska & Kaczmarek, 2015
, only reported from the type locality in Argentina, by the appearance of cuticle (faint pseudopores visible only with a high quality PCM on the caudal part of the dorsal cuticle in
M. alpigenum
vs well-visible pseudopores in
M. beatae
on the entire dorsum with a standard PCM), by a more slender buccal tube (standard width/length ratio 21–38% in
M. alpigenum
vs standard width/ length ratio 58–66% in
M. beatae
).
•
M. bohleberi Bartels, Nelson, Kaczmarek & Michalczyk, 2014
, recorded from North Carolina and Tennessee, USA, by a more slender buccal tube (standard width/length ratio 21 – 38% in
M. alpigenum
vs standard width/ length ratio 54–64% in
M. bohleberi
).
•
M. brachyungue Binda & Pilato, 1990
, recorded from the type locality in Chile and south Argentina ( Roszkowska et al. 2016), by a higher pt of primary branches of all claws ( 36.0– 63.2 in
M. alpigenum
vs 22.9– 33.1 in
M. brachyungue
).
•
M. burgessi Schlabach, Donaldson, Hobelman, Miller & Lowman, 2018
, reported from Kansas, USA, by a higher pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
vs 52.9–68.5 in
M. burgessi
) and by a lower pt of primary branches IV ( 46.9–63.2 in
M. alpigenum
vs 66.6–96.2. in
M. burgessi
).
•
M. dornensis Ciobanu, Roszkowska & Kaczmarek, 2015
, recorded from Romania ( type locality), Poland ( Kaczmarek et al. 2018) and Tunisia ( Gąsiorek et al. 2017b), by the appearance of cuticle (faint pseudopores visible only with a high quality PCM on the caudal part of the dorsal cuticle in
M. alpigenum
vs well-visible pseudopores in
M. dornensis
on the entire dorsum with a standard PCM) and by a statistically lower pt of the buccal tube standard width ( 21.0–38.4, on average 31.4 in
M. alpigenum
vs 37.8–51.6, on average 44.1 in
M. dornensis
; t 43 = 10.473, p<0.001).
•
M. eurystomum Maucci, 1991
, recorded from Greenland ( type locality), Chile and Argentina ( Maucci 1996), and Mongolia ( Kaczmarek & Michalczyk 2006), by a more slender buccal tube (standard width/length ratio 21 – 38% in
M. alpigenum
vs standard width/length ratio 62–65% in
M. eurystomum
).
•
M. longiungue Tumanov, 2006
, reported from the type locality in the Himalayas ( India) and China ( Beasley & Miller 2007), by the presence of accessory points on primary branches, a lower pt of primary branches III ( 39.7–51.7 in
M. alpigenum
vs 57.1–73.5 in
M. longiungue
), and by a lower pt of primary branches IV ( 46.9– 63.2 in
M. alpigenum
vs 81.8–92.4 in
M. longiungue
).
•
M. minutum Pilato & Lisi, 2016
, only reported from the type locality in Sicily, by a lower pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
vs 38.6–42.4 in
M. minutum
).
•
M. sandrae Pilato & Lisi, 2016
, only reported from the type locality in Hawaii, by a higher pt of the stylet support insertion point ( 61.1–70.3 in
M. alpigenum
vs 58.0– 60.5 in
M. sandrae
) and by a lower pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
vs 44.9–48.0 in
M. sandrae
).
•
M. shilohae Meyer, 2015
, only reported from the type locality in Hawaii, by a lower pt of the stylet support insertion point ( 61.1–70.3 in
M. alpigenum
vs 75.5–77.5 in
M. shilohae
), a lower pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
vs 47.1–55.9 in
M. shilohae
), and by a higher pt of external spurs I–III ( 11.3–17.8 in
M. alpigenum
vs 1.9–7.5 in
M. shilohae
).
•
M. swansoni Young, Chappell, Miller & Lowman, 2016
, only reported from the type locality in USA, by a higher number of peribuccal lamellae (six in
M. alpigenum
vs four in
M. swansoni
), a lower pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
vs 39.2–42.2 in
M. swansoni
), a lower pt of the posterior buccal tube width ( 23.7–39.4 in
M. alpigenum
vs 39.9–42.2 in
M. swansoni
), and by a lower pt of primary branches I ( 36.0– 47.9 in
M. alpigenum
vs 48.4–53.7 in
M. swansoni
). It should be noted that the number of peribuccal lamellae in
M. swansoni
was identified only with the use of PCM, thus until SEM observations are made, the number of lamellae should be treated as a working hypothesis.
•
M. tumanovi Pilato, Sabella & Lisi, 2016
, only reported from the type locality in Crimea, by a higher pt of the stylet support insertion point ( 61.1–70.3 in
M. alpigenum
specimens 383–983 µm long vs ca. 52.3 in
M. tumanovi
in a specimen 774 µm long) and by a lower pt of the buccal tube standard width ( 21.0– 38.4 in
M. alpigenum
specimens 383–983 µm long vs ca. 55.1 in
M. tumanovi
in a specimen 774 µm long).
•
M. validum Pilato, Sabella, D’Urso & Lisi, 2017
only reported from the type locality in the Antarctic, according to the measurements presented in the description of
M. validum
all pt ranges overlap, but a comparison of specimens of a similar body length ( 414–509 µm in
M. alpigenum
and 424–482 µm in
M. validum
) shows that
M. alpigenum
has a shorter buccal tube (33.0–43.0 µm in
M. alpigenum
vs 44.1–55.6 µm in
M. validum
), moreover the two species differ in the shape of secondary branches (slender in
M. alpigenum
vs robust in
M. validum
, compare Fig. 1
View FIGURE 1
G–H here and Fig. 6B–D in Pilato et al. 2017), and in the shape of spurs (of typical width in
M. alpigenum
vs very thin in
M. validum
).
•
M. inceptum
sp. nov. (described below), recorded from Germany, Japan, Switzerland and Bulgaria—please see the section “Delineation of
M. alpigenum
and
M. inceptum
sp. nov. ” below for a detailed differential diagnosis between these two pseudocryptic species.
•
M. zsalakoae Meyer & Hinton, 2010
, recorded from Arizona and New Mexico ( USA), by the presence of accessory points on primary branches and by a lower pt of primary branches of all claws ( 36.0– 63.2 in
M. alpigenum
vs 64.4–102.9 in
M. zsalakoae
).
Genotypic differential diagnosis: All sequences obtained for
M. alpigenum
were unique and distinct from the sequences deposited in GenBank. The ranges of the uncorrected p-distances between neotype
M. alpigenum
and sequences of other congeners are as follows:
• 18S rRNA: 1.1%–3.6% (2.6% on average), with the most similar being
M. inceptum
sp. nov. from Europe (
MH000383
View Materials
, present study) and the least similar being an undetermined species from Marion Island in the sub- Antarctic (
EU266922
View Materials
, Sands et al. 2008).
• 28S rRNA: 4.5%–8.0% (6.1% on average), with the most similar being an undetermined species from the USA (
JX888585
View Materials
–7, Adams et al, unpublished) and the least similar being
M. tardigradum
s.s. from Poland (
KC138809
View Materials
, Zawierucha, unpublished).
• ITS-2: 20.4%–23.2% (20.2% on average), with the most similar being
M. tardigradum
s.s. from Germany (
JF951049
View Materials
, Michalczyk et al. 2012a) and the least similar being
M. tardigradum
s.s. from France (
MG923555
View Materials
, Morek et al. 2019).
• COI: 14.8%–25.8% (17.4% on average), with the most similar being
M. variefidum
from the UK (
KT951663
View Materials
, Morek et al. 2016a) and an undetermined species from the USA (
KX306950
View Materials
, Fox et al., unpublished), whereas the least similar being an undetermined species from the Antarctic (
KP013598
View Materials
, Velasco-Castrillón et al. 2015).