Polydora cf. haswelli Blake & Kudenov, 1978
|
publication ID |
https://doi.org/10.5281/zenodo.174538 |
|
DOI |
https://doi.org/10.5281/zenodo.6256898 |
|
persistent identifier |
https://treatment.plazi.org/id/F72C87EB-FF9E-FFA7-057E-FBDC8E9337A2 |
|
treatment provided by |
Plazi |
|
scientific name |
Polydora cf. haswelli Blake & Kudenov, 1978 |
| status |
|
Polydora cf. haswelli Blake & Kudenov, 1978 View in CoL
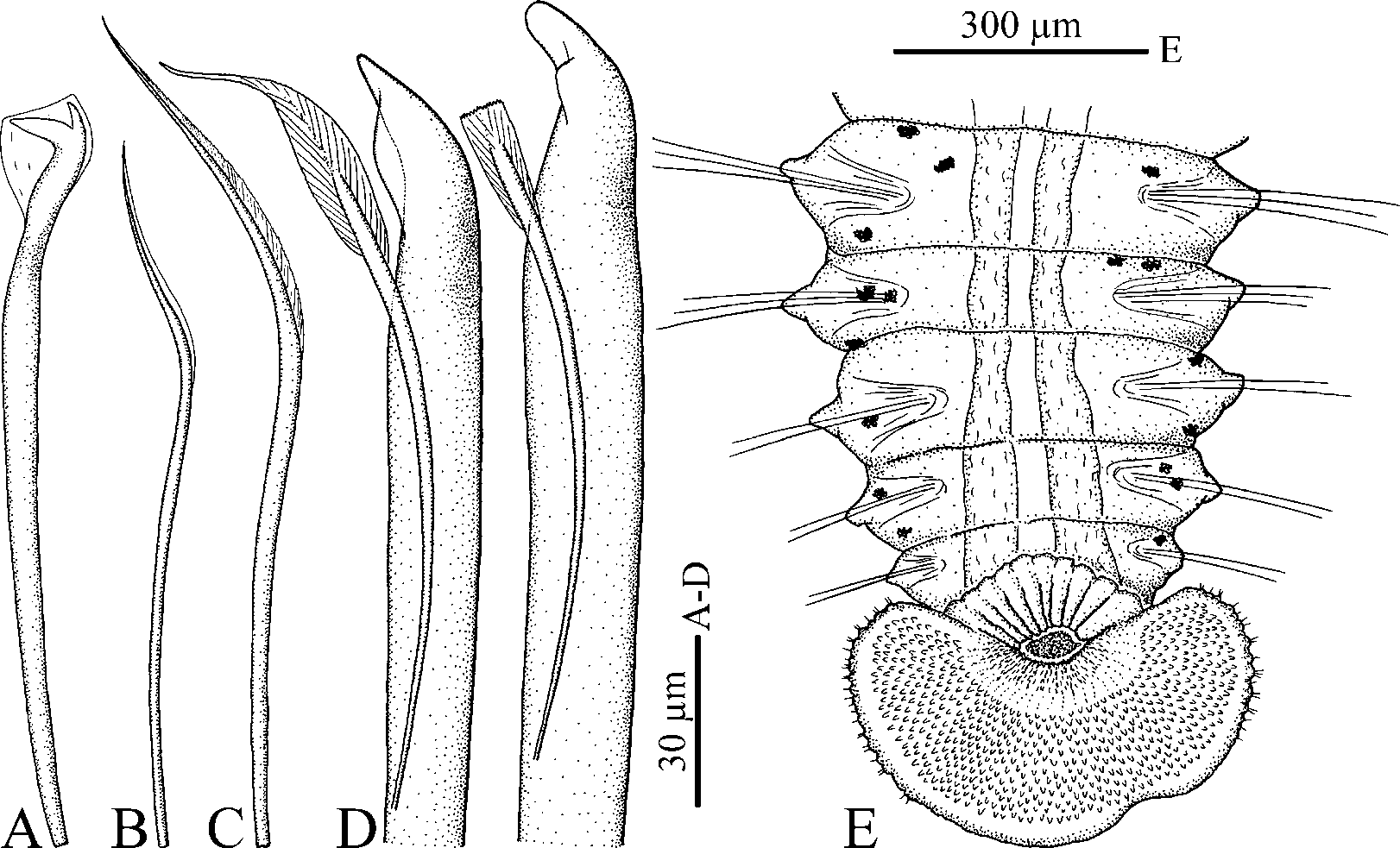
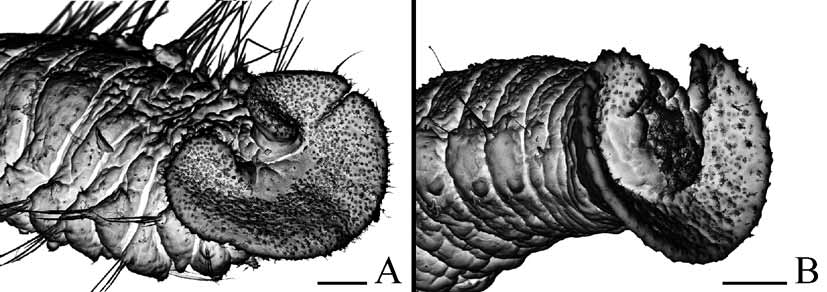
( Figs 8 View FIGURE 8 & 9 View FIGURE 9 )
Polydora haswelli Blake & Kudenov (1978, pp. 259–260, Fig. 44).
Polydora websteri View in CoL : Bolívar & Lana (1987, pp. 115–116, Figs 8 View FIGURE 8 –20). — Blankensteyn & Moura (2002, p. 718, table 2). Not Polydora websteri Hartman View in CoL in Loosanoff & Engle, 1943.
Material
BRAZIL, Espírito Santo: Espírito Santo Bay: Ilha do Frade, 20°17´S, 40°17´W, intertidal, from shells of the oyster C. rhizophorae , coll. R.C. Nalesso and V.I. Radashevsky, 29 May 2003, MZSP 175 (12); 25 Feb 2004, MZSP 310 (5). São Paulo: Alcatrazes Is., 24°06´S, 45°42´W, 5–6 m, from live stony coral Mussismilia hispida (Verrill, 1808) , coll. J.M.M. Nogueira, 4 Dec 1996, MZSP 173 (2), USNM 1022188 (2). Praia Fazenda, 23°22´S, 44°50.3´W, intertidal, sandy beach, from shell of a gastropod inhabited by hermit crab Dardanus insignis (de Saussure, 1858), 0 9 May 2001, coll. V.I. Radashevsky, MZSP (1+). Praia São Francisco, 23°45´S, 45°25´W, intertidal, from shells of living oysters and gastropods, and from gastropods inhabited by hermit crabs, 16 Apr 2003, coll. V.I. Radashevsky, MZSP (1+). Praia Araçá, 23°48.8´S, 45°24.2´W, sandy intertidal, coll. V.I. Radashevsky, from shell of oyster C. rhizophorae , 17 Apr 2003, MZSP (1+). São Sebastião Island, Praia do Curral, 23°51.5´S, 45°25.9´W, 3–5 m, from empty shells of the gastropod Stramonita haemastoma inhabited by hermit crabs, 13 May 2004, coll. V.I. Radashevsky, (10+, notes on live material, not fixed). São Sebastião, Praia do Saco Grande, 23°49.7´S, 45°25.5´W, 1–5 m, from shells of living saddle oyster Anomia ephippium Linnaeus, 1758 and gastropods Astraea olfersii (Philippi, 1846) , Morula nodulosa (Adams, 1845) , Pisania auritula (Link, 1807) , Pisania pusio (Linnaeus, 1758) , Siratus senegalensis (Gmelin, 1791) , Stramonita haemastoma , Strombus pugilis , and Tegula viridula inhabited by hermit crabs Paguristes tortugae and Pagurus brevidactylus , 30 Jun 2004, coll. V.I. Radashevsky, MZSP 179 (50). Paraná: Paranaguá Bay: Ilha das Cobras, 25°28.9´S, 48°26´W, intertidal, from oyster shell, 23 Jul 1985, coll. P.C. Lana, CEM/ UFPR 38 (2). Brasilia village of Ilha do Mel, 25°33´S, 48°19´W, 0.5 m, 25 Aug 2001, coll. V.I. Radashevsky, from shell of the gastropod Crepidula plana Say, 1822 , MZSP 178 (1); from shells of gastropods Pugilina morio and Stramonita haemastoma inhabited by hermit crab Clibanarius vittatus , MZSP 176 (80). 25°28´S, 48°27´W, 2 m, from shells of the cultured oyster C. rhizophorae , 13 Sep 2001, coll. V.I. Radashevsky, MZSP 174 (10). Santa Catarina, Florianópolis: Praia da Ponta de Sambaqui, 27°28.5´S, 48°33.7´W, 1 m, from shells of the cultured oyster C. gigas , coll. Y.M.B. Neptune, 15 Aug 2003, MZSP 177 (18).
Adult morphology
Up to 30 mm long and 1 mm wide at chaetiger 7 for 170 chaetigers. Body pale or light tan in life. Pigmentation on palps greatly variable: fine black lines or separate black bars present along longitudinal ciliated groove; occasionally pigmentation lacking. Pigment pattern and numbers of bars on palps not correlated with total number of chaetigers in an individual. Light yellow pigment often present along ciliated groove between black bars. Black pigment absent on body or black narrow strips present on either side of anterior part of prostomium, and indistinct paired patches present on dorsal side of peristomium and 1–3 anterior chaetigers. Small distinct yellow chromatophores present on dorso-lateral sides of posterior chaetigers in large individuals ( Fig. 8 View FIGURE 8 E). Yellow pigment on palps and posterior chaetigers absent in formaldehyde-fixed specimens.
Prostomium incised anteriorly. Caruncle extending to end of chaetiger 3. Occipital antenna absent. Two pairs of black eyes usually present; occasionally one to three eyes present or eyes lacking. Palps as long as 10–20 chaetigers, with longitudinal groove lined with fine frontal cilia, latero-frontal motile compound cilia bordering groove, short lateral papillae with non-motile cirri arranged along either side of groove, and short compound non-motile cilia arising directly from palp surface and scattered on lateral and abfrontal palp surfaces.
Chaetiger 1 with short capillaries in neuropodia and postchaetal lamellae in both rami; notochaetae absent. Posterior notopodia with only capillary chaetae.
Chaetiger 5 greatly modified, almost twice as large as chaetigers 4 or 6, with 3–5 dorsal superior winged capillaries ( Fig. 8 View FIGURE 8 C), 5–8 major modified spines alternating with bilimbate-tipped companion chaetae and arranged in a slightly curved, diagonal row, and 5–8 winged ventral capillaries ( Fig. 8 View FIGURE 8 B); postchaetal lamellae absent. Dorsal superior and ventral capillaries shorter and fewer than those on chaetigers 4 and 6. Major spines falcate, with lateral accessory flange of variable size ( Fig. 8 View FIGURE 8 D).
Hooded hooks in neuropodia from chaetiger 7, bidentate, with constriction on shaft ( Fig. 8 View FIGURE 8 A), up to 8 in a series.
Branchiae from chaetiger 7, full-sized from chaetigers 9–10, distributed to middle or usually almost end of body, becoming much smaller on posterior chaetigers.
Pygidium cup-shaped to disc-like, with dorsal gap to narrow incision, white due to numerous glandular cells; small notch often present on ventral side of pygidium, usually positioned asymmetrically ( Fig. 8 View FIGURE 8 E). Small knobs with short non-motile, probably sensory, cilia arranged along edge of pygidium, mainly along its dorso-lateral edge.
Lateral ciliated organs as small pits between noto- and neuropodia on all chaetigers except 4 and 5.
Glandular pouches from chaetiger 7, large in chaetigers 7 to 9, then greatly diminishing in size.
Digestive tract without gizzard-like structure.
Metanephridial segmental organs from chaetiger 7, opening to exterior laterally on anterior, sterile chaetigers and dorsally on gametogenic chaetigers; paired nephridia opening separately on all chaetigers.
Habitat
In Brazil, P. cf. haswelli was found intertidally and in shallow water in shells of live oysters Crassostrea brasiliana , C. gigas , C. rhizophorae and Anomia ephippium , the gastropod Crepidula plana , and empty shells of the gastropods Astraea olfersii , Pisania auritula , Pisania pusio , Pugilina morio , Siratus senegalensis , Stramonita haemastoma , Strombus pugilis , and Tegula viridula inhabited by hermit crabs Clibanarius vittatus , Paguristes tortugae and Pagurus brevidactylus , and also in live scleractinian corals. Up to 10 worms occurred per cm2 of shell surface. Worm burrows were U-shaped with walls lined with fine silt. The burrows of P. cf. haswelli resemble those of P. ecuadoriana . The two species occurred together in shells in various proportions.
Reproduction
Polydora cf. haswelli is gonochoristic. Spermatids were interconnected in tetrads. Spermatozoa were introsperm with an elongated straight head about 1 µm in diameter, head+middlepiece 12 µm long, acrosome 1.5 µm, nucleus 6 µm, middlepiece 4.5 µm, and flagellum 45 µm long. Females deposited eggs about 100 µm in diameter into capsules which were joined to each other in a string and attached to the inner wall of the burrow by two stalks. Larvae developed inside the capsules until the 3-chaetiger stage, when they hatched and continued development in the water column until the 16–18-chaetiger stage, feeding on the plankton. Morphology of the capsules and early, encapsulated larvae resembled those of P. ecuadoriana . Larval development of the species will be described elsewhere.
Remarks
Worms identified herein as P. cf. haswelli resemble P. ecuadoriana in many characteristic features. The two species occur together in shells, have similarly variable pigmentation on palps and anterior chaetigers, an incised prostomium, caruncle extending posteriorly to the end of chaetiger 3, spermatids joined in tetrads, similar dimensions of oocytes and spermatozoa, and morphology of early larvae. The extreme forms of each species can easily be distinguished by the shape of pygidium: those referred to P. ecuadoriana have a large, scoop-shaped pygidium, while P. cf. haswelli individuals have a small, disc-like to cup-shaped pygidium. However, the morphology of the pygidium is variable in both species, including form intermediate between the cup- and scoop-shaped pygidia ( Fig. 9 View FIGURE 9 B). Other characteristics that might be diagnostic, such as the dentition of chaetiger 5 falcate spines (single lateral flange versus lateral tooth and small subterminal flange), and the presence of small yellow chromatophores on the posterior segments, are also variable, making delineation of the species ambiguous. Lateral additional structures on old falcate spines (situated in the anterior part of the row) were worn and indistinct, while on new spines (situated in the posterior part of the row) they appeared as one complete large flange or lateral tooth and small subterminal flange, depending on the view of observation and quality of the preparation. Yellow chromatophores were absent in some live worms with all the other characteristics corresponding to P. cf. haswelli . This variability raised a problem of conspecificity of worms with large, scoop-shaped and small, disc-like pygidia (see below doubts about identification as P. haswelli ).
Pelagic Polydora larvae caught in a creek entering Espírito Santo Bay in the state of Espírito Santo provided support for the idea of two sympatric species. Two kinds of larvae were found in the plankton and only P. ecuadoriana and P. cf. haswelli were present on the bottom, boring in oyster shells. The larvae appeared very similar in most diagnostic characteristics but differed unambiguously in the shape and position of melanophores on the lateral peristomial lips. It is plausible that two Polydora species co-occur in the area and they are able to interbreed on occasion, resulting in individuals with intermediate shape of the pygidium. Further molecular investigations are certainly needed to clarify this issue. Meanwhile worms with scoop-shaped and disc-like pygidia are distinguished taxonomically.
Another problem to solve was the identification of the two species under discussion. The diagnostic features and taxonomic status of P. ecuadoriana are discussed above in this paper, while reasoning for P. cf. haswelli is provided here. Although both species demonstrate variable pigmentation (even absent in some individuals), they can be referred to a group of Polydora species having characteristic black bars on palps. Those species were reviewed by Williams & Radashevsky (1999: Table 1) and Radashevsky & Hsieh (2000: Table 1) but the Brazilian worms fit none of them. Both reviews, however, overlooked P. ecuadoriana and P. haswelli . The original description of the latter species referred ambiguously to “additional pigment on palps” ( Blake & Kudenov 1978: p. 259) but James A. Blake clarified that "pigment spots occurred along the palps" (e-mail of 3 November 2003 to VIR). This clarification made P. neocaeca Williams & Radashevsky, 1999 (originally described from Rhode Island, U.S.A.) and P. haswelli Blake & Kudenov, 1978 (originally described from New South Wales, Australia) similar to each other and raised a problem of their identity. Both species are shell-borers with almost identical main characteristic features, including body and palp pigmentation and the shape of the pygidium. They differ in the shape of additional structures on major falcate spines of chaetiger 5, with large accessory tooth and smaller vertical flange above the tooth present in P. haswelli ( Blake & Kudenov, 1978: fig. 44C–E), and an obliquely curved flange present in P. neocaeca ( Williams & Radashevsky 1999: fig. 1D). These differences are not, however, unambiguous and should be studied more carefully since both kinds of spine lateral structures may vary. Sperm morphology might be informative for the species delineation and is used herein for a tentative identification of the Brazilian worms. Polydora neocaeca has spermatids joined in octads (Williams 2000) and spermatozoa with acrosome 0.9 ± 0.1 µm, nucleus 4.8 ± 0.4 µm, middlepiece 4.2 ± 0.4 µm ( Williams & Radashevsky 1999; Williams 2000). Polydora cf. haswelli collected in Brazil have spermatids joined in tetrads, and the spermatozoa differ in measurements from those of P. neocaeca . The number of spermatids joined together by cytoplasmic bridges is species specific, 4 or 8 in examined Polydora species, and has been used for distinguishing between sibling species ( Radashevsky & Pankova 2006). Information about spermatid aggregation and sperm morphology of P. haswelli from Australia is lacking. Pending more details on morphology of the Australian material, we refer to the Brazilian specimens as P. cf. haswelli .
Remarkably, P. h a s w e l l i has not been reported outside of Australia, and P. ecuadoriana was recorded only from Ecuador. Further molecular investigations would clarify the identification of the Brazilian worms referred to these species.
Distribution
Australia: New South Wales; Brazil: Espírito Santo south to Santa Catarina.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.