Dispio uncinata Hartman, 1951
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4178.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:C533EE2A-5831-49A2-A4ED-2E7CD94EC663 |
|
DOI |
https://doi.org/10.5281/zenodo.5661059 |
|
persistent identifier |
https://treatment.plazi.org/id/CA1187AD-CA4B-E850-FF30-A7F0DE5AF8B1 |
|
treatment provided by |
Plazi |
|
scientific name |
Dispio uncinata Hartman, 1951 |
| status |
|
Dispio uncinata Hartman, 1951 View in CoL
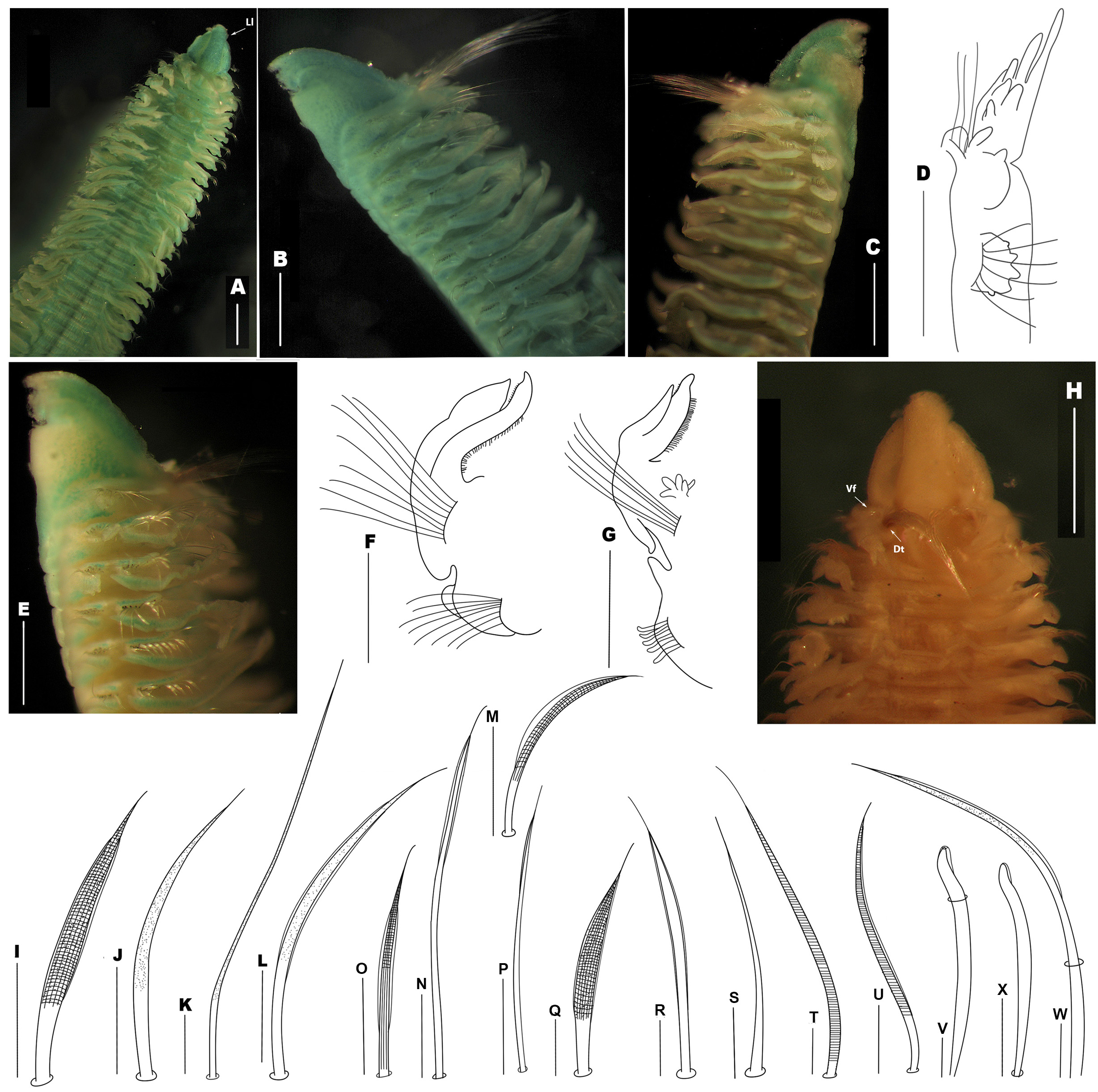
Figures 2 View FIGURE 2 A–X
Spio setosa Behre, 1950 View in CoL , p. 13.
Dispio uncinata Hartman, 1951: 87 View in CoL , pl. 22, figs. 1–5, pl. 23, figs. 1–4. Foster (1971:73–79; figs. 161–174) (in part). Johnson (1984: 6/32–33; figs 6–23/24) (in part).
Material examined. Gulf of Mexico, USA, Florida, Franklin County, Alligator Point , 29º41’11’’N, 84º56’39’’W coll. L.M. Henry, 9 March 1950, holotype and paratype ( LACM-AHF POLY 0634–5 ) GoogleMaps . Lousiana, Jefferson Parish Barataria Bay, Grand Isle , 29º14’42’’N, 89º59’14’’W, sandy beach, coll. J.H. Roberts, 10 July 1942. Non type, 2 specimens ( LACM-AHF POLY 6246 ) GoogleMaps ; Lousiana, Jefferson Parish Barataria Bay, Grand Isle , 29º14’42’’N, 89º59’14’’W, sandy beach, coll. Ellinor H. Behre, 2 August 1943. Non type, 1 specimen ( LACM-AHF POLY 6245 ) GoogleMaps .
Redescription. Holotype incomplete, anterior fragment with 56 chaetigers, 21 mm long, 2 mm wide at chaetiger 15 (holotype in original description: 120 chaetigers, 32 mm long, 2–2.5 mm wide). Paratype incomplete, anterior fragment with 74 chaetigers, 25 mm long, 2 mm wide at chaetiger 15. Color in alcohol light brown, no other pigmentation present.
Prostomium spindle-shaped, pointed anteriorly with curved tip at distal end, with a pair of lateral emarginations on large lateral lobes that comprise peristomium, widest posteriorly ( Fig. 2 View FIGURE 2 A, B). Prostomium posteriorly tapered, with long, narrow caruncle to end of chaetiger 1 ( Fig. 2 View FIGURE 2 A). Eyes absent. Peristomium long, collar-like, surrounding prostomium, separated from chaetiger 1, forming moderate lateral wings ( Figs. 2 View FIGURE 2 B–C). Palps lost in types, but in original description were described as thick palps, longitudinally grooved, marked by widely spaced black transverse bars on side opposite of longitudinal groove.
Notopodial postchaetal lamellae of chaetigers 1–2 shifted dorsally and deeply serrated. Lamellae of chaetiger 1 bearing 5 – 6 digitiform papillae along distal and middle margins, basal margin rounded, small, wider, with small dorsal papilla ( Fig. 2 View FIGURE 2 D) (holotype: right lamella entire). Notopodial lamellae of chaetiger 2 with 5 – 8 digitiform papillae along middle and distal margins, basal margin entire ( Fig. 2 View FIGURE 2 B); lamellae of chaetigers 3 – 9 with ruffled margins ( Fig. 2 View FIGURE 2 E) (Paratype: right lamellae of chaetiger 3 with a small digitiform papilla on distal margin); lamellae of subsequent chaetigers with entire margin ( Fig. 2 View FIGURE 2 F); lamellae from chaetiger 31 and succeeding chaetigers with pointed ventral border ( Fig. 2 View FIGURE 2 G). Ventral and dorsal edges of notopodial and neuropodial lamellae not overlapping or touching on any chaetiger ( Fig. 2 View FIGURE 2 C). Notopodial prechaetal lamellae large, oval on chaetigers 1– 6, thereafter increasing in size, rounded, wider on chaetigers 7–40 ( Fig. 2 View FIGURE 2 B), from about chaetiger 41 triangular, large; all lamellae not basally fused with notopodial postchaetal lamellae. Each segment with pair of dorsal Cshaped double bands of cilia, with transverse band of cilia between them. Each segment with dorsal, transverse band of cilia ( Fig. 2 View FIGURE 2 A). Lateral organs between notopodial and neuropodial postchaetal lamellae insubstantial.
Neuropodial postchaetal lamellae on chaetigers 1–3 serrated and shifted to dorsal side ( Fig. 2 View FIGURE 2 C). Neuropodial lamellae of chaetiger 1 with 5 digitiform papillae along margin ( Fig. 2 View FIGURE 2 D), and lamellae of chaetigers 2 – 3 with 4 digitiform papillae ( Fig. 2 View FIGURE 2 C); lamellae of chaetigers 4 – 12 rounded and smooth ( Fig. 2 View FIGURE 2 C, F) later becoming wider on chaetigers 13 – 30, becoming pointed upper border ( Fig. 2 View FIGURE 2 G) from around chaetiger 31 up to end of fragments, gradually diminishing in size on subsequent chaetigers. Neuropodial prechaetal lamellae large, rounded and wide; larger in middle region, then gradually decreasing in size on posterior chaetigers; all lamellae not basally fused with neuropodial postchaetal lamellae.
Branchiae present from chaetiger 1 ( Fig. 2 View FIGURE 2 A) to end of body; tapered, elongate, smooth, partially fused to notopodial lamellae, branchial tips free, pointed distally on all chaetigers; branchiae longer than notopodial lamellae ( Fig. 2 View FIGURE 2 F–G). Each branchia with a dense band of cilia along inner and outer edges ( Fig. 2 View FIGURE 2 F–G). Accessory branchiae present from chaetigers 14 – 16, initially with simple, long digitate lobe arising from dorsolateral side of body behind notopodial base; lobes arranged in two rows, with number increasing gradually up to 5 or 8 ( Fig. 2 View FIGURE 2 G) on posterior chaetigers.
Notochaetae of chaetiger 1 in three groups ( Fig. 2 View FIGURE 2 H): a dorsal tuft with about 30–50 very long, smooth, slender capillaries directed upwards, longer than ventral fascicle, and a ventral fascicle arranged in two rows: anterior row comprised of stout, heavily reticulated, granulated, bilimbated capillaries ( Fig. 2 View FIGURE 2 I), posterior row of slender, granulated, alimbated capillary chaetae ( Fig. 2 View FIGURE 2 J), longer than those on first row, and fewer than those comprising dorsal tuft. Arrangement of chaetae on chaetiger 2 similar to chaetiger 1, dorsal tuft chaetae with about 10 long, slender, granulated capillaries ( Fig. 2 View FIGURE 2 K), shorter than notochaetae from tuft on chaetiger 1, and more numerous than chaetae making up rows. Arrangement of chaetae on subsequent chaetigers similar to that of chaetiger 2, with 2 or 3 uppermost dorsal tuft chaetae ( Fig. 2 View FIGURE 2 L) have wider, granulated, bilimbated capillaries, diminishing gradually in size and number. In addition, capillaries of anterior row of two types: 1) Uppermost capillaries: stout, heavily reticulated, granulated, bilimbated ( Fig. 2 View FIGURE 2 I); 2) Lower capillaries: slender, heavily reticulated, granulated, unilimbated (wide limbation) ( Fig. 2 View FIGURE 2 M); width of limbation of chaetae apparently depending on orientation. Chaetae of posterior notopodia arranged in similar to those of anterior and middle chaetigers, only with dorsal chaetae long, smooth and distally bilimbate ( Fig. 2 View FIGURE 2 N), chaetae on anterior row bilimbated, slightly reticulated in middle and distal end of shaft, and striated in middle and basal end of shaft ( Fig. 2 View FIGURE 2 O); those of posterior row slender, smooth, and slightly unilimbated distally ( Fig. 2 View FIGURE 2 P). Notopodial hooded hooks absent.
Neurochaetae of chaetiger 1 arranged in 2 rows of about 20 slender chaetae each: anterior row comprised of stout, heavily reticulated, granulated bilimbate capilliaries ( Fig. 2 View FIGURE 2 Q), and posterior row of long, smooth, slightly bilimbate capillary chaetae ( Fig. 2 View FIGURE 2 R); anterior capillaries shorter than posterior ones; in addition, a ventral tuft of 6 slender, shorter, smooth, alimbated capillaries ( Fig. 2 View FIGURE 2 S) located in position of sabre chaetae; neurochaetae on chaetiger 2 and subsequent chaetigers similar to those of chaetiger 1, but with an additional 2 – 3 sabre chaetae, chaetae longer, stout, heavily reticulated, granulated, alimbated ( Fig. 2 View FIGURE 2 T) on anterior and middle chaetigers; stouter, unilimbated sabre chaetae ( Fig. 2 View FIGURE 2 U) on posterior chaetigers; all chaetae with long, pointed tips. Unidentate neuropodial hooded hooks ( Fig. 2 View FIGURE 2 V), hooks completely hooded, replacing anterior row of capillary neurochaetae from chaetigers 25 – 27, up to 8 present per neuropodium, accompanied by a row of pointed, slender, granulated and unilimbated capillary chaetae ( Fig. 2 View FIGURE 2 W). Hooded hooks distally entire and slightly curved, hood extends distally to, or slightly beyond apex of hook ( Fig. 2 View FIGURE 2 X).
Pygidium lost in type material: however, in original description it was described with anal end tapering gradually to a short, narrow collar with a slight middorsal notch and a posterior aperture; without flange or cirrus.
Ovigerous segments from about chaetiger 52 to end of the fragment in paratype.
Remarks. Dispio uncinata is recorded from the Gulf of Mexico but deserves to be redescribed in order to clarify some doubtful features in the original description, particularly in regards to the morphology of the notopodial and neuropodila lamellae along the body. This has caused confusion and has led to undescribed species from other regions to be treated as synonyms of D. uncinata ; we anticipate that species from other regions will continue to be treated as synonyms with this species without careful consideration of these features.
We re-examined the holotype and paratypes of Dispio uncinata and the description of the species provided here agrees generally with that of Hartman (1951), except for differences in the starting point and arrangement of the accessory branchiae. Further information on noto- and neurolamellae morphology along the body is also provided. Additionally, the original description did not mention whether the ventral and dorsal edges of the notopodial and neuropodial lamellae overlap or touch each other on any of the chaetigers, or give details about the shape of the notopodial and neuropodial capillary chaetae. Hartman (1951) described four prostomial eyes almost completely concealed in the grooves between the prostomial caruncle and palpal bases. They are arranged in a trapezoid with the anterior pair further apart than the posterior pair. However, these were not observed in the type material, possibly due to discoloration of the preserved samples over time.
Foster (1971) gave a description of D. uncinata based on specimens from Massachusetts , Virginia , North Carolina, the Bahamas , Florida, Puerto Rico , Texas, Port Aransas , Chile and California . However, according to the re-examination of the D. uncinata specimens from Florida, and other material from Venezuela , Panama and California it is probable that Foster’s (1971) collection contains more than one species. Thus, the specimens from Texas and Florida examined by Foster may be D. uncinata , but the specimens from Massachusetts, Virginia , North Carolina, Bahamas , Puerto Rico, Chile and California should be re-examined in order to verify their identity.
Also, Johnson (1984) gave a very general description of notopodial and neuropodial lamellae and structure of chaetae of D. uncinata and compared his specimens with the description of D. uncinata by Foster (1971). Johnson (1984) concluded that the variability in the number of serrations on anterior parapodial lamellae, the number of chaetigers on which serrations occur, and the presence of bidentate hooded hooks falls within the range discussed by Foster (1971:76) for Gulf and Caribbean specimens. Other differences observed between the specimens examined in this study and the description given by Johnson (1984) include the latter the noto- and neuropodial lamellae that may overlap laterally, accessory branchiae present from chaetigers 18 – 28, and neuropodial hooded hooks from chaetigers 20 – 30. In contrast, the present material examined had ventral and dorsal edges of notopodial and neuropodial lamellae not overlapping or touching on any chaetiger, accessory branchiae present from chaetigers 14 – 16, and neuropodial hooded hooks from chaetigers 25 – 27. However, these characters could be influenced by the size of the specimens. Another discrepancy is where the sabre chaetae appear (20 – 30 vs. 2), but this should be confirmed. However, according to the re-examination of type and non-type material of D. uncinata in this paper it is probable that Johnson´s (1984) collection contains more than one species and require additional study in order to verify their identity.
Light (1978) gave a description of D. uncinata based on the specimens from San Francisco Bay, and compared his specimens with the description of D. uncinata by Foster (1971). Light (1978) mentioned that the material from central California conforms to the observations of Foster (1971) in that their specimens present an extreme variability in the number of serrations seen on the anterior notopodial lamellae and in the number of chaetigers bearing such serrated lamellae, even between individuals from the same locality. However, Light (1978) found important morphological differences with the description of Foster (1971); for example, serration on the neuropodial postsetal lamellae serrated were not always found (also not present on specimens from central California), the length of caruncle of some specimens reaching middle of chaetiger 1 or anterior margin of chaetiger 2, eyes arranged trapezoidally or as wide crescent, postchaetal notopodial lamellae those of first 1 to 10 chaetigers with a extreme variability of digitiform papillae, conferring to notolamellae a deeply serrated appearance, although occasionally these papillae are absent, postchaetal notopodial lamellae fused to branchiae partially or almost completely, branchiae prominent, long, cirriform or straplike, accessory branchiae present from chaetigers 18 – 28, neuropodial hooded hooks from chaetigers 16 – 37, in specimens from central california from chaetiger 30 – 37. This degree of morphological variation among their specimens, and according to the reexamination of type material of D. uncinata in this paper suggest that Light’s (1978) collection contains more than one species. Thus, the specimens from San Francisco Bay examined by Light (1978) should be re-examined in order to verify their identity.
Records of D. uncinata from other localities such as the Mediterranean ( Streftaris et al. 2005), Cantabrian ( Ibañez & Viéitez 1972), Trinidad ( Gobin 1990), Japan ( Imajima 1990) and Costa Rica Pacific coast ( Dean 2009), Chile ( Cañete et al. 2000; Castilla & Neill 2009) should also be reviewed. Dispio remanei Friedrich, 1956 and D. schusterae Friedrich, 1956 were considered synonymous with D. uncinata by Foster (1971). We were unable to examine specimens of these species as the type material was lost ( Fiege & Wehe 2004).
Ecology. Sandy beach
Geographical distribution. Gulf of Mexico. USA, Type locality: Florida, Franklin County, Alligator Point , Florida, and Grand Isle, Lousiana, Jefferson Parish Barataria Bay .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.