Acanthogethes Reitter, 1871
|
publication ID |
https://doi.org/ 10.5281/zenodo.5319334 |
|
DOI |
https://doi.org/10.5281/zenodo.10542340 |
|
persistent identifier |
https://treatment.plazi.org/id/03BE87CC-F66C-FF8D-BA95-FB7BFCC3F9FA |
|
treatment provided by |
Felipe |
|
scientific name |
Acanthogethes Reitter, 1871 |
| status |
|
1. Acanthogethes Reitter, 1871 View in CoL View at ENA stat. nov.
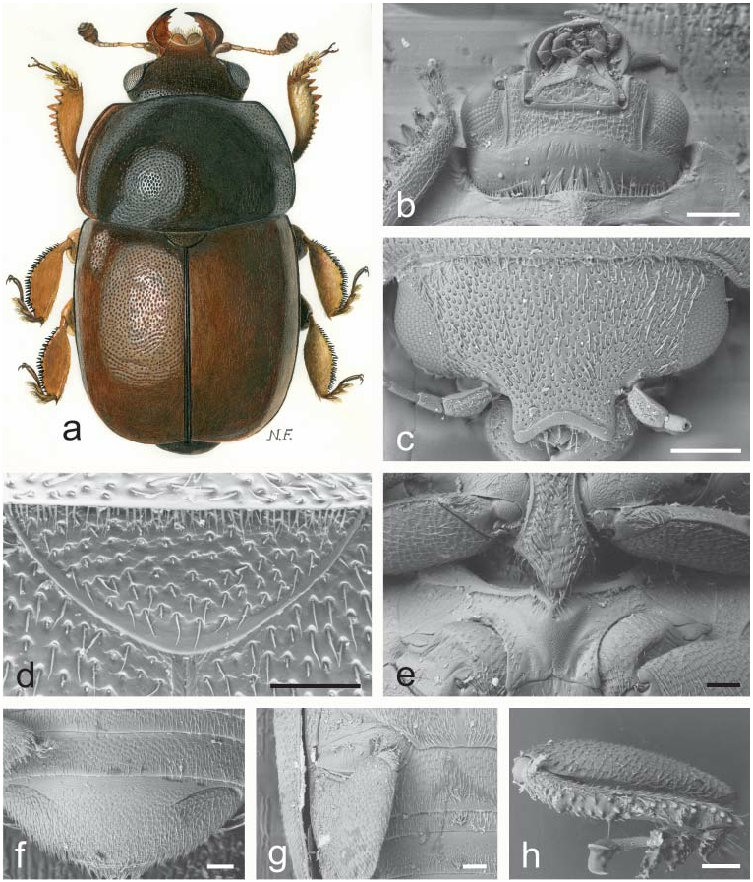
( Figs. 1 a–h View Fig )
Acanthogethes Reitter, 1871: 155 View in CoL (described as a subgenus of Meligethes Stephens, 1830 View in CoL ).
Type species. Sphaeridium fuscum A. G. Olivier, 1790: 10 (subsequent designation by PARSONS (1943)) [= Acanthogethes fuscus (A. G. Olivier, 1790) comb. nov.]
Generic redescription and diagnosis. Inclusive species range greatly in size (1.2 – 4.3 mm length), and share the following combination of characters.
Body color and pubescence: pubescence usually short and fine, recumbent, golden to silvery-whitish, never obscuring the variably colored (brown, blackish, reddish, or blackish with orange spots on elytra, e.g. Fig. 1a View Fig ) dorsal body surface; pronotal and elytral sides narrowly flattened, typically same color as disc, a few species with pale reddish sides. Lateral margin of pronutum and elytra with a series of faintly distinct, small and short setae, each seta 0.3 – 0.5× as long as those on elytral disc; posterior margin of pronotum with long, usually distally bifid or trifid microsetae, microsetae uniformly distributed in middle region anterior to scutellum ( Fig. 1d View Fig ).
Dorsal habitus: body markedly convex, short, wide and oval ( Fig. 1a View Fig ); dorsal punctures on discal portion of pronotum as large as, or larger than eye facets, usually deeply impressed and densely distributed; anterior margin of clypeus always strongly arcuately emarginate, simple, i.e. without small distinct medial bulge, and distinctly bordered ( Figs. 1a, c View Fig ); circumocular furrows (occipital sulci) on dorsal side of head reduced, somewhat distinct anteriorly, absent posteriorly ( Fig. 1c View Fig ); eyes large and usually moderately projecting laterally ( Figs. 1a, c View Fig ); pronotum with faintly distinct posterior angles, rounded to obtuse and never directed posteriorly ( Fig. 1a View Fig ); scutellum regularly punctured on most of exposed portion; elytra with simple punctation, never transversely strigose, occasionally with faint traces of orange peellike rugosity; elytral humeral angle bluntly rounded, not protruding laterally ( Fig. 1a View Fig ); elytral humeral striae faint; elytral pre-sutural striae visible, originating at scutellar vertex or slightly posterior, terminating at elytral apex, and delimited on each elytron by a faintly distinct, flat sutural border, widest at posterior third; border slightly narrower than proximal width of 3 rd antennomere; elytral apices faintly rounded in both sexes ( Fig. 1a View Fig ); pygidium partially exposed, moderately convex, apically rounded in both sexes ( Fig. 1a View Fig ) or distinctly produced and distally faintly explanate in females (Fig. 125 s in AUDISIO 1993b).
Ventral habitus: antennal furrows markedly delimited, nearly parallel-sided, or slightly diverging posteriorly; mentum subpentagonal ( Fig. 1b View Fig ); prosternal antennal furrows almost completely obliterated ( Fig. 1b View Fig ); prosternal process variably shaped, usually relatively narrow, subapical portion dilated, 2.7 – 3.0× as wide as maximum width of 1 st antennomere, apex usually blunt, not acute ( Fig. 1e View Fig ); lateral borders of prosternal process not delimiting shallowly impressed and distinct furrows, and distally terminating prior to predistal lateral expansions ( Fig. 1e View Fig ); posterior margin of mesoventrite distinctly incised at middle ( Fig. 1e View Fig ); usually distinct sexual dimorphism present, comprising shallow to deep impressions on metaventrite, more or less raised tubercles, or small lobes along posterior margin of metaventrite; first two visible abdominal ventrites usually simple in both sexes, without tufts of setae, but in males of the A. fuscus complex with faintly distinct, minute tufts of brown setae on first four abdominal ventrites; caudal marginal lines of metacoxal cavities always simple, parallel and contiguous to posterior margin of metacoxal cavities, without deep arched impression of outer ‘axillary’ line ( Fig. 1g View Fig ); ‘axillary’ space on first abdominal ventrite strongly reduced, ‘axillary’ angle acute ( Fig. 1g View Fig ); large shallowly impressed arched impressions on basal portion of last visible abdominal ventrite usually not covered by distal portion of penultimate visible abdominal ventrite ( Fig. 1f View Fig ).
Appendages: male 1 st antennomere 0.8 – 1.0× as long as width of protibiae excluding distal teeth ( Figs. 1a, b View Fig ); 3 rd antennomere in both sexes 2.8 – 3.0× as long as wide, 1.3 – 1.4× longer and distinctly thinner than 2 nd antennomere ( Figs. 1a, c View Fig ); 4 th and 5 th antennomeres in both sexes subequal, short, slightly longer than wide; antennal club compact, small, simple, comprising last 3 antennomeres in both sexes, never sexually dimorphic ( Fig. 1a View Fig ), much narrower than width of protibiae; labial palpi relatively short in both sexes ( Fig. 1b View Fig ), terminal segment 1.7 – 1.8× as long as wide; maxillary palpi moderately long and slender in both sexes ( Fig. 1b View Fig ), terminal segment 2.3–2.4× as long as wide; mandible usually large ( Fig. 1a View Fig ), nearly 1.5–1.6× longer than wide, with peculiarly acuminate apex and denticulate distal portion of inner margin, without evident differences in shape and size among sexes; tarsal claws strongly dentate at base (as in Fig. 17m View Fig ); tarsi of normal size and shape, 0.6–0.7× as long as corresponding tibiae ( Fig. 1a View Fig ); protibiae with a series of usually very large, uneven, sharp or blunt teeth on lateral margin ( Fig. 1a View Fig ; see also Figs. 126 a–e in AUDISIO 1993b); meso- and metatibiae on lateral margin bearing a nearly double and usually uneven row of large and robust blackish spurs ( Figs. 1a, h View Fig ), without U-shaped sinuosity at distal third; meso- and metatibiae of variable width, usually moderately flat but slender ( Fig. 1a View Fig ), never distinctly subtrapezoidal or axe-shaped; scarcely apparent sexual dimorphism in tibial shape; tarsal plates of prolegs slightly wider in males than females; posterior margin of metafemora simple in both sexes, without tubercles or projections.
Male genitalia: processes along inner side of parameres absent (Fig. 132 in AUDISIO 1993b), usually with deep and narrow excision along distal margin, without deep median longitudinal desclerotization from proximal portion of tegmen extending to a medial distal V-shaped excision; median lobe of aedeagus variable, without emargination laterally, rounded, subtruncate to acute apically, without distal minute excision or emargination.
Female genitalia (ovipositor): variably shaped, usually large; styli usually short but distinct, simple, cylindrical, not distinctly pigmented, inserted close to apex of contiguous gonostyloids; each gonostyloid lightly sclerotized and unpigmented distally, with a simple, never indentate outer portion of basicoxites (Figs. 153 a–f in AUDISIO 1993b), and a single, narrow, scarcely pigmented and sclerotized arcuate area along outer subdistal portion of gonostyloids; ‘central point’ of ovipositor usually more distad than middle, with or without proximally directed spicule.
Etymology. The generic name is derived from a combination of the Greek ‘ακανθα’ (= spine), to emphasize the strongly toothed tarsal claws, and ‘- gethes ’ which is indicative of the phylogenetic relationship with Meligethes . Gender masculine.
Biology. All species are strictly associated with flowers of Cistaceae for larval development, especially on small bushes of the following genera: Cistus L., Helianthemum Miller , Halimium (Dunal) Spach ( AUDISIO 1993b) .
Phylogenetic position. Available morphological datasets suggest a likely monophyletic clade that includes Acanthogethes , members of the Lariopsis complex of genera, and likely Clypeogethes Scholz, 1932 , and Xerogethes gen. nov. Molecular data weakly support these relationships. With regards to Acanthogethes , this genus possesses a combined series of several symplesiomorphic and few autapomorphic characters, and likely occupies a relatively basal phylogenetic position in Meligethinae .
Taxonomy and geographic distribution. Acanthogethes includes six described species distributed from the Canary Islands, western Europe and northern Africa to Caucasian areas, which were attributed to three formerly recognized species-complexes, the ‘ Meligethes fuscus ’, ‘ M. brevis ’, and ‘ M. solidus ’ species-complexes. Two additional species described from North Africa by EASTON (1955a), likely dubiously attributed to differentiated populations of Acanthogethes lamii ( Rosenhauer, 1856) , require further morphological and molecular analyses to definitively ascertain their true taxonomic status ( AUDISIO 1986, 1993b).
Acanthogethes brevis ( Sturm, 1845) comb. nov. Europe, N Turkey, Caucasus
Acanthogethes fuscus (A. G. Olivier, 1790) comb. nov. W Mediterranean region, W Greece
Acanthogethes hercules ( Audisio, 1986) comb. nov. Canary Islands: Tenerife
Acanthogethes lamii ( Rosenhauer, 1856) comb. nov. W Mediterranean region
Acanthogethes reyi (Guillebeau, 1885) comb. nov. S Europe, Turkey
Acanthogethes solidus (Kugelann, 1794) comb. nov. Europe, N Turkey, Caucasus
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Acanthogethes Reitter, 1871
| Audisio, Paolo, Cline, Andrew Richard, Biase, Alessio De, Antonini, Gloria, Mancini, Emiliano, Trizzino, Marco, Costantini, Lorenzo, Strika, Sirio, Lamanna, Francesco & Cerretti, Pierfilippo 2009 |
Acanthogethes
| REITTER E. 1871: 155 |