Amphiesma khasiense (Boulenger, 1890)
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3694.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:6F2F58C1-3927-4E66-B09C-AFAF4B4A2C71 |
|
DOI |
https://doi.org/10.5281/zenodo.6165420 |
|
persistent identifier |
https://treatment.plazi.org/id/855087F6-7E10-FFBF-FF5F-7236B05C6048 |
|
treatment provided by |
Plazi |
|
scientific name |
Amphiesma khasiense (Boulenger, 1890) |
| status |
|
Amphiesma khasiense (Boulenger, 1890)
( Fig. 3 View FIGURE 3 , 4 View FIGURE 4 D–F, 5)
Tropidonotus khasiensis Boulenger, 1890: 344 .— Type locality. “Khasi Hills”, in the State of Meghalaya, India.— Syntypes (4). BMNH 1946.1.12.80 (adult female) and 1946.1.12.81–82 (adult males; all formerly BM 70.11.30.33), collected by T. C. Jerdon; BMNH 1946.13.45 (juvenile; formerly 53.8.12.44); collected by Sir. J. Hooker.
Tropidonotus khasiensis .—Boulenger 1893a: 322; 1893b: 223, Pl. 13: Fig. 3 View FIGURE 3 - Fea 1897a: 475; 1897b: 95 - Annandale 1905: 210; 1912: 49 & 53 - Wall 1908: 316, 317: Fig. 2 View FIGURE 2 - Werner 1929: 24.
Natrix khasiensis .—Wall 1923: 601; 1926: 559 - Mell 1929: 148 (in part) - Bourret 1936a: 103, 113 & 132 (in part); 1936b: 69, Fig. 28 (in part for the text; see under Amphiesma boulengeri for the figure) - Smith 1940: 483; 1943: 289.—Deuve 1970: 84 & 88 (in part). - Anonymous 1977: 63 & 87 - Djao et al. 1977: 69 - Yang et al. 1978: 67; 1983: 43.
Amphiesma khasiensis . —Malnate 1960: 50, 52 & 57 - Tian et al. 1986: 116 & 149—Yang & Inger 1986: 7—Zhao 1986: 239— Zhao et al. 1986: 201; 1998: 52 & 62: Fig. 18—- Hu et al. 1987: 126—Welch 1988: 31—Zhao & Adler 1993: 226—Das 1996: 53—Zhao & Yang 1997: 201 & 207—Mathew 1998: 132—Sharma 1998: 94—Orlov et al. 2000: 71 (in part)—Ji 2002: 176 & 177—Hallermann et al. 2002: 150—Sharma 2003: 132 & 133; 2007: 206 & 209 (in part)—Zhao 2006: (I)164, (II)85: Fig. 48—Yang & Rao 2008: 258—Li et al. 2010: 166 & 209: Fig. 3 View FIGURE 3 -14—Luo et al. 2010: 74—Murthy 2010: 33.
Amphiesma khasiense .—Zhao et al. 2000a: 232; 200b: 205—Chanhome et al. 2001: 53 & 57.—Iskandar & Colijn 2001: 96— He & Zhou 2002: 167—Das 2003: 473; 2010: 150: Pl. 67: Fig. 9, 332 (in part for the text; at the exception of of the mentions of Thailand [Loei Province] and “northern Vietnam ”)—Whitaker & Captain 2004: 25, 218 & 219, Figure— David et al. 2007: 54 & 55—Sen & Mathew 2008: 140, 159, Fig. 160–161—Ahmed et al. 2009: 19—Pauwels et al. 2009: 75—Teynié & David 2010: 228, 284 & 308.
Natrix gilhodesi Wall, 1925a: 587 , Plate: Fig. 2, 2 View FIGURE 2 a–c. – Type locality. “Huton, Bhamo District (30 miles north-east of Bhamo; circa 4,500 feet; Lat. Circa 97°.33; Long. Circa 24°.24)”, now Hutung (24°15'N- 97°31'E; 1,715 m a.s.l.), Kachin State, Myanmar. – Holotype. BMNH 1946.1.13.62 (adult male; formerly BM 1925.4.2.8); collected by Father Gilhodes and deposited by F. Wall (see below). – Status. A junior subjective synonym of Tropidonotus khasiensis Boulenger, 1890 ; synonymized by Wall (1926: 559).
Natrix gilhodesi .—Wall 1925b: 808.
Natrix kashiensis (lapsus calami pro Natrix khasiensis ).—Mell 1931b: 225 & 238.
Amphiesma modesta (nec Tropidonotus modestus Günther, 1875 , a distinct species).—Cox et al. 1998: 45—Das 2002: 18.
Paranatrix modesta (nec Tropidonotus modestus Günther, 1875 , a distinct species).—Mahendra 1984: 247 (see below).
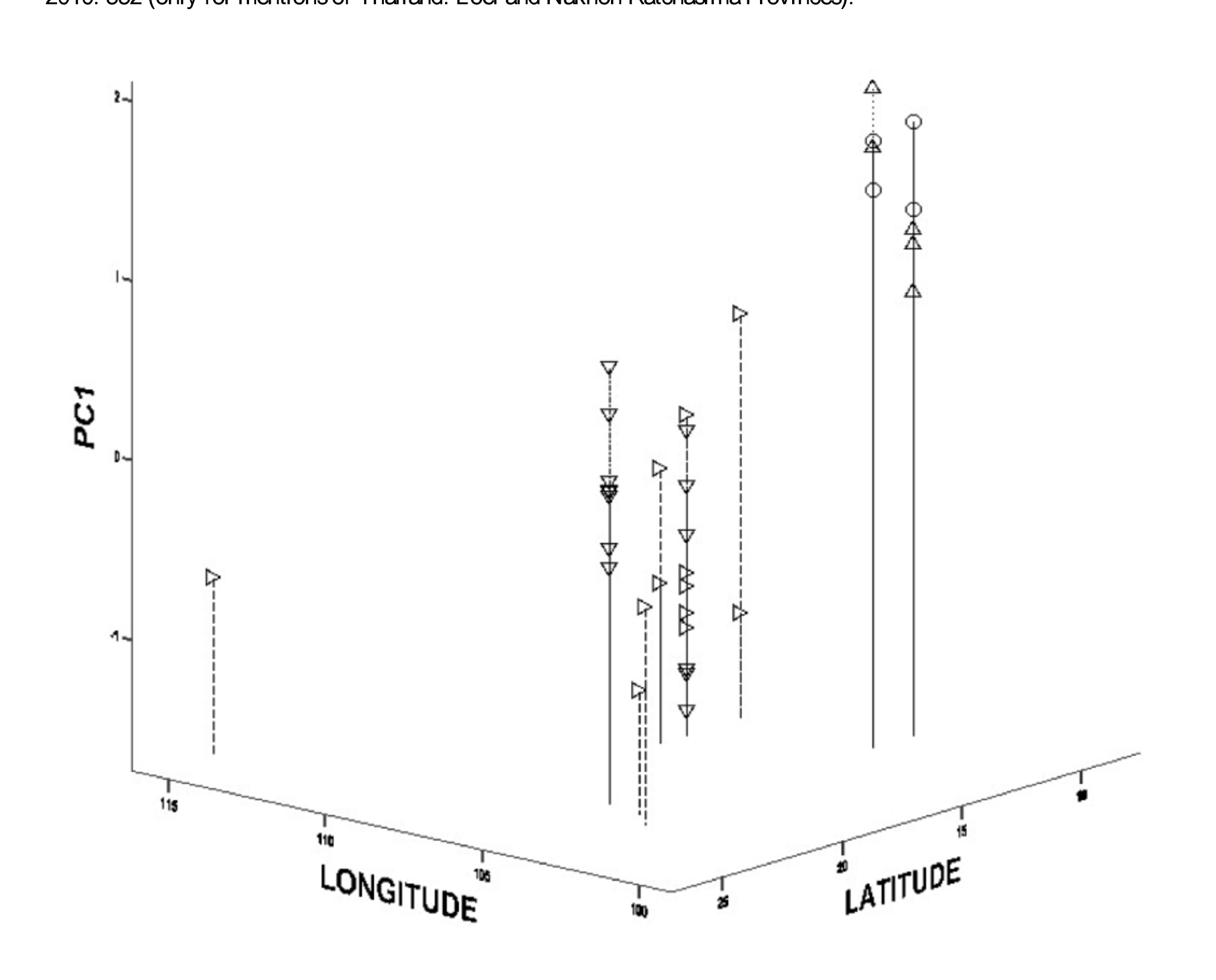
Amphiesma inas (nec Natrix inas Laidlaw, 1901 , a distinct species).—Chan-ard et al. 1999: 153 (top; specimen from Phu Luang, Loei Province, Thailand)—Nabhitabhata et al. 2004: 124 (in part: mention of Phu Luang, Loei Province)—Das 2010: 332 (only for mentions of Thailand: Loei and Nakhon Ratchasima Provinces).
Material examined (35 specimens). – India. State of Meghalaya. BMNH 1946.1.12.80–1946.1.12.82, BMNH 1946.1.13.45, Khasi Hills. State of Arunachal Pradesh. P 377–379, Dihang Dibang Biosphere Reserve, District of Dibang Valley; ZSI 23926, Changlang. State of Nagaland. KSC 140, Kohima. – Myanmar. Chin State. CAS 220023, Nat Ma Taung National Park (21°22' 20.1N 93°58' 34.6E), Min Dat Township, Min Dat District. Kachin State. BMNH 1946.1.13.62 (holotype of Natrix gilhodesi Wall, 1925 ), BMNH 1946.1.13.63, BMNH 1925.4.2.10– 15, BMNH 1925.4.2.15A, “Huton, Bhamo District (30 miles north-east of Bhamo; circa 4,500 feet; Lat. Circa 97°.33; Long. Circa 24°.24)”, now Hutung; BMNH 1974.884, Nawng Hkai, near Putao; CAS 221504, between Babaw and Rabaw (27°26' 28.4N 97°55' 06.3E), Naung Mon Township, Putao District; CAS 221525, Rabaw (27°26' 28.4N 97°55' 07.5E), Naung Mon Township, Putao District; CAS 221543, Rabaw (27°26' 14.9N 97°55' 21.1E), Naung Mon Township, Putao District; CAS 224654, Nagmung (27°30' 18.8N 97°48' 33.9E), Nagmung Township, Putao District; CAS 224694, Nagmung (27°29' 49.6N 97°49' 06.9E), Nagmung Township, Putao District. Kayah State. MNHN 1893.0399, Monts Karen. – People’s Republic of China. Yunnan Province. CIB 2000I 0009, Ruili; CIB Xi0089, Xichuangbanna. – Laos. Phongsaly Province. MNHN 2004.0248, Long Nai Tai. – Thailand. Chiang Rai Province. KZM 0 0 1, QSMI 542, near Ban Pa Miang Mae Hang, Moo. 7, Pagnew Subdistrict, Wieng Pa Pao District. Chiang Mai Province. CTNRC 980504, Doi Inthanon National Park; FMNH 251780–251781, Chiang Mai. Loei Province. QSMI 0 273, Phu Luang.
Taxonomic comments. Amphiesma khasiense is here redefined as an Indo-Himalayan species which enters the mountain ranges of North Thailand and northern Laos. In the literature, this species has often been confused with Amphiesma boulengeri as defined here, and, more rarely, with A. inas . These confusions explain the numerous citerations in part in the chresonymy given above. This species is monotypic.
We have examined the holotype of Natrix gilhodesi Wall, 1925 and we concur with Smith (1943) in regarding this taxon as a junior synonym of Amphiesma khasiense . Furthermore, Wall (1925a: 587) stated he had six specimens of Natrix gilhodesi at hand but that one was selected as the type. This specimen is registered as BMNH 1946.1.13.62. According to the catalogue of the Natural History Museum of London, other specimens of the original series are BMNH 1946.1.13.63 and BMNH 1925.4.2.10–15, BMNH 1925.4.2.15A, so a total of eight specimens.
Mahendra (1984: 244) erected the genus Paranatrix (type species: Tropidonotus modestus Günther, 1875 , now Amphiesma modestum , by original designation) for six species of the genus Amphiesma present in India. Furthermore, Mahendra (1984: 247) synonymized A. khasiense with A. modestum . This synonymy is unjustified as these species are morphologically distinct as shown above. However, the generic nomen Paranatrix is an available name if the genus Amphiesma has to be split.
Diagnosis. A species of the genus Amphiesma characterized by the combination of (1) body slender in males and females; (2) nostrils directed laterally; (3) 21–26 maxillary teeth, gradually enlarged, the last 2 or 3 moderately enlarged; (4) 19 dorsal scale rows at midbody, strongly keeled at the exception of scales the 1st dorsal scale row, smooth or weakly keeled on the anterior half of the body, weakly or distinctly keeled posteriorly; (5) dorsal pattern made of a series of reddish-brown or rusty dots or small blotches, usually aligned on a faint dorsolateral stripe; (6) each of the three or four posterior supralabials with a large, white, cream or ivory, rounded, subrectangular, triangular or vertically oval blotch, or, rarely, nearly entirely cream or ivory with dark edges; (7) pattern of the neck usually made of isolated rounded blotches, sometimes partly connected by a narrow pale line; (8) venter ivory or cream, with the outer quarter of each ventral dark brown, separated from the dark dorsal colour by a narrow pale line; (9) eye large, 1.8–2.8 times the distance between the lower margins of eye and of lip; (10) 141–155 VEN, 87– 111 SC; (11) internasals abruptly truncated anteriorly; (12) 9 (rarely 8) supralabials; (13) 1 anterior (rarely 2) temporals.
A. khasiense , A. boulengeri and A. inas are morphologically similar. Characters of use for separating these taxa are given below in the Discussion.
Variation (based on 35 examined specimens and Smith 1943). Body cylindrical, elongate, rather slender in males, slightly more robust in old females; head short, oval, distinct from the thick neck, flattened; snout short, blunt, amounting for 24.5–30.4 % of HL or 1.2–1.4 times as long as diameter of eye; nostrils large, rounded, directed laterally and piercing in the middle of the nasal; eye large, 1.8–2.8 times the distance between the lower margin of eye and the lower edge of lip, with a round pupil; tail long, thin and tapering.
The maximal total length known is 673 mm according to Wall (1923b: 601); the largest specimen seen by us is 604 mm (SVL 412 mm; TaL 192 mm; specimen BMNH 1946.1.12.80; female). The longest known male is 562 mm long according to Wall (1926); the longest male seen by us is 549 mm long (SVL 368 mm, TaL 181 mm long; BMNH 1925.4.2.11). Ratio TaL / TL: 0.296–0.346, without clear sexual dimorphism (see below).
Dentition. Maxillary teeth 21–26, gradually enlarged, the last 2 or 3 teeth distinctly enlarged.
Body scalation. DSR: 19–19–17 scale rows; dorsal scales rhomboedric, moderately to strongly keeled on all rows but 1st DSR, normal in shape or notched posteriorly; scales of 1st DSR weakly keeled on the anterior half of the body, rarely smooth, posteriorly weakly keeled in juvenile specimens but moderately to strongly keeled in adults.
Scale row reductions: first reduction (19→17) at VEN 89–96 (x = 92.5; s = 2.5); second reduction (17→15) at VEN 92–99 (x = 95.5; s = 2.1).
VEN: 141–155 (plus 1 or 2 preventrals); SC: 87–111, all paired, with a sexual dimorphism; ratio VEN / SC: 1.37–1.69; anal plate divided.
Position of the reduction from 8 to 6 scale rows around the tail: 8th–24th SC, with a strong sexual dimorphism. Ratio length of the portion of tail with 4 dorsal scale rows / length of the portion of tail with 6 dorsal scale rows: 0.88–1.31 with a weak sexual dimorphism.
Head scalation. Rostral wider than high, only barely visible from above; nasals subrectangular, elongate, vertically divided on their lower half, with the posterior part larger and higher than anterior one, internasals subrectangular, in broad contact, about 0.8–1.1 times as long as wide, abruptly truncated anteriorly with anterior margin about 0.55–0.75 times the width of the posterior margin; 2 wide prefrontals, 1.1–1.5 times as long as internasals; frontal shield-like with apex directed posteriorly, moderately enlarged, 1.3–1.5 times longer than wide, 2.0–2.5 (usually 2.1–2.2) times as long as prefrontal; 1 supraocular on each side, longer than wide, about as wide as internasals; two large parietals, 1.3–1.6 times longer than frontal; 1 / 1 subrectangular loreal scale, 0.8–1.0 times as high as long, in broad contact with the nasal; 9 (exceptionally 8) SL (9 / 9 in 37 cases out of 39, 8 / 8 in 1 case, 8 / 9 in 1 case), 3rd–9th longer than high; 1st and 2nd SL small and short, in contact with nasal; 2nd and 3rd SL or more rarely 2nd–4th SL in contact with the loreal; 4th–6th SL (or exceptionally 3rd–5th SL in 3 out of 70 occurrences) entering orbit; 7th and 8th SL largest; 1 or 2 preoculars on each side (2 preoculars in only 18 / 70 occurrences); 3 (exceptionally 2 in 1 / 70 occurrences) small postoculars; 1 anterior temporal (2 in 4 / 70 occurrences), narrow and elongate, with complete temporal formulas as 1+1+2, 1+2+2, more rarely 2+1+2 or 2+2+2; usually 10 / 10 infralabials (rarely 10 / 11, 9 / 9 in only 1 out of 35 specimens), first pair in contact each with other, 1st–5th IL in contact with anterior chin shields, 6th IL largest.
Coloration and pattern in alcohol. The dorsal and lateral surfaces of body are ochre-brown, greyish-brown, dark gray, dark grayish-brown or dark chestnut brown, often distinctly darker, i.e. dark grayish-brown or blackishbrown, on the five upper scale rows of the back, throughout more or less heavily variegated with much darker pigmentation; back and each side with 2 or 3 series of small, blackish-brown spots, subrectangular or elongated, more or less well defined and scattered in alternance each with the other; on each side, a faint, ochre or reddishbrown dorsolateral stripe extending from the neck on upper half of 5th and the whole of 6th dorsal scale rows, sometimes also on lower part of scales of the 7th row is often present although often not visible after midbody; on each dorsolateral stripe, a series of small, irregular pale, dark-edged spots, cream, yellow, ochre or rusty brown (coral, orange or red in life), covering only one scale and arranged every 1 to 3 or 4 dorsal scales, conspicuous on the anterior part of the body, progressively fainter and often not visible on the second third of body; some paler dashes may be present between the dorsolateral spots on the anterior part of the body; a short, narrow, ventrolateral stripe made of irregular cream spots extends from the neck to the anterior part of the body on the 1st dorsal scale row between the dark dorsal colour and the tips of ventrals, disappearing progressively after 15th–20th ventral. The tail is as the body, with minute dorsolateral cream or yellow (coral, orange or red in life) spots, vanishing progressively; posterior half of tail uniformly dark, usually variegated with irregular and faint darker pigmentation.
The head is ochre-brown, greyish-brown, dark gray or dark grayish-brown, paler than the background colour of the body, often paler above the snout than on the occiput, with irregular dark brown vermiculations or minute blackish-brown dots and with irregular paler areas on the frontal and parietal regions; a short cream streak just behind the suture of parietals present or absent; a pattern made of twin pale spots, one on each parietal, present or absent; 1st -5th, sometimes 1st -6th anterior supralabials white, cream or pale yellow (same in life), narrowly edged with dark brown or black; 1st supralabial sometimes darker than other anterior supralabials; 6th to 9th or 7th to 9th supralabials with a large, conspicuous blotch in their centre, pure white or cream and broadly edged with blackishbrown, rounded or elongate (i.e. subrectangular, slightly horizontally ovale or rarely triangular), straight or slightly oblique, more rounded in 9th SL, not in contact with each other and contrasting sharply with the dark colour of the head; exceptionally the posterior supralabials are nearly entirely white, cream or ivory with dark edges (see below); no postocular streak; on each side of the neck, from the corner of the mouth behind 9th supralabial, an oblique series of small, isolated, rounded or elongate white or cream blotches (same colour in life), usually not connected with each other or sometimes partly connected by a narrow pale line, usually not reaching the upper part of the neck; these blotches may even be reduced to a few pale spots and do not constitute a continuous stripe. The throat and chin are cream, ivory or very pale yellow (pale pink or reddish-gray in life); infralabials and mental more or less heavily dotted or speckled with small, irregular dark brown spots; sometimes minute dots on the preventral area. The pupil is black, the iris is dark red in life.
The venter is uniformly ivory, cream, pale yellow or pale ochre (bright pink or reddish-gray in life); about a quarter of the outer part of each scale with a well-defined, sharp dark brown or blackish-brown area, separated from the dark similar colour covering the ventral tip by a very narrow, cream or ivory line or streak at the base of the tip, crossing the scale or limited to a spot at the posterior edge of the ventral, producing a narrow, discontinuous ventrolateral stripe on each side of the venter and separating the dark colour of the tip from that of the inner part of the ventral; dark tips in contact without discontinuity with the background colour of the body except for a short distance on the anterior body, this pale space contributing the short ventrolateral stripe of the body. The tail is ivory or cream below (pink or red in life), with outer edges of subcaudals very dark brown; posteriorly, the tail becomes heavily speckled with minute dark brown dots, nearly entirely dark near its tip.
The pattern of the posterior supralabials, i.e. a rounded or elongate, isolate white, cream or ivory central blotch is diagnostic of this species. However, exceptionally, the posterial supralabials may be nearly entirely pale, with only dark edges. We have met only two such specimens, MNHN 1893.0399 (Karen Mts, Myanmar; identified as A. khasiense by E. Malnate according to the catalogue of the MNHN) and MNHN 2004.0248 (Long Nai Tai, Phongsaly, Laos; depicted in Teynié & David 2010: 229) respectively. These specimens may easily be identified as Amphiesma modestum but the keeled 1st dorsal scale row, pattern of the neck and the well-defined lateral blotches of the venter is different and diagnostic of A. khasiense .
Hemipenis. In situ, it is short and stout, undivided but with two short extensions at its tip, reaching in situ the 8th or 9th SC; sulcus spermaticus simple, S-shaped, reaching the top of the hemipenis, with prominent lips; hemipenis entirely covered with short but dense spines at the exception of the base which is covered with large calyces; lips of the sulcus also covered with short, thick spines; both extensions densely covered with rather numerous thin spines; 2 or 3 large, thick spines on the internal side of the organ at 90° of the sulcus.
Sexual dimorphism. It is expressed only in the difference in the number of subcaudals, with males: 96–111 (x = 103.3, s = 4.2) vs. females: 87–97 (x = 93.8, s = 3.5). Another weaker difference appears in the position (counted in number of subcaudals) of the reduction from 6 to 4 scale rows around the tail, with males: 15th–22nd SC (x = 18.7, s = 2.8) vs. females: 8th–21st SC (x = 13.4, s = 4.8).
Distribution. According to authors cited above in the chresonymy and our material, the distribution of A. khasiensis is currently known as follows: India. Known only from north-east India. State of Meghalaya: Khasi Hills and Garo Hills. State of Arunachal Pradesh: Changlang; Dibang. State of Nagaland: Kohima District; Wokha District.— Myanmar. Kachin State. Regions of Bhamo, Hutung, Putao and Sumka Uma (Mahtum, Sumka Uma). Chin State. Nat Ma Taung National Park, Min Dat District. Kayah State. Monts Karen.— People’s Republic of China. Yunnan Province. Xichuangbanna, in extreme southern Yunnan, and Ruili. Xizang Province. South of Motuo.— Laos. Recorded only from the north of the country. Phongsaly Province. Long Nai.— Thailand. Known only from the mountainous regions of the northern and western parts of the country. Chiang Rai Province. Near Ban Pa Miang Mae Hang, Pagnew, Wieng Pa Pao District. Chiang Mai Province. Chiang Mai. Loei Province. Phu Luang. Tak Province. Umphang; photographed specimen ( Fig. 5 View FIGURE 5 top left). See Pauwels et al. (2009) for a detailed discussion on the distribution of this species in Thailand.
Records from Vietnam, Cambodia and other parts of Thailand should be referred to Amphiesma boulengeri or A. inas (see below).
Biology. This rather uncommon species inhabits wet primary submontane and montane forests and wet secondary forests between about 600 and 1,400 m a.s.l. It is usually associated with the forest floor near the edge of fast-flowing mountain streams but is also present in the vicinity of still waters. A. khasiense has also been found in a ricefield located at the edge of a disturbed primary forest or seen active along a man-made pool surrounded by agricultural fields inhabited by newts of the species Tylototriton verrucosus Anderson, 1871 and various frogs. This active, terrestrial and semi-aquatic species is both diurnal and nocturnal. It lives mainly among leaves of the forest litter and in the vegetation surrounding of pieces of water. This snake feeds mainly on small frogs, tadpoles and, perhaps, insects. Wall (1926) recorded one or two frogs from the stomach of 12 specimens. This species is oviparous; Wall (1926) removed from 1 to 4 eggs from his series of specimens; Whitaker and Captain (2004) recorded new born specimens in June and October but nothing else is known on its reproductive habits.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.