Polydora ecuadoriana Blake, 1983
|
publication ID |
https://doi.org/10.5281/zenodo.174538 |
|
DOI |
https://doi.org/10.5281/zenodo.6256895 |
|
persistent identifier |
https://treatment.plazi.org/id/F72C87EB-FF88-FFA2-057E-FE998EED3635 |
|
treatment provided by |
Plazi |
|
scientific name |
Polydora ecuadoriana Blake, 1983 |
| status |
|
Polydora ecuadoriana Blake, 1983 View in CoL
( Figs 2–7 View FIGURE 2 View FIGURE 3 View FIGURE 4 View FIGURE 5 View FIGURE 6 View FIGURE 7 )
Polydora ecuadoriana Blake (1983, pp. 257–258, Fig. 26).
Material
ECUADOR, Manabí, Bahia de Santa Elena, 200 ms offshore, 1/ 2 mile NE of Punta Ballenita, ANTON BRUUN R/V, cruise 16, st. SE-6669, 02 °12.33´S, 80°52.17´W, 8–10 m, 8 May 1966, coll. S.A. Earle, USNM 59920 (20 paratypes); st. SE-6670, 02 °11.47´S, 80°56.52´W, 8–9 m, 8 May 1966, USNM 60549 (2 paratypes).
MEXICO, Caribbean Sea: Veracruz, Tamiahua Lagoon, st. E-6, 25 °35´N, 97°35´W, 1 m, from oyster Crassostrea sp., 10 Jan 1993, coll. V.H. Delgado-Blas, ECOSUR SPIO-75 (7); Quintana Roo, st. CP 32- 4 41-3, ECOSUR SPIO-76 (1).
BRAZIL, Espírito Santo: Espírito Santo Bay: Ilha do Frade, 20°18´S, 40°17´W, intertidal, from shells of the oyster C. rhizophorae , coll. R.C. Nalesso and V.I. Radashevsky, 29 May 2003, MZSP 167 (9), 16 Jan 2004, MZSP 171 (24), 25 Feb 2004, MZSP 315 (6), MZSP 313 (23); Maria Ortiz, 20°15´S, 40°18´W, intertidal, 20 Feb 2004, coll. K.M. Machado, MZSP 168 (16). São Paulo: São Sebastião, Praia das Cigarras, 23°43.7´S, 43°24´W, intertidal, from shells of various gastropods inhabited by hermit crabs, 24 Mar 2004, coll. V.I. Radashevsky (10+, notes on live material, not fixed). São Sebastião, Praia do Saco Grande, 23°49.7´S, 45°25.5´W, 1–5 m, from shells of gastropods Stramonita haemastoma (Linnaeus, 1767) , Strombus pugilis (Linnaeus, 1758) , and Tegula viridula (Gmelin, 1791) inhabited by hermit crabs Paguristes tortugae Schmitt, 1933 and Pagurus brevidactylus (Stimpson, 1859) , 30 Jun 2004, coll. V.I. Radashevsky, (10+, notes on live material, not fixed). São Sebastião Island, Praia do Curral, 23°51.5´S, 45°25.9´W, 3–5 m, from shells of the gastropod S. haemastoma inhabited by hermit crabs, 13 May 2004, coll. V.I. Radashevsky, (10+, notes on live material, not fixed). Paraná: Paranaguá Bay: Praia Grande of Ilha do Mel, 25°32.8´S, 48°17.6´W, intertidal, from shell of the barnacle Megabalanus sp., 13 Aug 1998, coll. V.I. Radashevsky and P.C. Lana, USNM 1022187 (9). Pontal do Sul, mouth of Perequê tidal creek, 25°33.8´S, 48°21.4´W, intertidal, from shells of live gastropod S. haemastoma and oyster C. rhizophorae , 22 Aug 1998, coll. V.I. Radashevsky, USNM 1022185 (32), 1022186 (1). 25°28´S, 48°27´W, 2 m, from shells of the cultured oyster C. rhizophorae , 13 Sep 2001, coll. V.I. Radashevsky, MZSP 166 (1). Brasilia village of Ilha do Mel, 25°33´S, 48°19´W, 0.5 m, from shells of gastropods Pugilina morio (Linnaeus, 1758) and S. haemastoma inhabited by the hermit crab Clibanarius vittatus (Bosc, 1802) , 25 Aug 2001, coll. V.I. Radashevsky, MZSP 172 (5). Guaratuba Bay, 25°53´S, 48°34´W, intertidal, from shells of the oysters C. brasiliena , C. gigas , and C. rhizophorae , 3 Jun 2002, coll. V.I. Radashevsky, MZSP 169 (22). Santa Catarina, Florianópolis: North Bight of Ilha de Ratones Grande, 27°28´S, 48°33´W, 1 m, from shell of the cultured oyster C. gigas , coll. Y.M.B. Neptune, 24 Sep 1998, SMF 13967 (3); Praia da Ponta de Sambaqui, 27°28.5´S, 48°33.7´W, 1 m, from shell of the cultured oyster C. gigas , coll. Y.M.B. Neptune, 25 Apr 2003, SMF 14010 (100+), 15 Aug 2003, MZSP 170 (55), SMF 13930 (45).
Adult morphology (based on material from Brazil)
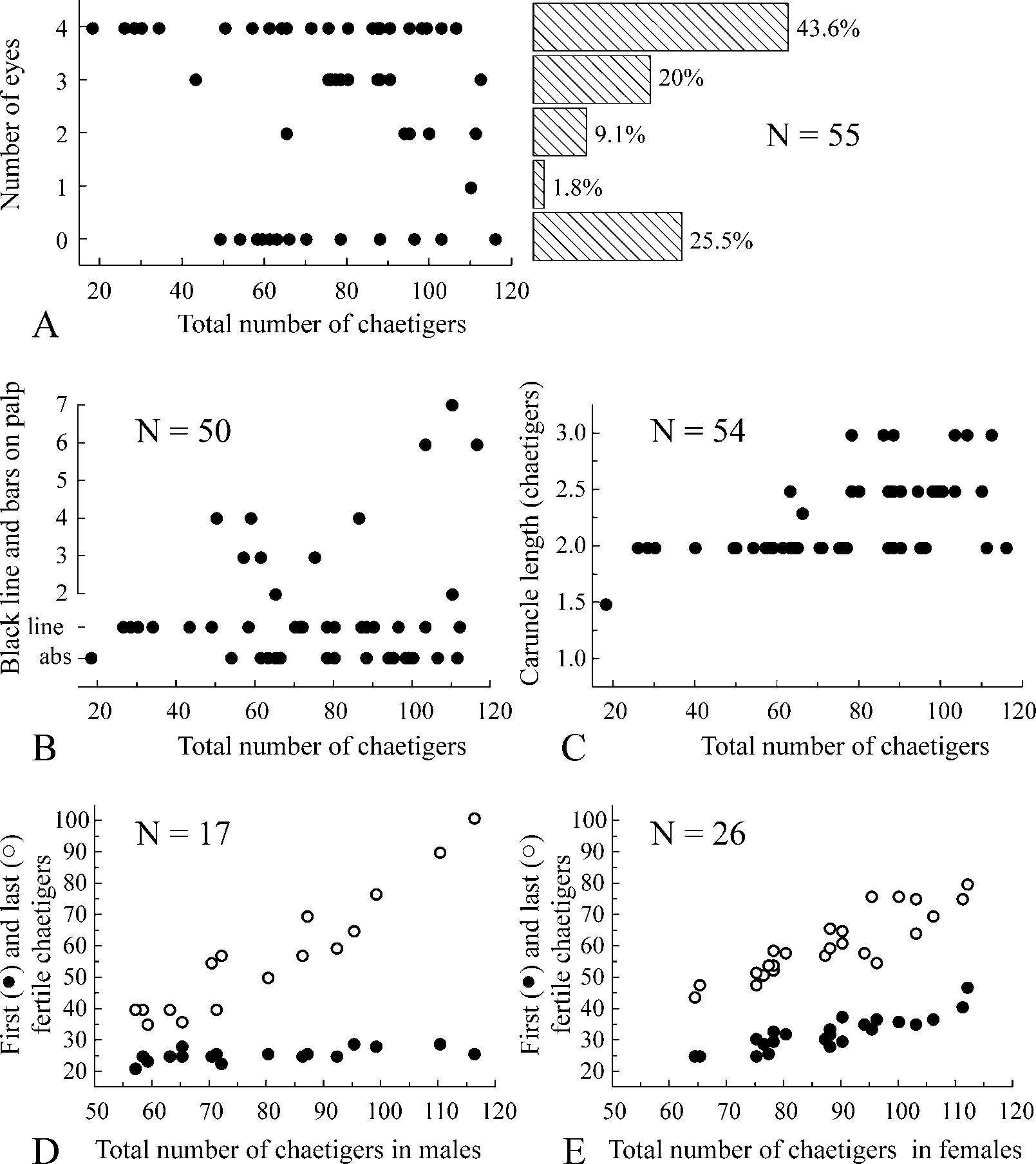
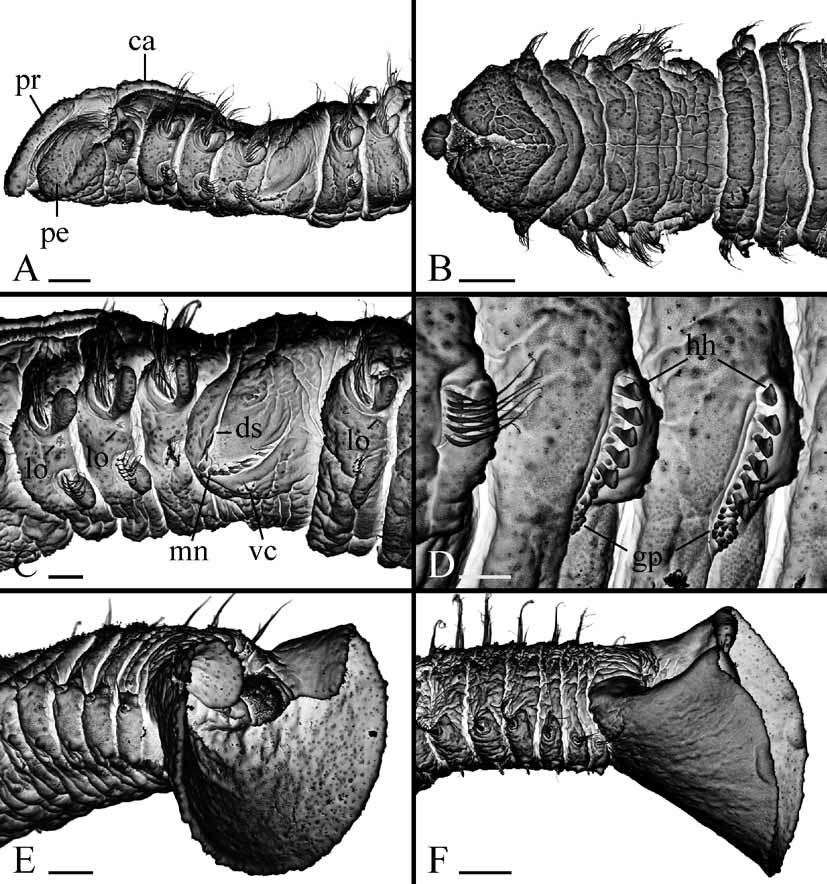
Up to 40 mm long and 1.2 mm wide at chaetiger 7 for 125 chaetigers. Body pale or light tan in life. Remains of larval black pigment often present on dorsal side of anterior chaetigers and on pygidium in individuals with less than 30 chaetigers; bigger individuals without pigmentation or fine black pigment scattered on ventral side of posterior chaetigers. Pigmentation on palps greatly variable, including fine black lines along longitudinal ciliated groove ( Fig. 2 View FIGURE 2 A), up to 7 separate black bars ( Fig. 6 View FIGURE 6 A) or lacking. Presence of pigmentation and its pattern not strongly correlated with total number of chaetigers in an individual ( Fig. 3 View FIGURE 3 B). Bars as paired gatherings of pigment on either side of groove, not extending all along palp periphery. Number of bars on left and right palps often different in same individual. Light yellow pigment present along ciliated groove between black bars on palps in some live individuals. Black pigment usually retained but yellow pigment absent in formaldehyde-fixed specimens.
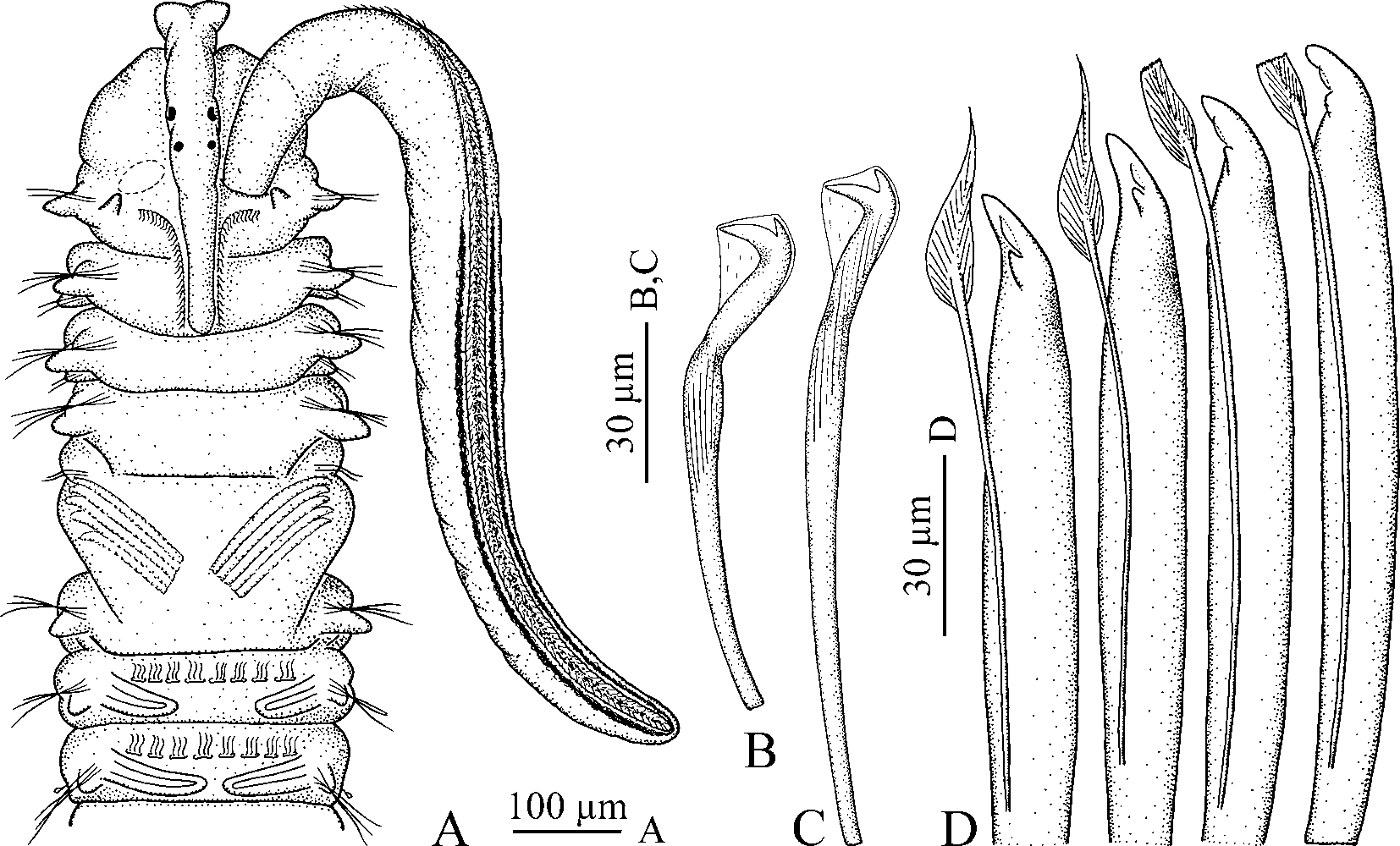
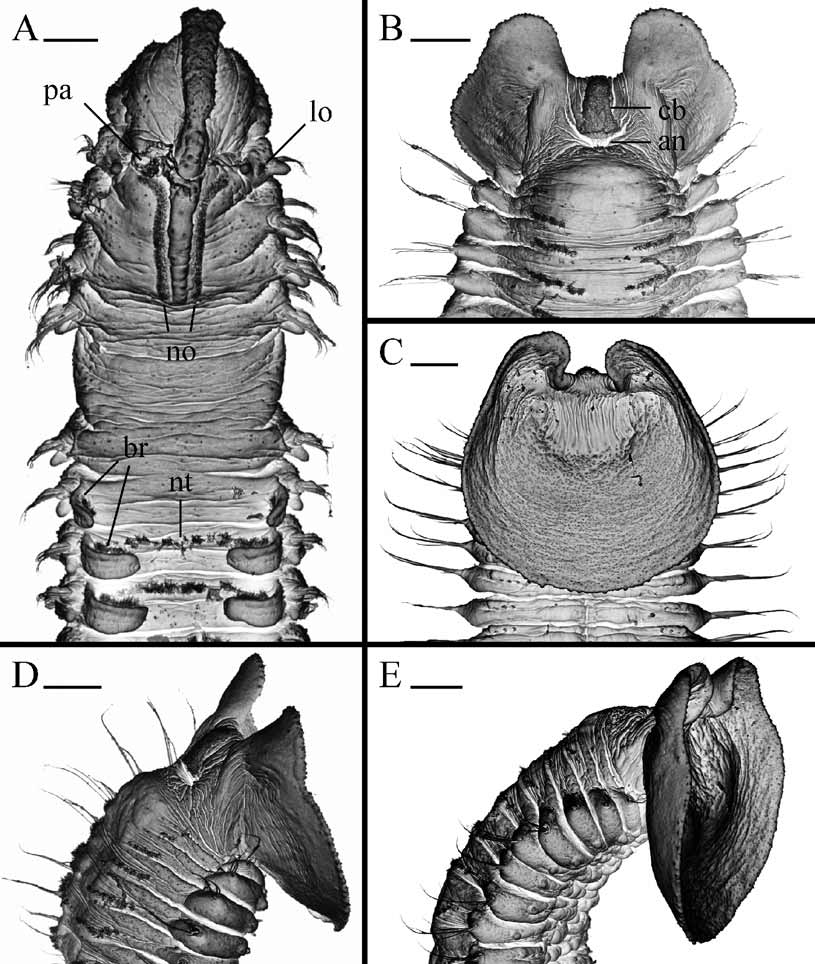
Prostomium anteriorly incised and in large individuals curved downwards ( Figs 4 View FIGURE 4 A, 5A), thus incision visible only in ventral view ( Fig. 5 View FIGURE 5 B). Caruncle extending to end of chaetiger 3, shorter in small individuals ( Fig. 3 View FIGURE 3 C). Occipital antenna absent. One to four black eyes present or eyes absent; their number not correlated with total number of chaetigers ( Fig. 3 View FIGURE 3 A). Palps as long as 10–20 chaetigers, with longitudinal groove lined with fine frontal cilia, latero-frontal motile compound cilia bordering groove, short lateral papillae with non-motile cirri arranged along either side of groove, and short compound non-motile cilia arising directly from palp surface and scattered on lateral and abfrontal palp surfaces.
Chaetiger 1 with short capillaries in neuropodia and well developed cirriform postchaetal lamellae in both rami; notochaetae absent. Capillary chaetae in notopodia gradually becoming fewer, shorter and alimbate in posterior chaetigers.
Chaetiger 5 greatly enlarged, overlapping chaetiger 6 dorsally, with 3–5 dorsal superior winged capillaries, up to 8 major modified spines alternating with bilimbatetipped companion chaetae and arranged in slightly curved, diagonal row, and 5–8 winged ventral capillaries; postchaetal lamellae absent ( Fig. 5 View FIGURE 5 C). Dorsal superior and ventral capillaries shorter and fewer than those on chaetigers 4 and 6. Major spines falcate, with accessory lateral tooth or flange and thin subdistal longitudinal flange located laterally on main fang above lateral tooth ( Fig. 2 View FIGURE 2 D); subdistal flange often worn and absent on spines in large individuals.
Hooks in neuropodia from chaetiger 7, bidentate, with constriction on shaft, up to 9 in a series ( Figs 2 View FIGURE 2 B, C, 5D).
Branchiae from chaetiger 7, full-sized from chaetigers 9–10, gradually diminishing in size along posterior half of body, distributed to almost end of body or absent on posterior one fourth to one half of body.
Pygidium large, scoop-shaped in juveniles and adults, same color as body, with wide dorsal gap, dorsal anus, postanal ciliary band and very few secretory cells ( Figs 4 View FIGURE 4 B–E, 5E, F).
Lateral ciliated organs as small pits between noto- and neuropodia on all chaetigers except 4 and 5 ( Figs 4 View FIGURE 4 A, 5C).
Glandular pouches from chaetiger 7, large in chaetigers 7 to 9–10, then gradually diminishing in size, single throughout. Large, flask-shaped secretory cells of pouches opening to exterior individually and appearing externally as small papillae below vertical rows of neurochaetae ( Fig. 5 View FIGURE 5 D).
Digestive tract without gizzard-like structure.
Metanephridial segmental organs from chaetiger 7, opening to exterior laterally on anterior, sterile segments and dorsally on gametogenic segments; paired nephridia opening separately on all segments. Distal part of segmental organs in gametogenic segments in females inflated, whitish in life, in formaldehyde-fixed specimens usually absorb methyl green stain intensely. Middle part of segmental organs in gametogenic segments in males formed by large urn-shaped cells and expanded.
Habitat
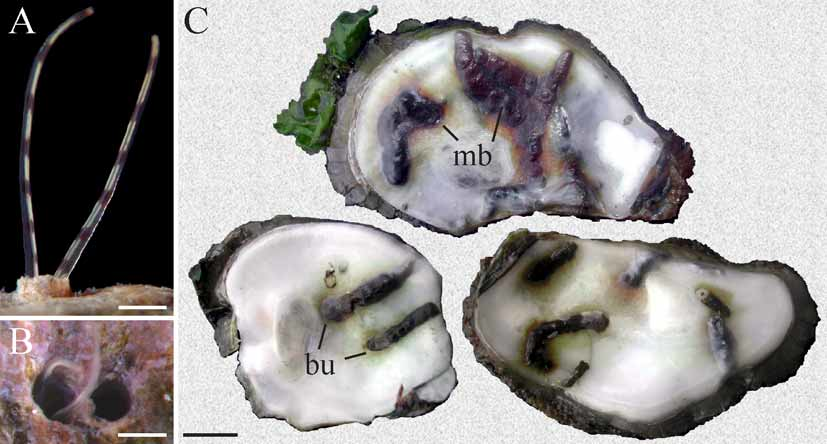
Polydora ecuadoriana is a borer in various calcareous substrata. In Ecuador it was found in shallow water boring into coralline algae and mollusk shells ( Blake 1983). In Brazil it occurred intertidally and in shallow water boring into shells of live oysters Crassostrea brasiliana , C. gigas , C. rhizophorae , the barnacle Megabalanus sp. and empty shells of the gastropods Pugilina morio , Stramonita haemastoma , Strombus pugilis , and Tegula viridula inhabited by hermit crabs Clibanarius vittatus , Paguristes tortugae and Pagurus brevidactylus . The worms resided in U-shaped burrows ( Fig. 6 View FIGURE 6 C) within the shell. Each burrow opened to the exterior via two joined apertures forming a characteristic 8-shaped opening ( Fig. 6 View FIGURE 6 B). Walls of the burrows were lined with fine silt and each aperture was extended by a smooth silty tube up to 10 mm high ( Fig. 7 View FIGURE 7 B). Paired palps of a worm can usually be seen protruding out of one of the tubes and collecting food particles suspended in the water and deposited on the surrounding surface ( Fig. 6 View FIGURE 6 A). The worms often caused the formation of dark blisters on the inner shell surface both in native and cultured oysters ( Fig. 6 View FIGURE 6 C). Severely infested mollusks had up to 50% of the inner shell surface covered by blisters. Infestations were seasonal and their intensity varied substantially between locations. Up to 10 worms occurred per cm2 of shell surface and more than one hundred worms were found in one oyster valve.
Regeneration
Worms regenerating lost anterior and posterior parts were occasionally found in shells. To investigate the regeneration process, various number of segments were cut off of live complete individuals and the fragments were maintained in dishes with filtered sea water in the laboratory at room temperature. In total, 45 worms were used in the experiment.
The epithelium healed the same day as the worms were cut, and regenerating buds on both the anterior and the posterior fragments developed in 1–2 days. In 2–4 days, the anterior blastema differentiated into head and body anlage. Palps began to develop immediately on the head anlage. The body anlage elongated gradually and in 4–5 days after the cut became segmented, probably simultaneously, into a definite number of segments. Chaetae, postchaetal lamellae and other chaetiger attributes only developed once segmentation was completed. No more chaetigers were added anteriorly in further growth. The number of chaetigers regenerated anteriorly depended on the number of lost chaetigers. The two numbers were equal if eight or less chaetigers were cut off. For example, if the head and five anterior chaetigers were cut off in the experiment, the head and five chaetigers were differentiated from the anterior blastema. If, however, nine or more chaetigers were cut off (30 worms cut in the experiment), only eight chaetigers regenerated anteriorly. All the anterior regenerates developed nototrochs from chaetiger 3 onwards apart from chaetiger 5.
The posterior blastema differentiated into pygidium and the prepygidial growth zone in 3–5 days after a cut. New posterior segments developed successively one by one following the formation of the growth zone.
Reproduction
Polydora ecuadoriana is gonochoristic. Of 46 mature individuals, 28 were females and 18 were males. Gametes developed along segmental blood vessels in middle segments, from chaetigers 21–47 to 35–101. The position of the first gametogenic chaetiger varied equally (from chaetiger 21 to 29) in small and large males ( Fig. 3 View FIGURE 3 D), whereas fertile chaetiger started more posteriorly (from chaetigers 25–47) in large females than in small ones ( Fig. 3 View FIGURE 3 E).
In males, the testis contained only spermatogonia; individual primary spermatocytes, diads of secondary spermatocytes, tetrads of spermatids, and individual spermatozoa floated freely together in the coelomic cavity. The smallest male with spermatozoa in the coelomic cavity was about 7 mm long for 57 chaetigers. Spermatids were spherical, 3 µm in diameter. Spermatozoa were introsperm with an elongated straight head about 1 µm in diameter, head+middlepiece 12 µm long, acrosome 2 µm, nucleus 6 µm, middlepiece 4 µm, and flagellum 46 µm long. On one occasion in the laboratory, a male shed spermatophores through the nephridiopores on fertile segments. The spermatophores were fine, sinuous, 3-5 mm long and 8 µm in diameter ( Fig. 7 View FIGURE 7 A). They were composed of regularly packed spermatozoa enveloped by a fine matrix. In seawater, spermatophores broke apart and spermatozoa started to move in a half an hour.
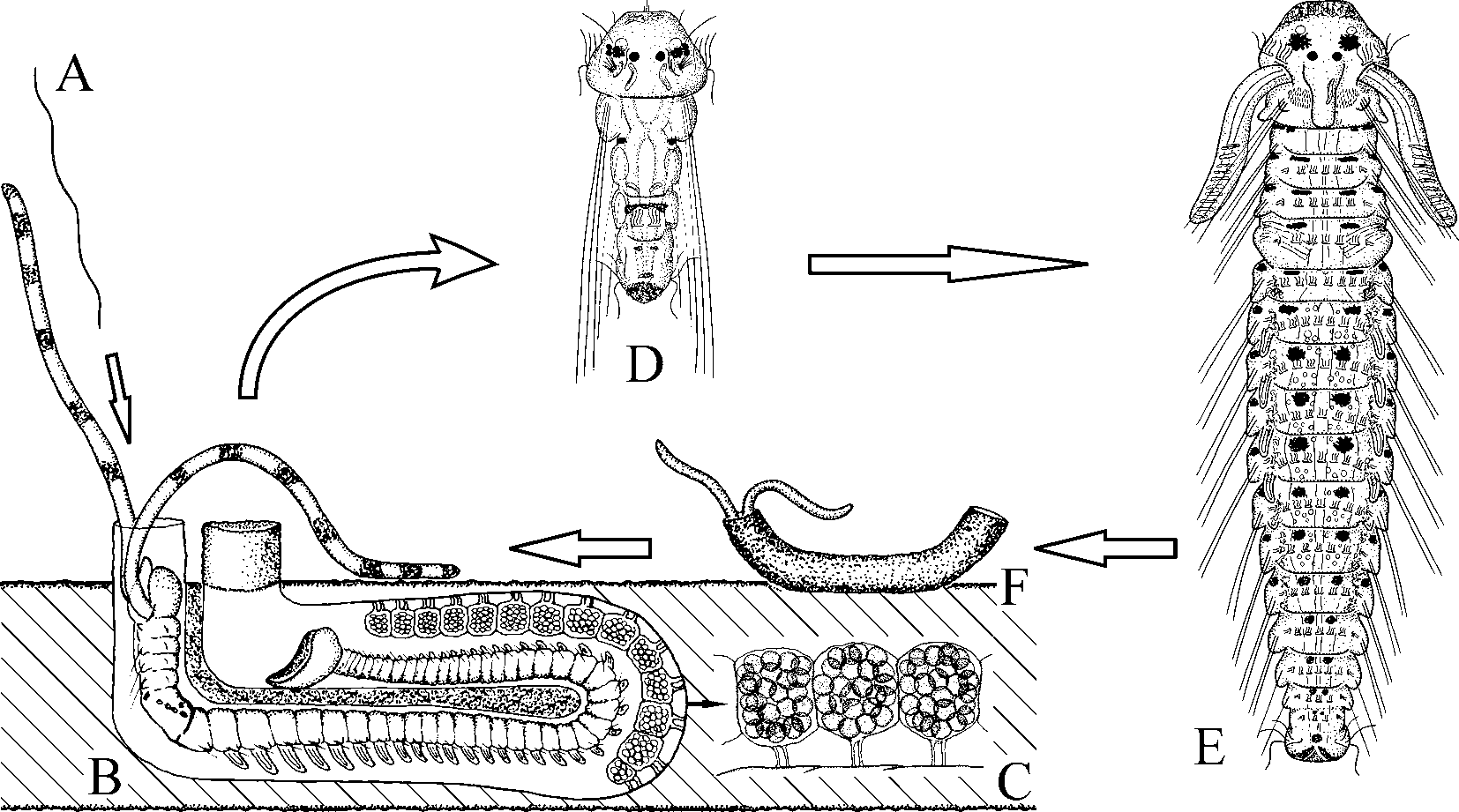
In females, the ovaries contained previtellogenic and vitellogenic oocytes of various diameters. Large oocytes occurred freely in the coelomic cavity. The smallest female with large oocytes in the coelomic cavity was about 10 mm long for 64 chaetigers. Paired intraepithelial seminal receptacles were present on the dorsal side of the female gametogenic chaetigers, posterior to the nototrochs, medial to the base of the branchia. The receptacles were oval chambers, about 20x25 µm in diameter, filled up with clusters of inactive spermatozoa. Females deposited up to 2500 eggs into 20–50 capsules which were joined to each other in a string. Each egg capsule was attached by two thin stalks to the inner wall of the burrow and contained from 10 to 60 eggs ( Fig. 7 View FIGURE 7 B, C). The eggs were spherical, 100–110 µm in diameter, filled with yolky globules. The majority of eggs in broods developed synchronously into larvae, but up to 10% of the eggs did not undergo cleavage and were consumed by developing larvae. Synchronous beating of nototrochs on the dorsal side of the female produced a constant water flow inside the burrow and provided oxygen for the developing larvae. When no yolky globules were left in the digestive tract, the 3-chaetiger larvae about 280 µm long hatched, escaped from the mother’s burrow and entered the plankton. Females brooding larvae in capsules had the next generation of vitellogenic oocytes up to 50 µm in diameter developing in the ovaries. Egg capsules in burrows occurred from February to September.
Life history
Males and females become mature when they have grown to about 55 and 65 chaetigers, respectively. Males produce spermatophores and pass them to females ( Fig. 7 View FIGURE 7 A). Females accumulate the sperm in seminal receptacles on the dorsal side of the gametogenic segments. The oocytes are steadily produced in ovaries and large oocytes are accumulated in the coelomic cavity within an extended period of reproduction. Females regularly lay broods which mostly contain eggs developing into larvae ( Fig. 7 View FIGURE 7 B, C). Fertilization is thought to occur in the egg capsule. Larvae develop 3 chaetigers in the capsule, then are released and continue development in the seawater, feeding on the plankton ( Fig. 7 View FIGURE 7 D). They are able to settle when they have grown to 16–18 chaetigers ( Fig. 7 View FIGURE 7 E). In the absence of suitable substrata, the larvae are able to postpone settlement and grow in the plankton until the 20–22-chaetiger stage. Settlement is accompanied by gradual metamorphosis and loss of provisional larval features. The switch to a new mode of feeding after settlement is enabled by rapid elongation of the palps, modification of the prostomium, enlargement of the ventral peristomial lip and transformation of the lateral peristomial lips into the dorso-lateral ciliary folds. Newly settled individuals first construct a small silty tube on the shell surface ( Fig. 7 View FIGURE 7 F) and then begin boring into the shell. Boring is due to a combination of glandular secretion and abrasion, by which process the Ushaped burrow is permanently elongated in its middle part ( Fig. 7 View FIGURE 7 B). After settlement, larval pigmentation is totally reduced or retained only on 1–3 anterior chaetigers, and black and yellow pigments usually appear on palps in further development. The life span of the species is unknown but preliminary examination of the size structure of the population allows us to assume that adults are able to survive over 2 years in suitable conditions. Larval development of the species will be described elsewhere.
Remarks
Specimens from Brazil fit well the main characteristics of P. ecuadoriana originally described by Blake (1983) from Ecuador. Black bars on palps, incised prostomium, major falcate spines of chaetiger 5 with lateral tooth and subdistal longitudinal flange, and large, scoop-shaped pygidium are the diagnostic features of the species. Blake (1983: p. 258) reported the posterior chaetigers in the species “with 4–5 long, thin capillaries and 3–4 short pointed spines”; however, the paratypes of P. ecuadoriana (USNM 59920, 60549) were examined and notopodial slender capillaries were found gradually transforming into spine-like capillaries in posterior notopodia, the same as in the specimens from Brazil. Such gradually modified capillaries differ from true heavy spines which appear abruptly in posterior notopodia in addition to capillaries in some Polydora species ( Blake 1979). Thus, the specimens from Brazil were identical to P. ecuadoriana and therefore referred to this species. The Brazilian worms exhibited significant variation in palp pigmentation; fine black lines and short prominent bars were present or pigmentation was totally absent.
Besides specimens with the typical scoop-shaped pygidium, there were worms in Brazil which appeared surprisingly similar to P. ecuadoriana but had small disc-like pygidia. These worms are here referred to P. cf. haswelli . The two species occurred together in a shell in various proportions, thus one or another was usually more abundant in different areas. The differences between them are discussed below, in the Remarks section after the description of P. cf. haswelli .
Some Polydora specimens boring into oyster shells from the Gulf coast of Mexico were also studied (ECOSUR SPIO-75, 76). They were anterior fragments lacking a pygidium, thus a definitive identification could not be made. However, other features, such as black bars on the palps, caruncle extending to the end of chaetiger 3, and major falcate spines of chaetiger 5 with lateral tooth and subdistal longitudinal flange, appeared similar to those of P. ecuadoriana . These specimens were tentatively referred to P. ecuadoriana .
With its boring mode of life and an unusual scoop-shaped pygidium, P. ecuadoriana is similar to Polydora pygidialis Blake & Woodwick, 1972 from California. The two differ, however, in that P. ecuadoriana has black bars on palps, an incised prostomium, caruncle extending posteriorly to the end of chaetiger 3, and major falcate spines with lateral tooth and subdistal flange, whereas P. pygidialis has plain palps, entire prostomium, caruncle to the end of chaetiger 2, and major spines with only a large lateral tooth.
Distribution
Ecuador; Gulf coast of Mexico; Brazil: Espírito Santo south to Santa Catarina.
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.