Culicoides (Diphaomyia) haematopotus Malloch
|
publication ID |
https://doi.org/ 10.5281/zenodo.6391684 |
|
publication LSID |
lsid:zoobank.org:pub:CBD29188-143B-44DF-BE21-1654D50D8621 |
|
persistent identifier |
https://treatment.plazi.org/id/E8511E53-FFE5-EF47-6A8A-FF16FDA5FE85 |
|
treatment provided by |
Felipe |
|
scientific name |
Culicoides (Diphaomyia) haematopotus Malloch |
| status |
|
Culicoides (Diphaomyia) haematopotus Malloch View in CoL
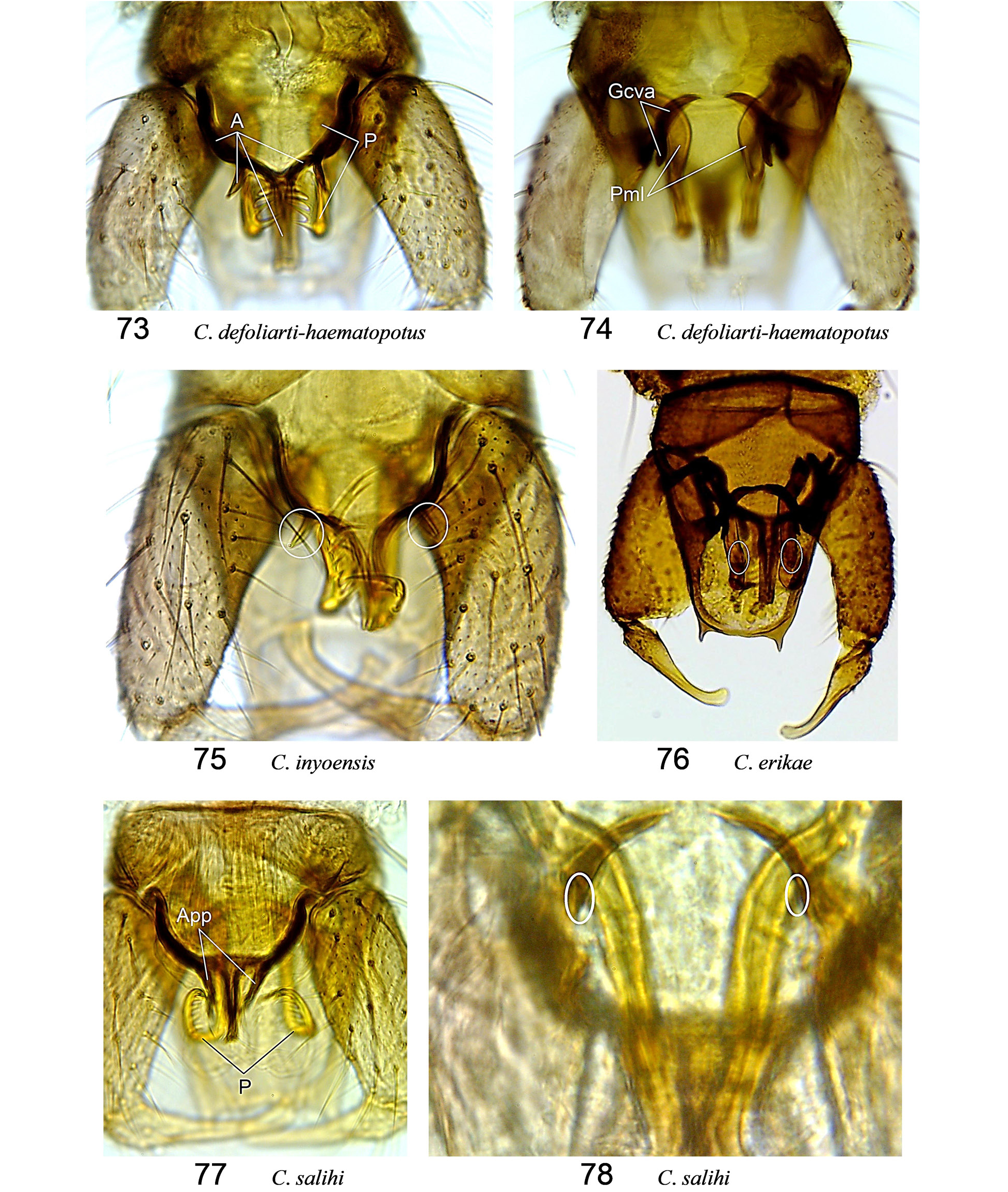
( Fig. 73, 74 View Figures 73–78 , 127, 205, 268)
Culicoides haematopotus Malloch, 1915: 302 View in CoL (key; male, female; fig. male genitalia, antenna, female wing; Illinois). Hoffman 1925: 299 (key; female; fig. wing, mesonotum). Root and Hoffman 1937: 161 (key; female; male genitalia; fig. male genitalia). Thomsen 1937: 69 (key; larva, pupa; fig. respiratory trumpet). James 1943: 149 (seasonal distribution; Colorado). Knowlton and Fronk 1950: 114 (Utah: Grand County). Wirth 1952a: 182 (in part; key; female; male genitalia; distribution; fig. dorsal thoracic pattern, female palpus, wing). Bullock 1952: 21 (key; female; Utah: Salt Lake County). Rees and Bullock 1954 (Utah: Salt Lake County). Foote and Pratt 1954: 23 (key; diagnoses of female, male, pupa; fig. female wing, mesonotum, palpus, male genitalia).
Culicoides (Oecacta) haematopotus: Khalaf 1954: 37 View in CoL (assignment to subgenus Oecacta View in CoL ). Fox 1955: 240 (in part; key and diagnoses of subgenera; species key; taxonomy). Wirth and Bottimer 1956: 263 (Texas ecology).
Culicoides (Diphaomyia) haematopotus: Vargas 1960: 40 View in CoL (assignment to subgenus Diphaomyia View in CoL ). Jones 1961a: 739 (in part; key; pupa; fig. respiratory trumpet, operculum, chaetotaxy, anal segment). Jamnback 1965: 70 (key; female; male genitalia; pupa, larva; biology; fig. male genitalia, female wing, antenna, palpus, eye separation, pupa, larva). Atchley 1967: 987 (in part; key; numerical characters; female; male genitalia; variation; fig. female wing, palpus, tibial comb, spermathecae, male genitalia, parameres). Childers and Wingo 1968: 14 (key; biology; fig. female wing, spermathecae). Jorgensen 1969: 17 (quantitative characters; key; female, male; seasonal distribution; fig. female wing, spermathecae, palpus, antenna, male genitalia, parameres). Battle and Turner 1971: 47 (female; male genitalia; larval habitats; feeding habits; seasonal distribution; fig. female eye separation, palpus, wing, spermathecae, male genitalia, parameres). Blanton and Wirth 1979: 95 (key; numerical characters; female; male genitalia; pupa, larva; fig. female antenna, palpus, wing, eye separation, spermatheca, leg, male genitalia, parameres; larval habitat; feeding habits; seasonal distribution). Atchley and Wirth 1979: 537 (key; numerical characters; female; male genitalia; pupa, larva; fig. female antenna, palpus, wing, eye separation, spermathecae, leg, male genitalia, parameres). Downes and Wirth 1981: 415 (fig. male genitalia). Wirth et al. 1985: 18 (numerical characters; fig. female wing). Wirth et al. 1988: 32 (numerical characters; fig. female wing). Murphree and Mullen 1991: 324 (key; larva; numerical characters; fig. head, epipharynx, hypostoma, caudal segment, mandible). Borkent and Spinelli 2000: 30 (in Neotropical catalog). Borkent 2012: 73 (fig. pupal abdominal segment 4). Borkent 2014: 24 (key to genera of pupae of Ceratopogonidae View in CoL ; fig. pupal abdominal segment 4, thorax, abdominal segment 9).
Diagnosis. ( Tables 14, 15) Wing pattern distinct; r 2 dark; isolated pale spots straddling at ~0.3 on M 1 and at ~0.5 on M 2; r 3 mostly dark with distal pale spot small and entirely within distal 0.2 of cell and reaching wing margin; pale areas anterior along CuA broken by dark areas at base and after midpoint; spermathecae unequal by ~1.2, sclerotized necks>2× longer than wide; ventral apodeme of gonocoxite with two widely divergent processes, footlike; basal arms of aedeagus each with spurlike process on posterior margin, median process of aedeagus narrow parallel-sided, aedeagal ratio ~0.5; parameres separate, each with bulbous submedian lobe and subapical fringe of spines.
Distribution. Southern Canada (British Columbia to Nova Scotia), through the United States and Mexico, to Honduras. Utah: Grand, Salt Lake counties.
Biology. In 1979, C. defoliarti was described from specimens of C. haematopotus collected in the western United States where their distributions are now recognized to overlap; thus, earlier western bionomic records for these species are conflated. For this reason, unless indicated as possibly for C. defoliarti , the following information is from outside the known range of C. defoliarti and, hence, likely specific for C. haematopotus .
Larval ecology. Jones (1961b) collected pupae he identified as C. haematopotus , which may be C. defoliarti , from two Utah sites (see C. defoliarti biology). Others have collected or reared C. haematopotus immatures from heavily vegetated and bare moist mud with rotting leaves at pond margins, a shaded stream margin with decaying leaves ( Williams 1955), stream edges with damp sand and leaf mold ( Murray 1957), moist alkaline dung-polluted direct sunlit soil, leaves in stream and pond margins, low-organic freshwater pond margins ( Hair et al. 1966), mud flats, freshwater seepage ponds ( Rowley 1967), low-organic pond and stream margins ( Blanton and Wirth 1979), pond margins, and muddy areas in pastures ( Kline and Greiner 1985). Pfannenstiel and Ruder (2015) found C. haematopotus along with C. sonorensis and C. crepuscularis in mud in relict (long unused) and active bison ( Bison bison ) wallows in Kansas about two weeks after they were flooded by rain.
Erram et al. (2019) reared C. haematopotus from mud substrate samples collected over three months in Florida from the edges of various stream, puddle, and seepage habitats, which produced 280, 69, and 2 adults, respectively. They also characterized the habitat samples for P, K, Mg, Ca, Cu, Mn, Zn, organic matter, pH, moisture, electrical conductivity, and microbes and found that adult production in the stream samples was positively correlated with Zn and P concentrations, pH, moisture, and microbe levels, but was negatively correlated with Mn concentration and electrical conductivity.
Adult behavior. Snow (1955) reported C. haematopotus blood-feeds at different levels in a forest ecosystem, starting in low shady areas late in the afternoon, moving into the shrub and tree canopy as light intensity and temperatures drop, feeding in the canopy through warm nights with an early crepuscular activity peak, and returning to the understory at dawn. However, Murray (1957) reported C. haematopotus adults were most active in lowland fields and pastures rather than wooded areas; but this habitat distinction may be biased by his use of light traps, which are more effective in open areas. Clarifying this, McGregor et al. (2018) found C. haematopotus significantly more abundant in UVLTs 6–9 m up in the forest canopy rather than at ground-level within the forest—a distinction Murray did not make.
Using only ground-level NJLTs, Hair (1966) found flight activity in Virginia greatest during 2100–0300 hours, with half as much activity during 0300–0600 hours. However, his use of light traps misses diurnal and underreports crepuscular activity. More thoroughly, Nelson and Bellamy (1971) used truck traps at 2 h intervals in Kern County, California, and found C. haematopotus (may be C. defoliarti ) flight activity through the night with activity peaks near dusk and dawn.
Culicoides haematopotus is an opportunistic feeder with a preference for birds. Known hosts are human ( Edmunds and Keener 1954; Snow 1955; Hair 1966; Hair and Turner 1968; Sloyer et al. 2019a), crow ( Corvus brachyrhynchos ) ( Fallis and Bennett 1961a), mourning dove ( Greiner 1975), cow ( Bos taurus ) ( Hayes et al. 1984; Sloyer et al. 2019a), turkey ( Meleagris gallopavo ) ( Atkinson 1988; Sloyer et al. 2019a), northern cardinal ( Cardinalis cardinalis [Linnaeus] Cardinalidae ), red-eyed vireo ( Vireo olivaceus [Linnaeus], Vireonidae ) ( McGregor et al. 2018), white-throated sparrow ( Zonotrichia albicollis ) ( Swanson and Turnbull 2014), chicken ( Gallus gallus ), and white-tailed deer ( Odocoileus virginianus [Zimmermann], Cervidae ) ( Sloyer et al. 2019a). Also, Sloyer et al. (2019a) found no statistically significant seasonal host preference variation in Florida.
Hair (1966) collected blood-engorged C. haematopotus from drop traps baited with domestic rabbit ( Oryctolagus cuniculus ), eastern cotton-tail rabbit ( Sylvilagus ), guinea pig ( Cavia porcellus ), opossum ( Didelphis ), rat ( Rattus ), chicken, turkey, mallard duck ( Anas boschas ), bobwhite quail ( Colinus virginianus ), and mourning dove.
Vector potential. Culicoides haematopotus has been found naturally infected with the magpie filarial worms Eufilaria longicaudata ( Wirth and Hubert 1989) and Chandlerella striatospicula Hibler (Nematoda: Filarioidea), which Hibler (1963) showed are transmitted by C. haematopotus . Robinson (1971) found active Chandlerella quiscali microfilaria in the head and mouthparts of C. haematopotus 10 d after feeding on an infected grackle. Greiner (1975) collected C. haematopotus feeding on mourning doves during a period when avian malarial Haemoproteus Kruse (Aconoidasida: Haemoproteidae ) was being transmitted among doves in Nebraska, though the midges were not tested for the avian malarial parasite; however, Atkinson (1988) found Haemoproteus mansoni Castellani and Chalmers sporozoites (as Haemoproteus meleagridis Levine ) in C. haematopotus salivary glands in Florida. Though not previously considered a virus vector, one C. haematopotus was found infected with bluetongue virus (BTV) in Louisiana ( Becker et al. 2010).
Symbionts. Culicoides haematopotus is sometimes heavily parasitized by mermithid nematodes, which produce intersexes in surviving adult midges. Smith and Perry (1967) collected 123 mermithid-induced intersexes (42% of males) in Florida, with intersex rates up to 51%; Atchley (1967) collected an intersex specimen (possibly C. defoliarti ) in New Mexico, however, he did not say if it was parasitized; and, Erram et al. (2019) found 4% of the adults reared from streambank and puddle samples collected over three months in Florida were mermithidparasitized intersexes. Ten indeterminate C. defoliarti - haematopotus intersex specimens were collected in the present study: one apparently unparasitized, eight parasitized by mermithid nematodes, and one parasitized by a mermithid nematode and a larval mite ( Table 10). In addition, several normal indeterminate male C. defoliarti - haematopotus and a female C. haematopotus were parasitized by larval mites ( Table 10).
Erram (2016) studied the bacterial flora on adult female C. haematopotus and found that Proteobacteria were predominant, likely because of its relatively unpolluted larval habitat. In addition, Wolbachia infections, which can alter dipteran reproduction by killing male embryos, inducing gamete incompatibility, or feminizing genetic males ( Stouthamer 1999), have been found in a C. haematopotus population in Florida ( Covey 2020), suggesting the possibility of using Wolbachia to control C. haematopotus populations or reduce pathogen transmission.
Remarks. Culicoides haematopotus conflates with C. defoliarti in records from the southwestern United States before 1979. See C. defoliarti remarks.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Culicoides (Diphaomyia) haematopotus Malloch
| Phillips, Robert A. 2022 |
Culicoides (Diphaomyia) haematopotus: Vargas 1960: 40
| Borkent A. 2014: 24 |
| Borkent A. 2012: 73 |
| Borkent A & Spinelli GR 2000: 30 |
| Murphree CS & Mullen GR 1991: 324 |
| Wirth WW & Dyce AL & Spinelli GR 1988: 32 |
| Wirth WW & Dyce AL & Peterson BV & Roper I. 1985: 18 |
| Downes JA & Wirth WW 1981: 415 |
| Blanton FS & Wirth WW 1979: 95 |
| Atchley WR & Wirth WW 1979: 537 |
| Battle FV & Turner EC 1971: 47 |
| Jorgensen NM 1969: 17 |
| Childers CC & Wingo CW 1968: 14 |
| Atchley WR 1967: 987 |
| Jamnback H. 1965: 70 |
| Jones RH 1961: 739 |
| Vargas L. 1960: 40 |
Culicoides (Oecacta) haematopotus:
| Wirth WW & Bottimer LJ 1956: 263 |
| Fox I. 1955: 240 |
| Khalaf KT 1954: 37 |
Culicoides haematopotus
| Foote RH & Pratt HD 1954: 23 |
| Wirth WW 1952: 182 |
| Bullock HR 1952: 21 |
| Knowlton GF & Fronk LE 1950: 114 |
| James MT 1943: 149 |
| Root FM & Hoffman WA 1937: 161 |
| Thomsen LC 1937: 69 |
| Hoffman WA 1925: 299 |
| Malloch JR 1915: 302 |