Leiobunum gracile Thorell, 1876
|
publication ID |
https://doi.org/ 10.5852/ejt.2016.216 |
|
publication LSID |
lsid:zoobank.org:pub:2F526459-E23A-458B-9829-31F4C1BC6C46 |
|
DOI |
https://doi.org/10.5281/zenodo.3853168 |
|
persistent identifier |
https://treatment.plazi.org/id/BA4E87D3-FFE6-BB1A-7258-FCBBCD17FE03 |
|
treatment provided by |
Valdenar |
|
scientific name |
Leiobunum gracile Thorell, 1876 |
| status |
|
Leiobunum gracile Thorell, 1876
Figs 2 View Fig , 3 View Fig C–D, 4C–D, K–J, 5D–F
Partly refers to the record within the distribution of L. gracile according to Fig. 2 View Fig .
Phalangium bicolor Fabricius, 1793: 429 View in CoL .
Liobunum gracile Thorell, 1876: 496 (type series SMNH, examined).
Liobunum laeve Thorell, 1876: 497 (type series SMNH, examined). syn. nov.
Liobunum norvegicum Strand, 1900: 7 . syn. nov.
Nelima melanogranulata Morin, 1931 (nomen nudum), 1934 (valid description) syn. to rupestre fide Staręga 1978: 208.
Leiobunum tisciae Avram, 1968: 115 (neither declaration of type specimens nor depository for specimens, material of the original description dedicated by Avram to J.M., now in CJM3526, examined). syn. nov.
Phalangium bicolor View in CoL – Kulczyński 1876: 61. Remark: see L. rupestre .
Liobunum gracile – Tullgren 1906b: 216 (syn. with L. rupestre ).
Liobunum laeve – Tullgren 1906b: 216–217 (syn. with L. rupestre ).
Liobunum norvegicum – Tullgren 1906b: 217 (syn. L. rupestre ).
Leiobunum tisciae – Martens 1978: 408–412 (partim). — Staręga 2004: 80–81. — Stol 2010b: 35. — To- masson et al. 2014: 153.
Liobunum rupestre – Tullgren 1906a: 211; 1906b: 216. — Roewer 1910: 203–204.
Leiobunum rupestre – Roewer 1923: 890 (partim). — Heinäjoki 1944: 22–23. — Staręga 1978: 100–103. (partim). — Staręga 1979: 177–178. — Lengyel & Murányi 2006: 121. — Enghoff 1988: 68–6 9. — Chevrizov 1979: 14. — Spuņgis 2008: 21.
Nelima gracilis – Roewer 1910: 239, 250 (redescription). — Roewer 1923: 916.
Nelima laevis – Roewer 1910: 239, 251 (redescription). — Roewer 1923: 916.
Nelima norvegica – Roewer 1910: 251 (redescription). — Roewer 1923: 916–917. — Staręga 1976: 100.
Nelima norwegica – Roewer 1910: 239. — Staręga 1976: 100 (sic, lapsus calami).
Liobunum norwegicum – Müller 1920: 72 (lapsus calami).
Strandibunus obliquus – Bartoš 1939: 309.
Nelima glabra – Kolosváry 1965: 111–113. — Kolosváry 1966a: 123. — Kolosváry & Homonnay 1967: 77–79.
Nelima nigripalpis – Kolosváry 1963: 192. — Kolosváry 1966a, b: 123.
Leiobunum glabrum – Šilhavý 1981: 204–207.
Taxonomic history
A rather complex situation developed because old species names with type localities in various areas in Scandinavia or in (present) eastern locations were never checked in detail. This is partly due to the fact that names were based on juvenile specimens and partly to the unavailability of former authors to access specimens of northern (Baltic) and southern (Alpine) origin for direct comparison. Consequently, northern populations, originally described as Liobunum gracile Thorell, 1876 and Liobunum laeve Thorell, 1876 , were permanently affiliated to L. rupestre , starting with Tullgren (1906a, b), followed by Heinäjoki (1944), Staręga (1976) and Martens (1978). The situation became even worse when Avram (1968) described L. tisciae Avram, 1968 from the Tisza valley in Hungary. By external morphology, this species is similar to L. rupestre , which Avram did not take into account. Martens (1978) accepted this novelty but at that time lacked suitable fresh material to identify populations from northern Germany and Denmark as conspecific. Instead, he erroneously treated one species under two names, namely North German and Scandinavian populations under L. ‘ rupestre ’ and those from the Carpathians and single individuals from the British Isles under L. tiscae , besides occurrences of L. ‘ rupestre ’ in the Carpathian Arc as well ( Martens 1978). Šilhavý (1981) was the first to recognize two very similar Leiobunum species in (former) Czechoslovakia: L. rupestre in the West (now Czech Republic) and L. ‘ glabrum ’ in the East (now Slovakia).
Thorell (1876) based his species L. gracile Thorell, 1876 and L. laeve Thorell, 1876 on juvenile specimens from South Sweden, although he mentioned only a single male. His original material (kept at the Museum of Natural History Stockholm) definitely concerns the types and was examined. It was found, that it indeed only contains juveniles, as already Tullgren (1906b) assumed they represented juveniles of L. rupestre . Likewise, Tullgren (1906b) assumed L. norvegicum Strand, 1900 to be identical with L. rupestre , but refrained from making a final decision, not having seen the type material. Describing L. norvegicum, Strand (1900) depicted a female of the L. rupestre complex (from Kristiania [now Oslo]; leg. Sept. 1899) and later Martens (1978) agreed with Tullgren’s view, including L. norvegicum in the synonymy of a broad species L. rupestre .
Considering the allopatric distribution of L. rupestre and L. ‘ tisciae ’ we now can firmly state that all Leiobunum ‘ rupestre ’, based on material from Scandinavia, belong to L. ‘ tisciae ’. But as L. ‘ tisciae ’ is a recently proposed name, older names are to be preferred. Albeit L. gracile and L. laeve are oldest, their description is based on subadults ( Tullgren 1906b). Yet, both have been described from Sweden, Province of Scania, from where Tullgren (1906b) confirmed an adult specimen. Therefore, it seems a reasonable decision to re-establish the oldest available name, Leiobunum gracile Thorell, 1876 , and place L. laeve Thorell, 1876 , L. norvegicum Strand, 1910 and L. tisciae Avram, 1968 in its synonymy. L. gracile has page and even line priority over L. laeve .
Diagnosis
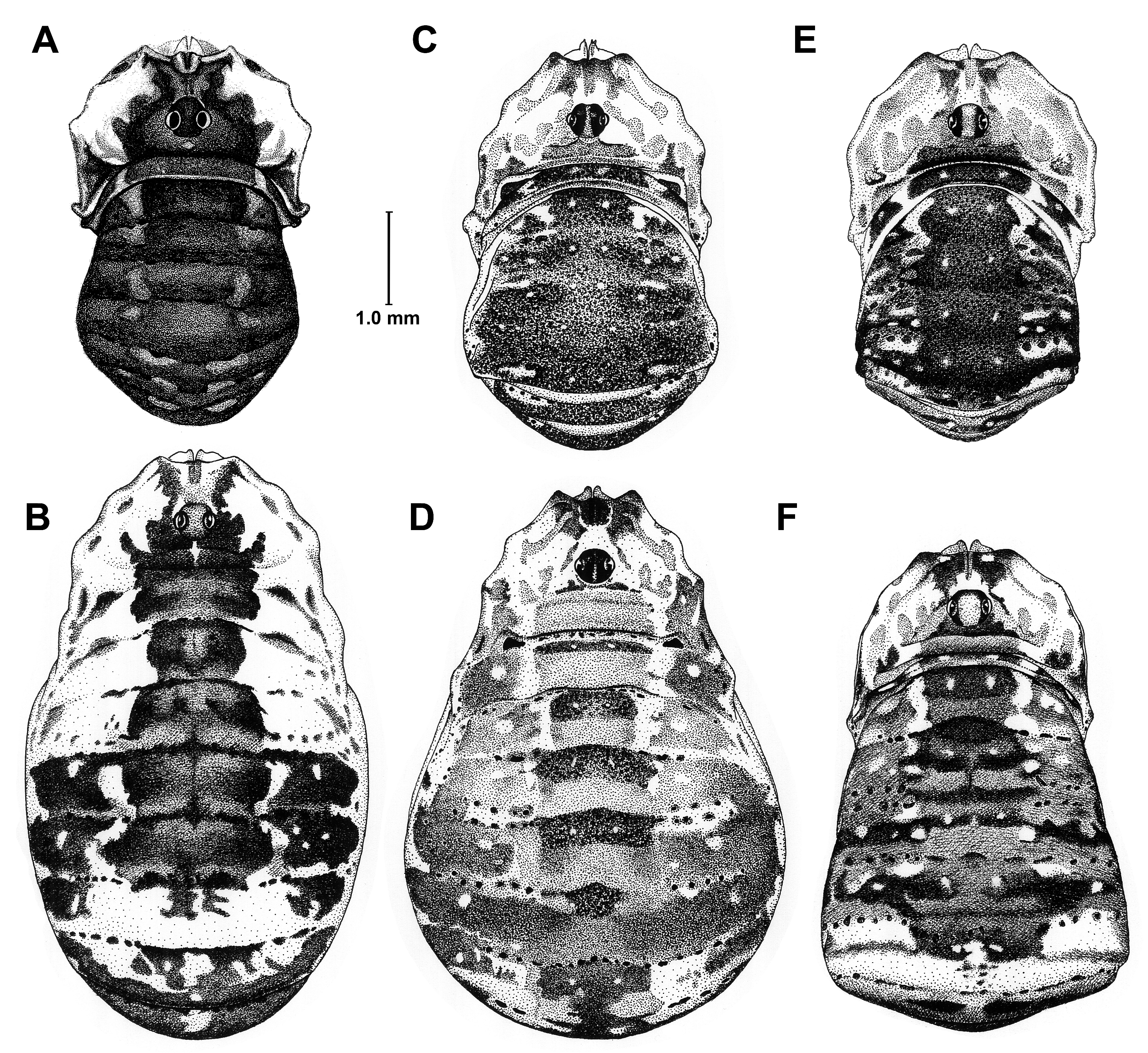
A medium-sized Leiobunum species with blackish upper side, except for broad white markings of Ceph disto-laterally in male ( Fig. 3C View Fig ), broad blackish irregular markings on abd area I–V of the body, in female with mottled white markings laterally from Ceph to posterior areae, white lateral marking on abd area V ( Fig. 3D View Fig ). In both sexes two paramedian small white spots on abd areae I–V, more conspicuous in male (most similar to L. apenninicum ). Contrasting yellowish underside including coxae of all appendages. Wings of truncus penis slender, slightly tapering proximally.
Description
BODY ( Fig. 3 View Fig C–D). In both sexes dorsal granulation consists of flat, pointed granules, regularly spaced, well visible at 250× (compare Šilhavý 1981: fig. 2).
DORSAL PATTERN ( Fig. 3 View Fig C–D). See Diagnosis. Tu oc in both sexes smooth, without spines, in rare cases one spine present.
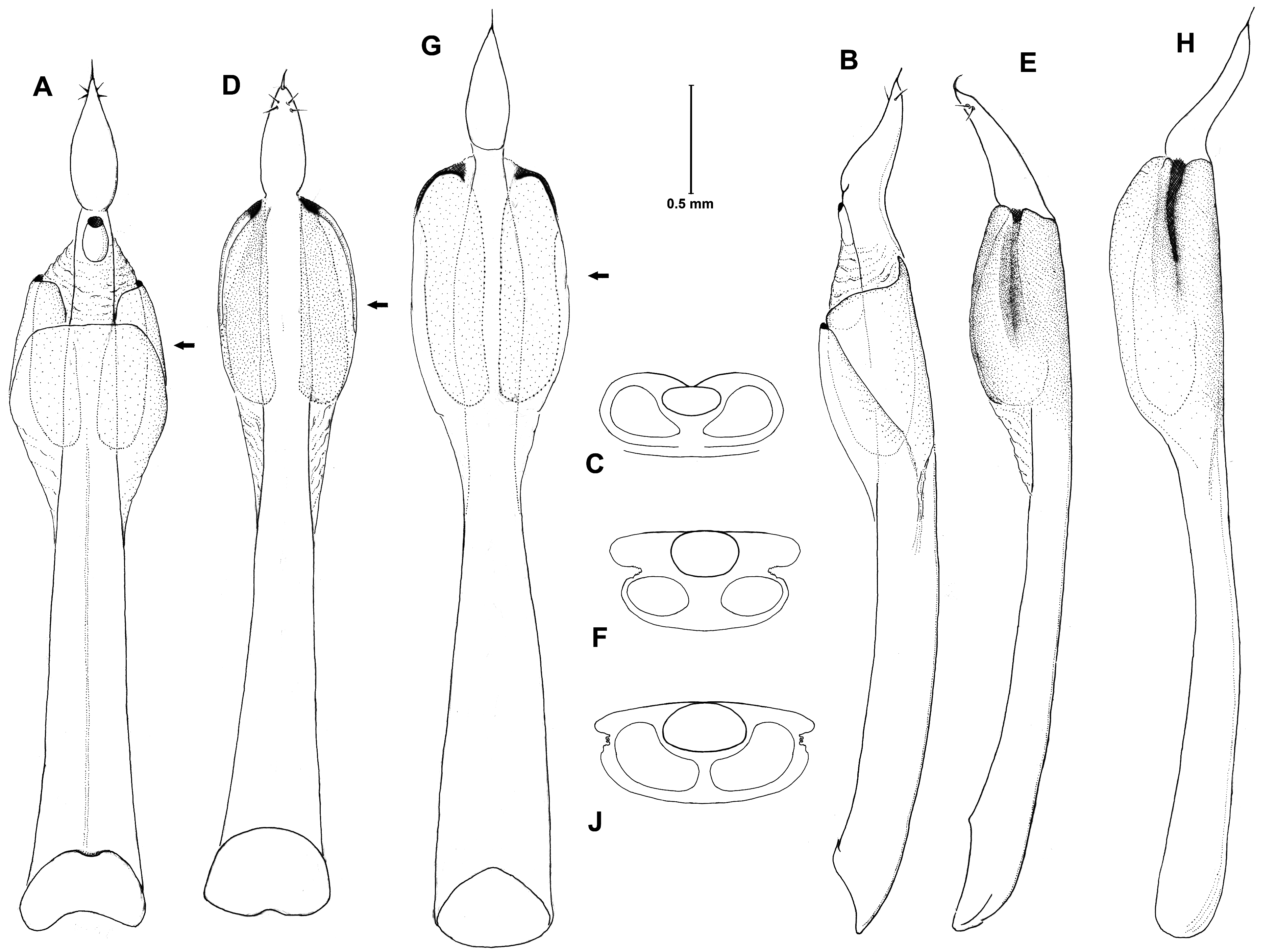
PEDIPALPS. Male ( Fig. 4 View Fig C–D): Fe with irregular rows of large triangular spines ventrally often with single hair on top; dorsally few on distal part, ventral spines broader and more massive than in L. rupestre , where they are more slender and without hair on top. Pt with scattered denticles la and do; Ti slightly concave ventro-distally, forming a flat S-bend, denticles and hairs on ventral side of palpal tibia light-coloured, hard to be seen; Ta mostly straight, bent only in distal third. Female ( Fig. 4 View Fig J–K): in all members similar to male, but armament less conspicuous, Ti more slender, Ta strait.
GENITAL MORPHOLOGY ( Fig. 5 View Fig D–F). Penis similar to that of L. apenninicum . Truncus stout, from ventral/ dorsal view from basal opening to insertion of glans slightly and continuously tapering to the internal sac of the wings; lateral wings (ventral view) markedly enlarged, smoothly rounded thus nearly eggshaped (irrespective the lateral membranes running down the truncus). At its very distal end the wing structure close to the glans insertion opens to a small opening, extending into two long nearly parallelsided double-walled sacks to the lower end of the wings. A lateral somewhat invaginated discharged membrane in the distal half of the wings unites the dorso-lateral and the latero-ventral part of the wings (lateral view) allowing for a possible inflation of the wing structure when the sacs are filled with secretion. From lateral view truncus slightly curved (concave on ventral side), slightly tapering towards basis of stylus. Wings enlarged on ventral side, slightly rounded convexly. Glans broad at truncus insertion, in lateral view continuously tapering towards stylus.
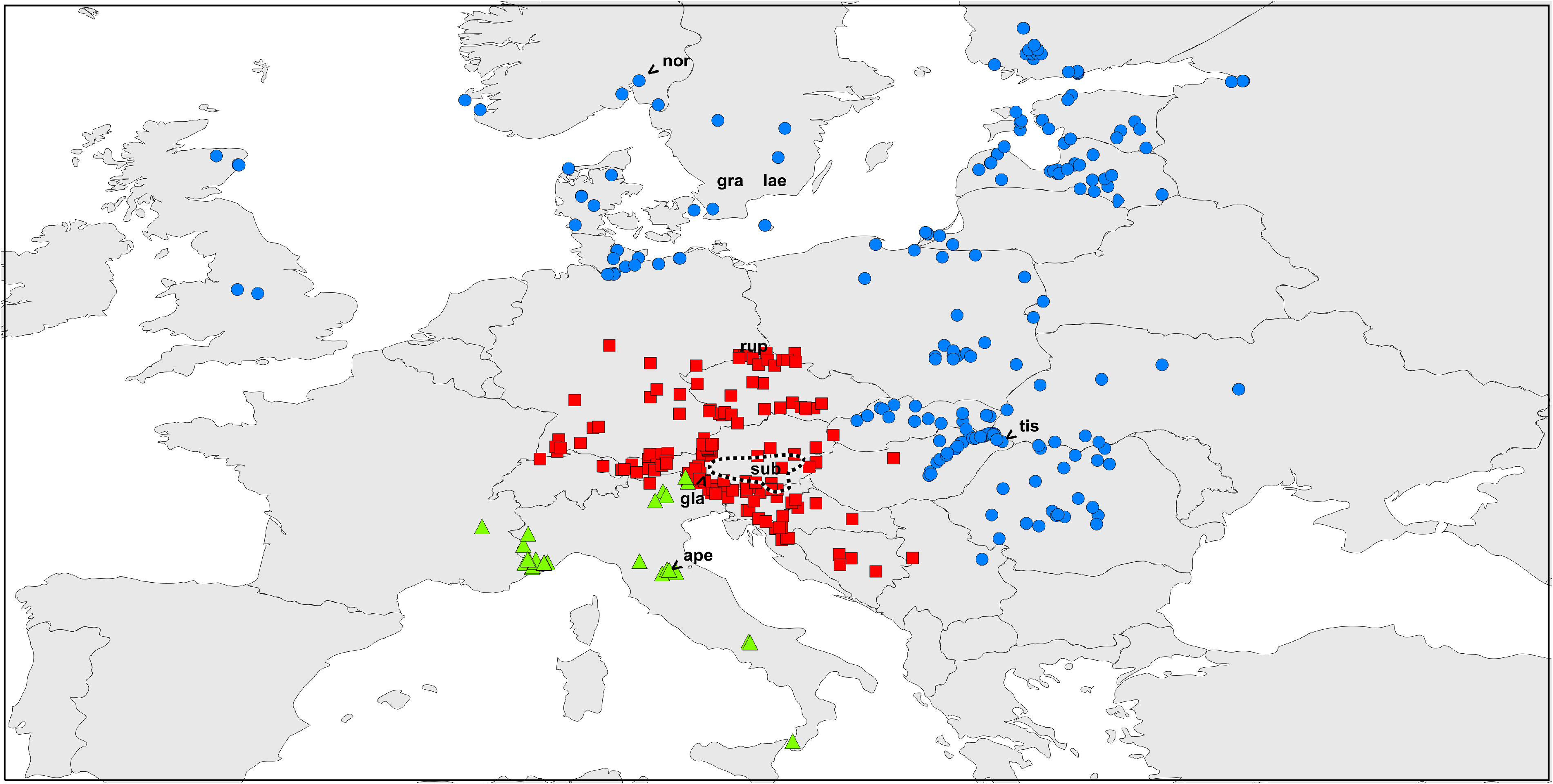
Distribution ( Fig. 2 View Fig )
From Slovakia ( Šilhavý 1981, glabra ), northern Hungary including the Tisza valley (type locality of L. tisciae Avram ; various records documented by Kolosváry 1963, 1965, 1966a, b, 1969 as nigripalpis and glabra , and Kolosváry & Homonnay 1967), no additional records in Komposch (2004) for Hungary. Carpathian Arc with many localities documented in collections, but few published papers available, not very far eastward and not in the southern lowlands (Murányi & Lengyel 2006; Weiss 1996; Cirdeî 1960). In the Southwest the area extends into Serbia (Bor Dubasnica, Mala tisnica, CJM 6363). There are scattered records for the western part of Ukraine, mainly as an extension of the Carpathian area ( Bartoš 1939; Cîrdei 1960; Staręga 1978; Morin 1931, 1934; L. Koch 1870). The many records in Poland pertain to the eastern two thirds of the country, extending to the Baltic Sea ( Staręga 1978, 2004, rupestre ; for true Polish rupestre see above) and including former Westpreussen. Occurrences in Russia are reported from former Ostpreussen ( Le Roi 1914) and from St. Petersburg (CJM 3032), extending to Northeast Poland ( Staręga 1976, 2004). Estonia: records along the Baltic Sea Coast ( Tomasson et al. 2014). Latvia: partly detailed locality maps ( Tumšs 1963; Spuņgis 2008). Finland: only in the southern part, with detailed map based on monitoring records ( Heinäjoki 1944), presence confirmed by Uddström et al. (2013). Germany: records along the Baltic Sea Coast and its hinterland (up to Hamburg), dating back to Kraepelin (1896), Le Roi (1914) and Rabeler (1929), but apparently presently very rare, last record in Schleswig-Holstein, Lübeck in 2007 (CJM 6111; see Martens 1978). Denmark: early records by Hansen (1884), more recent records from all over the country remained largely unchanged between about 1960 ( Meinertz 1964) and 1987 ( Enghoff 1988), with slight distributional differences, formerly missing from Funen and rare in South Jutland and Himmerland, but very common and abundant there in 1987 ( Enghoff 1988), including Funen, South Jutland and Himmerland. Since then, it has vanished, has not been recorded for many years, but it was rediscovered recently and is presently extremely rare. In 2008, four individuals were present on four out of 64 sites ( Enghoff et al. 2014). Sweden: except for the early records of Thorell (1876) in Västergötland, Östergötland, Skåne and Småland, there is a single new one from Uppsala in 2009 (examined, H. Enghoff leg.). Norway: Stavanger (SMF) and Oslo (= Kristiania; Strand 1900, norvegicum ). Great Britain: Derbyshire ( Martens 1978), unrecorded until 2008 when it was found around Aberdeen and Dunbennan, Huntly ( Davidson 2009, CJM 6358-6361), in Scotland apparently spreading and in Great Britain presently known from seven counties ( British Arachnological Society 2016).
The present distribution of L. gracile and that in the recent past obviously represent two different ecological-defined origins. One belongs from lowland to montane and riverine forests, the other one comprises secondary urban habitats to which the species was (most likely) transferred by human activity. Such “anthropogenic” populations may suddenly increase, occupy a large area and sometimes collapse again, for reasons little understood. Others are spot-like with little influence on local faunas. At least for a period of roughly 120 years such dynamics can tentatively be reconstructed (see Discussion).
Ecology
A euryoecious species. In Slovakia, including the Carpathian Arc, recorded from sheltered places in oldgrowth forests on tree trunks and on rock faces ( Šilhavý 1981). It is common in the inundation zone of rivers like the Tisza, e.g., frequently reported from tree hollows of Populus ( Avram 1968, tisciae ) and Salix trunks ( Avram 1968; Csizmazia et al. 1966, nigripalpis ; Kolosváry 1963, 1965, 1966a, b). This also holds true for East Poland where gracile lives in forested habitats (Neple, Bug River, AXLS 958). Weiss (1996) indicated its occurrence from Romanian Transylvania forests of the hilly and montane belt. In all other areas, which we believe to be secondary, L. gracile is confined to strongly modified and human-influenced habitats. There it competes with other species preferring house walls like Opilio saxatilis C.L. Koch, 1839 , O. parietinus (Degeer, 1778) and recently with O. canestrinii Thorell, 1876 . In central Europe the latter presently is by far the dominant species in human habitats and may have partly caused the noticeable decline of O. saxatilis , O. parietinus and L. gracile (see Discussion).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Leiobunum gracile Thorell, 1876
| Martens, Jochen & Schönhofer, Axel L. 2016 |
Leiobunum glabrum
| Silhavy V. 1981: 204 |
Nelima melanogranulata
| Starega W. 1978: 208 |
Leiobunum tisciae
| Stol I. 2010: 35 |
| Starega W. 2004: 80 |
| Martens J. 1978: 408 |
Leiobunum tisciae
| Avram S. 1968: 115 |
Nelima glabra
| Kolosvary G. & Homonnay S. 1967: 77 |
| Kolosvary G. 1966: 123 |
| Kolosvary G. 1965: 111 |
Nelima nigripalpis
| Kolosvary G. 1963: 192 |
Strandibunus obliquus
| Bartos E. 1939: 309 |
Leiobunum rupestre
| Spungis V. 2008: 21 |
| Muranyi D. & Lengyel G. D. 2006: 121 |
| Enghoff H. 1988: 68 |
| Starega W. 1979: 177 |
| Chevrizov B. P. 1979: 14 |
| Starega W. 1978: 100 |
| Heinajoki M. 1944: 22 |
| Roewer C. F. 1923: 890 |
Liobunum norwegicum
| Muller A. 1920: 72 |
Nelima gracilis
| Roewer C. F. 1923: 916 |
| Roewer C. F. 1910: 239 |
Nelima laevis
| Roewer C. F. 1923: 916 |
| Roewer C. F. 1910: 239 |
Nelima norvegica
| Starega W. 1976: 100 |
| Roewer C. F. 1923: 916 |
| Roewer C. F. 1910: 251 |
Nelima norwegica
| Starega W. 1976: 100 |
| Roewer C. F. 1910: 239 |
Liobunum gracile
| Tullgren A. 1906: 216 |
Liobunum laeve
| Tullgren A. 1906: 216 |
Liobunum norvegicum
| Tullgren A. 1906: 217 |
Liobunum rupestre
| Roewer C. F. 1910: 203 |
| Tullgren A. 1906: 211 |
| Tullgren A. 1906: 216 |
Liobunum norvegicum Strand, 1900: 7
| Strand E. 1900: 7 |
Liobunum gracile
| Thorell T. 1876: 496 |
Liobunum laeve
| Thorell T. 1876: 497 |
Phalangium bicolor
| Kulczynski W. 1876: 61 |
Phalangium bicolor
| Phalangium bicolor Fabricius, 1793: 429 |