Ablabes hamptoni Boulenger, 1900
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4457.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:60B855A1-F4A8-4F0D-9507-04B5CFF27188 |
|
DOI |
https://doi.org/10.5281/zenodo.5993149 |
|
persistent identifier |
https://treatment.plazi.org/id/03C787A6-0868-FF94-D890-F96FFE9068F9 |
|
treatment provided by |
Plazi |
|
scientific name |
Ablabes hamptoni Boulenger, 1900 |
| status |
|
Ablabes hamptoni Boulenger, 1900
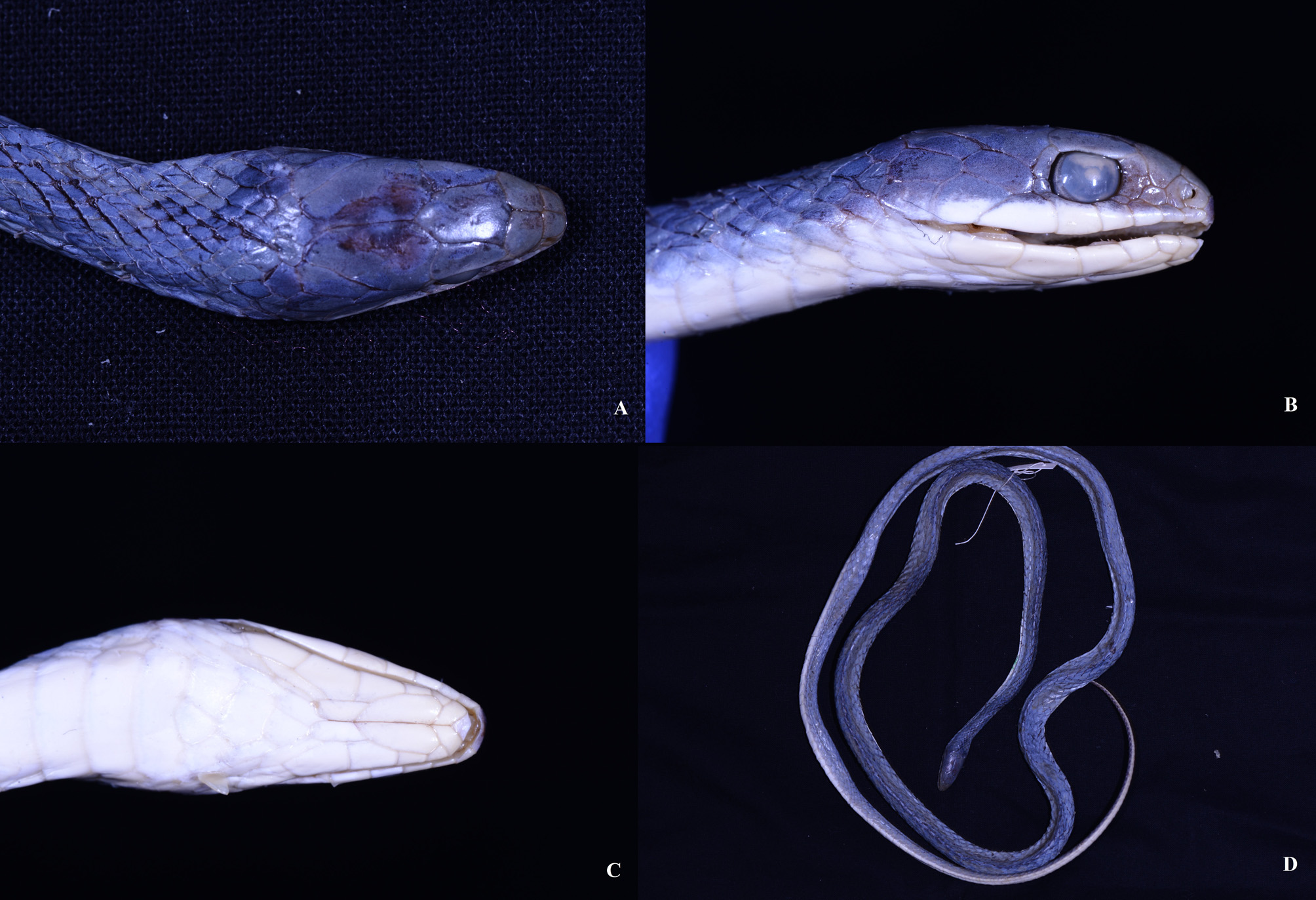
Redescription: ( Table 1, Figure 1 View FIGURE 1 , 2 View FIGURE 2 , 3 View FIGURE 3 ) A small to medium sized snake with a maximum SVL of 843 mm (in the holotype of P. hamptoni ); body slender; tail long (TAL/total length 0.21–0.24), head more or less distinct from neck; HW greater than HH; snout moderately elongated (ESD/HLB 0.37–0.39), obtusely pointed; from the prefrontal region the forehead slopes down steeply towards snout tip; eye moderately large (ED/HLB 0.23–0.28), with round pupil; rostral wider than high and contacts 6 scales, namely 1st SL, internasals and anterior nasals; suture between internasals smaller than that between prefrontals; lateral extensions of prefrontals descend onto upper parts of loreal region; loreal present or absent—for example, in ZSI 20503, 20504 and Regd. No. 40107 loreal is absent whereas a small loreal is present between posterior nasal, 2nd SL, pre-ocular and the prefrontal in NHML 1946.1.5.32, MCZ R 44714, VR/ERS/ZSI 501(A) and 501(J); amongst the specimens without a loreal, preocular contacts posterior nasal in two (ZSI 20503 and 20504) specimens whereas in Regd. No. 40107 the lateral extension of the prefrontal contacts the 2nd SL (however, an incomplete suture is present on the downward lateral extension of the prefrontal)—the pattern indicates that in the former loreal had fused with the pre-ocular while in the latter a fusion occurred between loreal and prefrontal; frontal pentagonal, longer than its distance from rostral, and contacts prefrontals, supraoculars and parietals; both the parietals and the suture between them are longer than the frontal; nasal divided and posterior nasal larger than its anterior counterpart; 1preocular; a very small presubocular either present or absent; postoculars 2 or 3 (Regd. No. 40107); TEMP (R/L) 1+2/1+2, lower posterior TEMP sometimes prevented from touching anterior TEMP by 7th SL (ZSI 11939, 20503, VR/ERS/ZSI 501(A), MCZ R 44714); SL (R/L) 6/6 (MCZ R 44714) or 7/7 (other specimens), 1 st and 2nd SL touch nasals; in most of the specimens 4th and 5th SL contact eye while in ZSI 20503 and MCZ R 44714, 3rd also in contact with eye; 7th SL large, followed by a moderately large scale (regarded by some authors as the 8th SL located behind the corner of the mouth, so not considered here to be a supralabial); mental triangular; IL (R/L) 6/6 or 7/7, 1st to 4th IL contacts anterior genial while posterior genial is usually contacted by 4th and 5th IL except in ZSI 20503 where 5th IL is just separated from posterior genial; anterior genial 1.6–2.2 times longer than the posterior genial; dorsal scales smooth, DSCH:M: V 15:15:15; VEN 173–194, in ZSI 20503 178th VEN is divided; Anal 1; SC 74–80 pairs.
Dentition was studied in ZSI 11939; this specimen possesses 26 or 27 recurved teeth on an elongated narrow maxilla; the number of maxillary teeth reported in the literature is 25–33 ( Boulenger 1900; Wall 1924b; Smith 1943).
Hemipenis was studied in ZSI 11939. The following description of hemipenis is from an in situ preparation as an everted preparation was not available and the description follows the terminology of Dowling & Savage (1960); the hemipenis extends upto 14th SC in situ; apart from the base of the organ which is devoid of ornamentation, the basal 2/3 part is covered with large spines; at about 2/3 length of the hemipenis, an abrupt transition from spinous to a calyculate area occurs; calyces away from distal end are large and formed of thick ridges, therefore appearing somewhat like flounces; the ridges of calyces are papillate; calyces become very small and closely set towards the end of distal part.
In life, the head and dorsum are uniformly verdant green which extends to the outer edges of VEN while the venter is white to whitish cream. In preservative, the dorsum becomes turquoise. One specimen, Regd. No. 40107, has a hitherto unreported colour pattern ( Figure 3 View FIGURE 3 ). The specimen has 4 black lateral stripes on a green (bluish-grey in preservative) dorsum. These stripes start at the midbody and while the lower one terminates at vent, the upper stripe runs for about half the length of tail. The lower stripes occupy 2nd and 3rd dorsal scale rows while the upper stripes run along 5th and 6th scales rows. This particular specimen, collected from Manipur by the first author, agrees with other specimens in every other aspect including lepidosis.
Distribution: Following the designation of a lectotype by Capocaccia (1961), the type locality of Ptyas doriae is “Monti ad Est di Bhamò: Kakhien Hills”; i.e. “Mountains situated east of Bhamo: Kachin hills”; the original locality given by Boulenger (1888) was merely “Kakhien Hills”. So far this species has been recorded from Kachin State and Mandalay Region of Myanmar, Manipur State of India and southern Yunnan Province of China ( Boulenger 1888, 1900; Wall 1924a, 1924b; Pope 1935; Smith 1943).
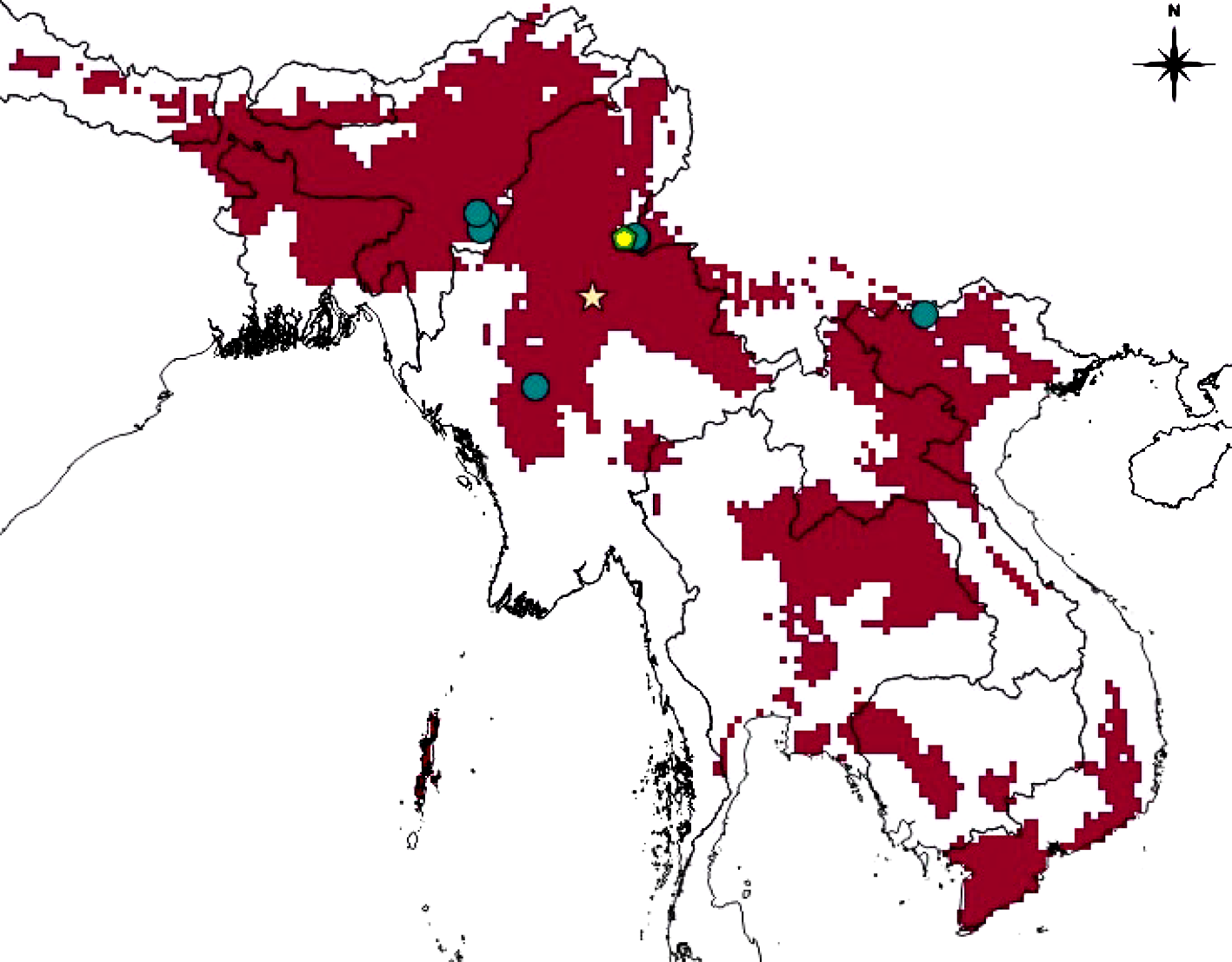
In the present study, we also predicted the range using Maxent modeling. The AUC value obtained from the Receiver Operating Characteristic curve was 0.94, indicating a very good model performance. The reclassified output ( Figure 4 View FIGURE 4 ) predicts a large area in Southeast Asia, including northeastern India, northern Myanmar and parts of China, Thailand, Laos and Vietnam, as being climatically suitable for the species. The suitable habitat includes both plains and montane region. This species has so far been recorded from an altitudinal range of ±600–1780 meters above sea level. Wall (1924b) claimed that this species occurs above 4000 feet (about 1212 meters) elevation but this was refuted by Pope (1935) who recorded it from a lower altitude as well. The occurrence data and the prediction of climatic suitability both support Pope (1935). Moreover, Pope (1935) questioned the validity of the record of this snake from the lower valley of Chang Jiang River (formerly Yangtze River) of China reported by Wall (1924a) as the specimen forming the basis of this record ( Wall 1903) was probably misidentified. The SDM analysis does not predict regions north of the extreme southern tip of Yunnan to be climatically suitable, thus once again supporting Pope’s opinion. The variable having the highest influence in determining distribution, as identified by jackknife analysis, is BIO18 (‘precipitation of the warmest quarter’) with percent and permutation contributions of 43.5 and 47.1 respectively. This species seems to be a denizen of forest and precipitation during the summer months may be important for maintaining forest cover, therefore acting as crucial scenopoetic determinant of this species’ distribution. From the location of known collection localities and predicted distribution, it seems that further exploration may reveal its presence in the Sagaing region of Myanmar andthe northeast Indian states adjacent to Manipur. It is noteworthy that Vietnam and Laos are inhabited by P. major and P. multicinctus (formerly placed in Cyclophiops ) ( Ziegler et al. 2007; Das 2010; Wallach et al. 2014) which may replace P. doriae there and strong prediction in those areas may actually indicate a conservatism of Grinnellian niche (also see Discussion).
Natural history: Very little is known about the natural history of these snakes. Two specimens were observed in September at Maryland village (Manipur, India). Another one was found dead on the road at NH-2 near Molnoi village on the way to Moreh (Manipur, India) in the same month. These snakes live in bushes and shrubs (mainly Lantana , sometimes also occurring in bamboo groves) up to a maximum of 6 feet above ground ( Figure 5). These snakes are said to be diurnal ( Das 2010). According to the local people, these snakes are not uncommon in forested hillocks of Manipur from March to October and local people further report that they generally encounter these snakes during ‘slash and burn’ cultivation works and also near villages. The vernacular name of these snakes in the Tangkhul language is ‘Shineiphara’ and ‘Naril’ in the Manipuri language. Nothing is known about their diet and reproductive habits.
| VEN |
Fundaci�n Instituto Bot�nico de Venezuela |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.