Docosaccus maculatus, Kahn, Amanda S., Geller, Jonathan B., Reiswig, Henry M. & Smith, Kenneth L., 2013
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3646.4.4 |
|
publication LSID |
lsid:zoobank.org:pub:6C451EF6-DA2C-48EA-8CF1-4AFFF22198A9 |
|
DOI |
https://doi.org/10.5281/zenodo.5629894 |
|
persistent identifier |
https://treatment.plazi.org/id/03D187AA-FF9C-BA22-E0DF-FF05E796FE39 |
|
treatment provided by |
Plazi |
|
scientific name |
Docosaccus maculatus |
| status |
sp. nov. |
Docosaccus maculatus View in CoL , new species
( Fig. 5–9 View FIGURE 5 View FIGURE 6 View FIGURE 7 View FIGURE 8 View FIGURE 9 , Table 2 View TABLE 2 )
Holotype. Stored at SIO-BIC (P1539), coll. A. S. Kahn using MBARI ROV Tiburon, dive T1094 from R/V Western Flyer, 3,953 m depth, Station M (34º50’N, 123º0’W), 0 5 June 2007.
Other material examined. Paratypes: CASIZ 190480, coll. H. Ruhl using MBARI ROV Tiburon, dive T1143, from R/V Western Flyer, 4,000 m depth, Station M, 23 September 2007; MBARI “Sponge 3”, coll. A. S. Kahn using MBARI ROV Tiburon, dive 1094 from R/V Western Flyer, 4,000 m depth, Station M, 0 6 June 2007.
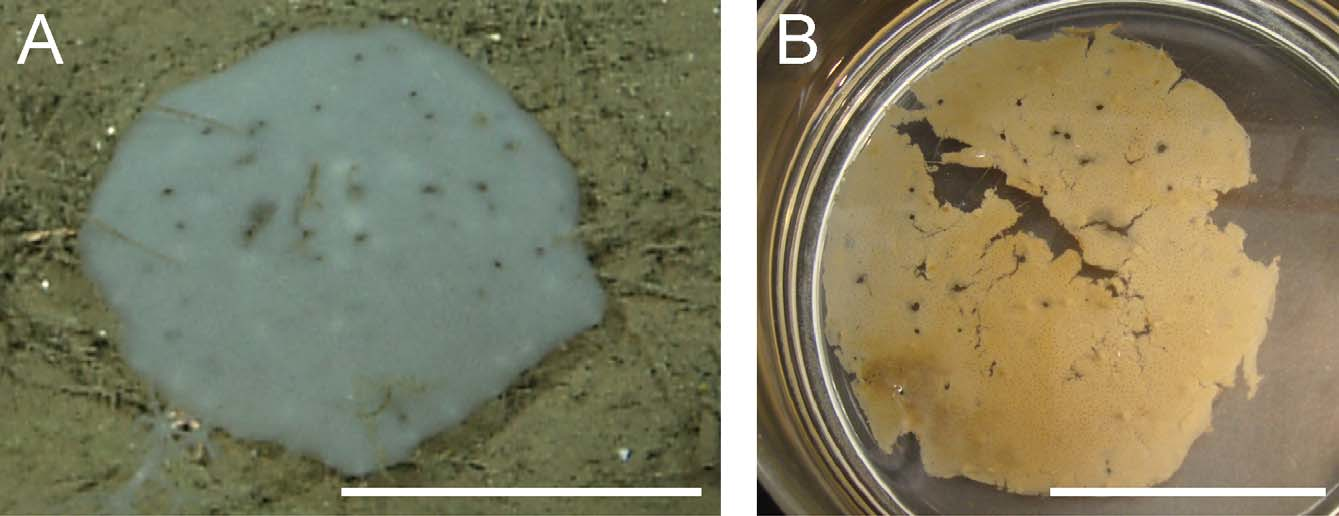
Diagnosis. Docosaccus with dermal layer of rough hexactins and atrial layer of rough pentactins; microscleres oxyhexasters, oxyhemihexasters, and floricomes. Body shape flat, plate-like, with smooth margins (no marginal prostalia). Lophophytous mode of attachment, each anchoring spicule tuft with bundles of diactins surrounding a giant prostal hexactin. Dermal surface facing downward, toward the seafloor. Parietal oscula regularly scattered across the surface; color translucent white.
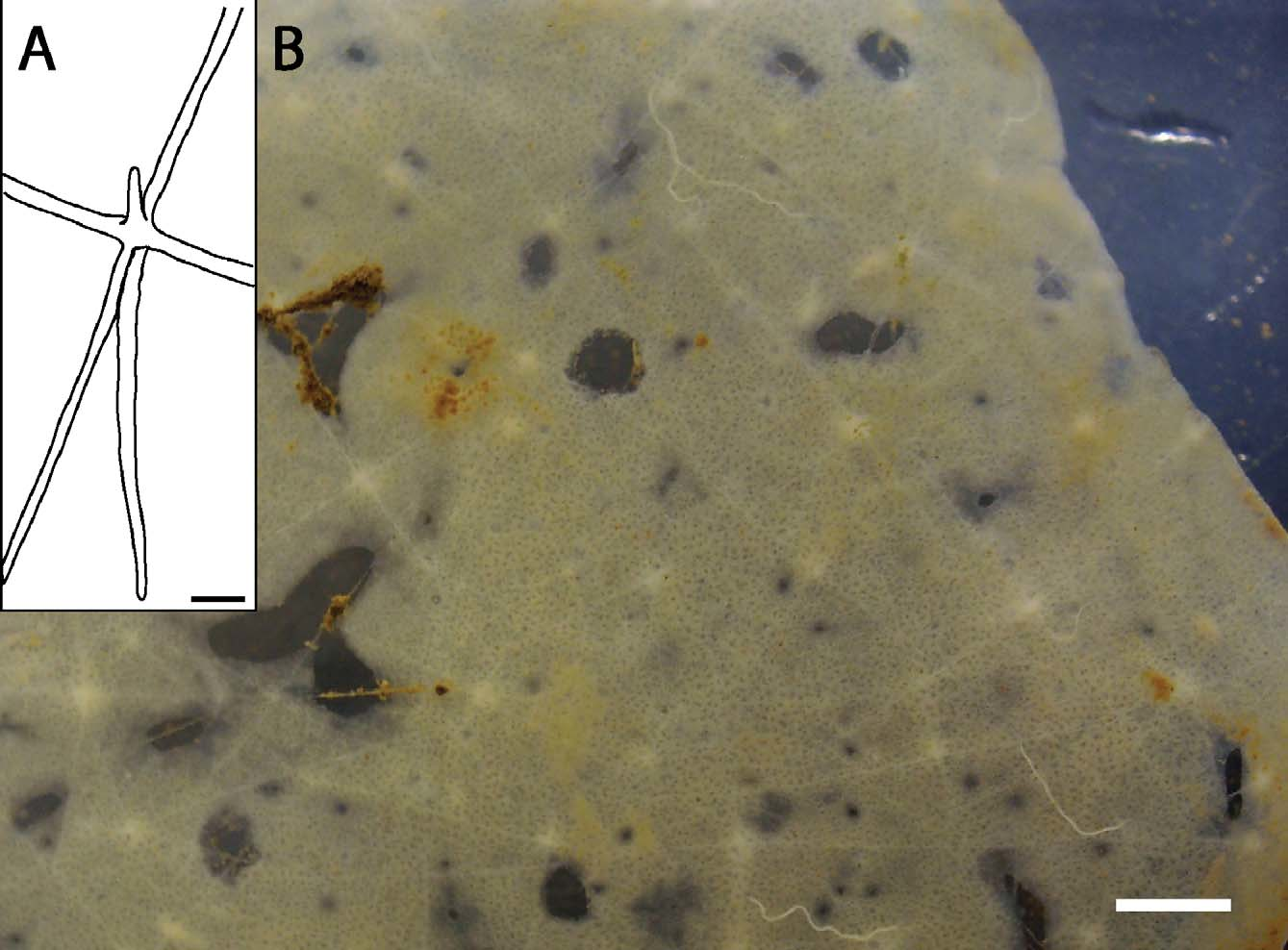
Description of holotype. Holotype ( Fig. 5 View FIGURE 5 ) 13.8 cm diameter at longest axis, 1–3 mm thick. The atrial surface is smooth, facing up away from seafloor, while the dermal surface shows several tufts of anchoring basalia, consisting of diactins surrounding a single, large distal ray of a prostal hexactin. Both surfaces have regular ostial or oscular apertures. Also scattered across the body are larger holes, parietal oscula, 2 to 5 mm in diameter, which perforate the entire body and appear as dark spots in photographs and ROV video. Both live and preserved specimens are translucent white, and in photographs appear to have white and black spots ( Fig. 5 View FIGURE 5 ). The black spots appear where parietal oscula perforate the sponge body while white spots appear where anchoring spicule tufts project from the dermal surface. Spicules are loosely arranged, resulting in a flexible, delicate texture that tears easily.
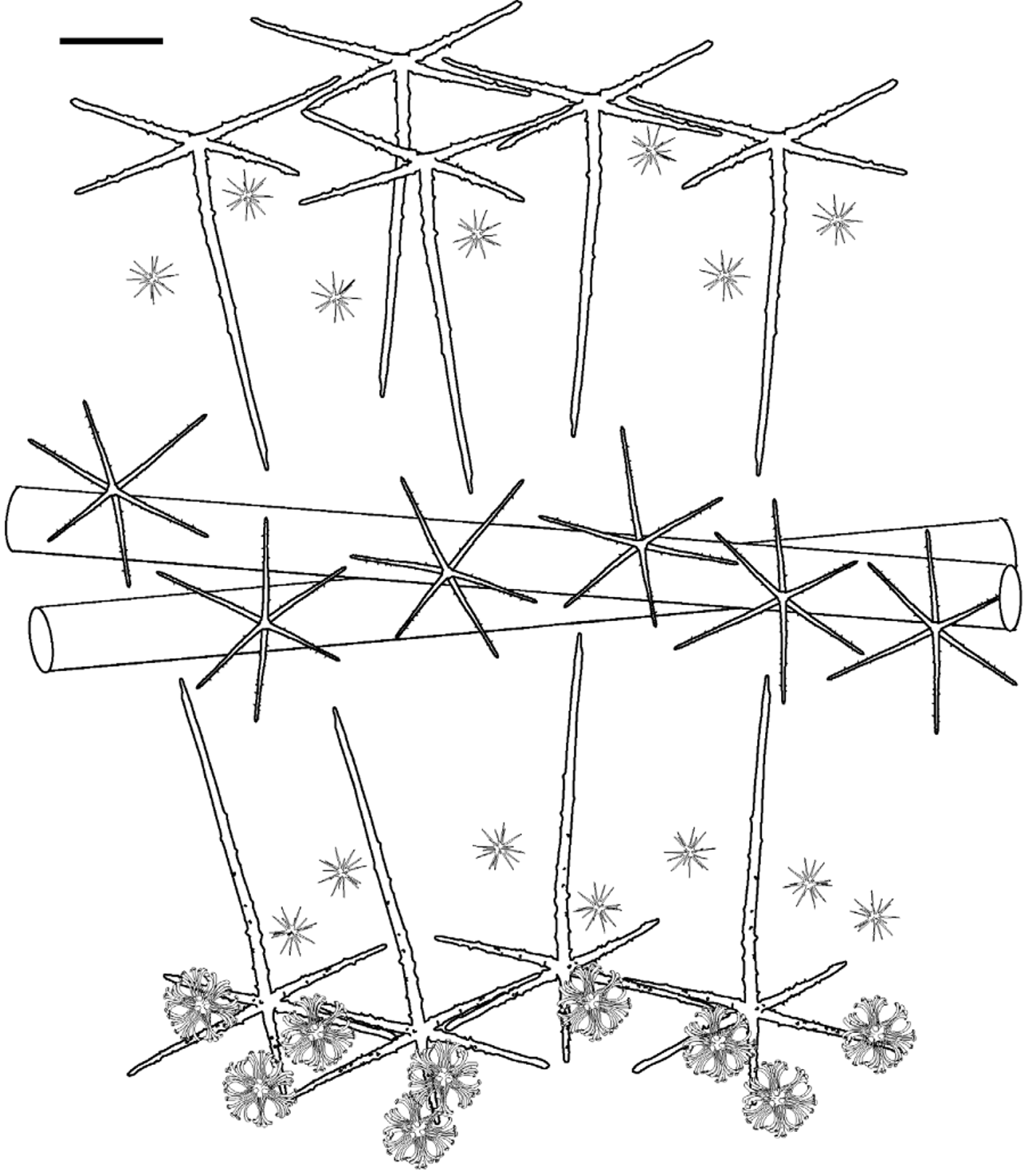
Lyssacine framework. Framework is loose, resulting in a flexible but delicate structure that is easily broken. Moving from the dermal to the atrial surface, the outermost layer of the dermal surface has a single layer of swordshaped hexactins with tangential rays forming an unfused, lyssacine network ( Fig. 6 View FIGURE 6 bottom). Proximal rays are much longer than the others, and project all the way through the choanosome to the atrial surface. Floricomes perch atop the distal ray of the hexactins. Below the dermal layer is a mesh of long diactins, generally pointing in all directions but sometimes forming bundles or tracts. Among the diactins are large, rough hexactins with even rays. Distal to the choanosome is the atrial membrane containing pentactins arranged with an overlapping network of tangential rays (summarized in Fig. 6 View FIGURE 6 upper).
The channel system is compressed into just two millimeters of body thickness, yet contains circuitous channels distributing water flow through the body. Openings of inhalant canals are regularly spaced across the dermal surface (about 100 to 250 µm diameter), and openings of exhalant canals (oscula) are regularly spaced and visible on the atrial surface (150 to 500 µm diameter). Internal channels leading from inhalant canal apertures and oscula curve laterally and presumably branch and weave through the choanosome.
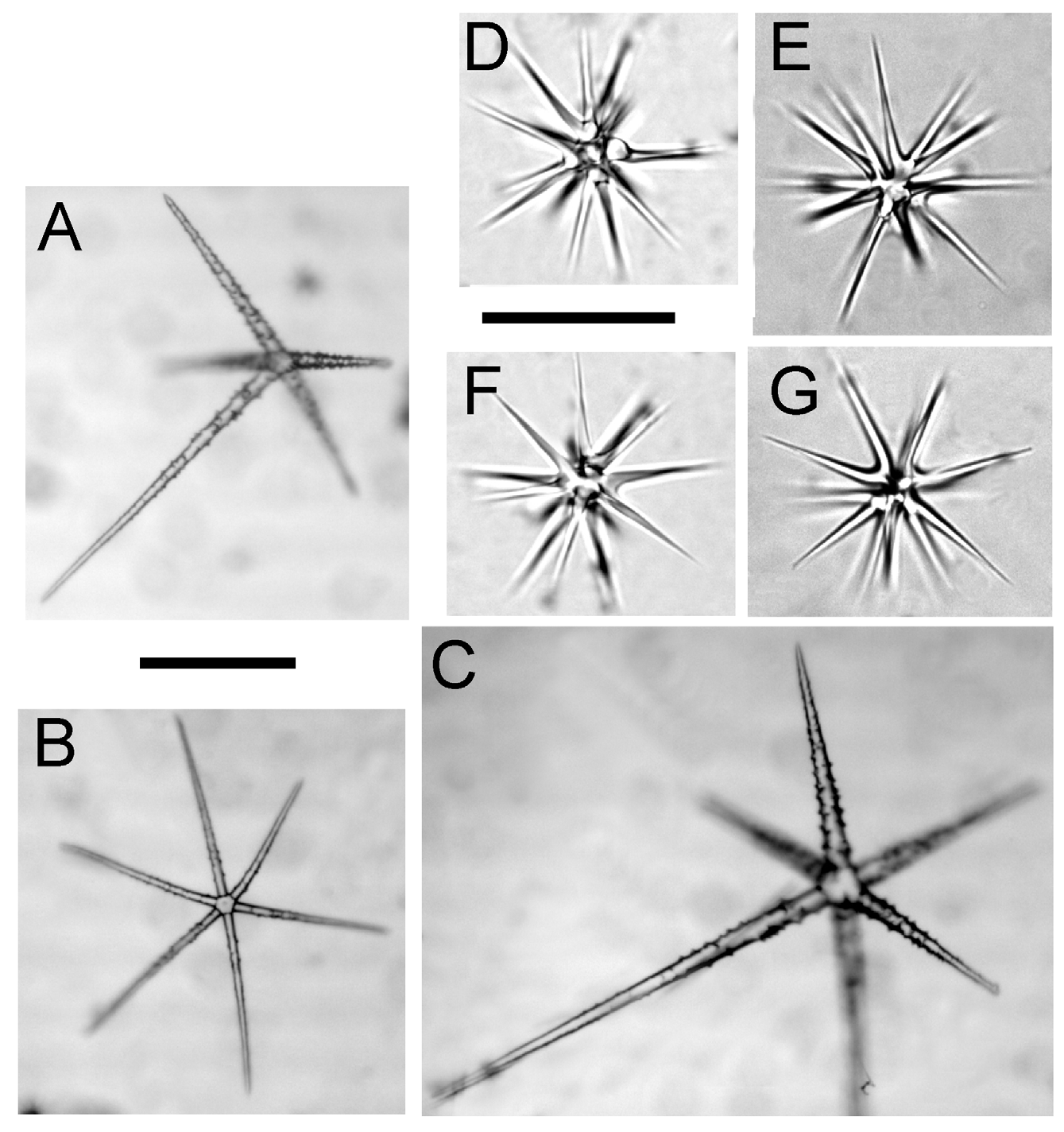
Spicules. Spicule forms are shown in Fig. 6 View FIGURE 6 through Fig. 9 View FIGURE 9 and dimensions are provided in Table 2 View TABLE 2 . Megascleres are hexactins of three varieties, rough atrial pentactins, and choanosomal and anchor-tuft diactins. Microscleres are oxyhexasters, oxyhemihexasters, and floricomes. The three types of hexactins will be described as 1) giant prostal hexactins, 2) dermal hexactins, and 3) choanosomal hexactins.
Giant prostal hexactins are smooth, with no spines along the entire length. A single giant prostal hexactin was found in each anchoring spicule tuft; the sinuous, thickened distal ray protrudes down into the sediments along with a bundle of diactins. The distal, anchoring ray and one tangential ray running through the choanosome are of similar dimensions (21- 25 - 30 mm, min- mean -max, n=4). Though not oriented in any regular direction, the long tangential ray overlaps with those of other giant hexactins and reinforces structure throughout the body ( Fig. 7 View FIGURE 7 ). Two other tangential rays are smaller (4- 7.2 - 14 mm, n=4) while the final tangential and proximal rays are rounded swellings of about 100 µm in length.
The sword-shaped dermal hexactins form a network of overlapping tangential rays, with longer proximal rays pointing into the choanosome ( Fig. 8 View FIGURE 8 c). These hexactins are covered with small spines; tangential rays are covered evenly while the proximal ray has more spines near the center and fewer at the tip. The distal ray is also covered with spines, and tapers to a point like the other rays.
Choanosomal hexactins are covered with spines evenly throughout the length of the six cylindrical, pointed rays ( Fig. 8 View FIGURE 8 b). Rays of choanosomal hexactins are of roughly equal size, unlike those of the dermal and giant hexactins.
Atrial pentactins form a network of overlapping tangential rays, with proximal rays pointing into the choanosome. Pentactins are rough with spines, but the spines are not as large as those of the dermal hexactins. Tangential rays are straight, while the proximal ray is either straight or bent slightly near the base ( Fig. 8 View FIGURE 8 a).
Microscleres include oxyhexasters, oxyhemihexasters, and floricomes. Full oxyhexasters with all six primary rays bearing two or more secondary rays are the most common form, but oxyhemihexasters with at least one set of multiple secondary rays (1–3 secondary rays for each primary ray) are also present. Both oxyhexasters and oxyhemihexasters are smooth, with short primary rays and tapering secondary rays that end in pointed (oxyoidal) tips ( Fig. 8 View FIGURE 8 d through 8g). They are found scattered throughout the sponge, from just below the dermal surface to the atrial membrane. Floricomes are found in the dermal membrane perched atop the distal rays of dermal hexactins. They have 9–12 S-shaped secondary rays radiating from each short primary ray ( Fig. 9 View FIGURE 9 ). Each secondary ray is roughened with small spines on the concave part of the distal curve, and widens to a flattened, eccentric toothed claw at the end with 4–6 teeth.
Etymology. The species name, maculatus , refers to the white and dark spots that appear through ROV video in situ. Parietal oscula perforating the entire body wall appear as dark spots while tufts of anchoring basalia, viewed through the atrial surface, appear as white spots.
Gene sequences. Ribosomal DNA from 18S, 28S, and 16S, plus mitochondrial COI were amplified and sequenced from the holotype in the same two previously published molecular phylogenies as for Bathydorus laniger (Dohrmann et al. 2009, 2012), where they were identified as Docosaccus n. sp. DNA vouchers from the holotype were deposited into the collection of G. Wörheide, voucher number GW5429. GenBank accession numbers FM946116 View Materials (18S), FM 946115 View Materials (28S), FM946105 View Materials (16S), FR848934 View Materials (COI).
Comparisons. To our knowledge, this species has not been reported from elsewhere in the world. Its only congener, Docosaccus ancoratus Topsent, 1910 , is found frequently but appears to be endemic to Antarctic waters (Barthel & Tendal 1994). The unique giant hexactin identifies the two species as closely related and belonging to the same genus, but geographic distribution and spicule composition confirm that they are different species. Docosaccus maculatus has an atrial membrane supported by pentactins while the atrial membrane of D. ancoratus is supported by hexactins. Oxyhexasters and oxyhemihexasters are much thicker in the Pacific species, appearing much more delicate in the figures of D. ancoratus provided by Topsent (1913). The dermal hexactins have pointed distal rays in D. maculatus , while they appear rounded into a spined swelling in the Antarctic species. Finally, the anchoring tufts are different between the two species. The diactins surrounding the giant hexactins of D. maculatus are straight and smooth with unevenly tapering tips while those of D. ancoratus are covered with spines recurved back toward the body and ends that look like grappling hooks (Topsent 1910, 1913). These differences confirm that the NE Pacific form is a new species, here designated as Docosaccus maculatus .
Remarks. The genus Docosaccus was considered to be endemic to Antarctic waters (Downey et al. 2012) until discovery of this plate sponge Docosaccus maculatus in temperate Pacific waters. Given the sparse nature of existing sampling of deep-sea habitats, exact distributions of neither the genus nor the species can be estimated, but overall this species expands the range of the genus.
TABLE 2. Spicule dimensions of Docosaccus maculatus sp. n., from Station M, California, USA excluding giant prostal hexactins (dimensions in µm).
| parameter | mean | SD | range | number |
|---|---|---|---|---|
| Megascleres | ||||
| Atrial pentactin | ||||
| tangential ray length | 326.9 | 42.7 | 241.3–401.0 | 16 |
| tangential ray width | 28.3 | 4.5 | 21.3–37.0 | 16 |
| proximal ray length | 653.2 | 139.0 | 426.6–880.0 | 17 |
| proximal ray width | 29.5 | 2.8 | 24.6–36.1 | 15 |
| Choanosomal hexactin | ||||
| ray length | 180.2 | 107.6 | 56.7–596.1 | 34 |
| ray width | 9.2 | 5.2 | 2.8–30.0 | 34 |
| Dermal hexactin | ||||
| tangential ray length | 340.9 | 68.8 | 195.0–466.8 | 27 |
| tangential ray width | 20.9 | 5.7 | 8.9–31.6 | 50 |
| proximal ray length | 596.5 | 164.7 | 356.5–1134.9 | 49 |
| proximal ray width | 22.3 | 5.8 | 10.7–37.3 | 49 |
| distal ray length | 141.9 | 38.9 | 66.2–232.8 | 50 |
| distal ray width | 20.1 | 5.1 | 10.4–32.4 | 50 |
| Microscleres | ||||
| Oxyhemihexasters & oxyhexasters | ||||
| diameter | 79.1 | 7.2 | 63.2–93.8 | 50 |
| primary ray length | 7.5 | 1.3 | 4.1–10.1 | 50 |
| secondary ray length | 31.9 | 3.8 | 24.3–41.7 | 50 |
| Floricome | ||||
| diameter | 79.1 | 7.2 | 63.2–93.8 | 50 |
| primary ray length | 8.3 | 1.6 | 5.2–11.9 | 22 |
| secondary ray length | 43.9 | 6.5 | 28.4–51.8 | 22 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.