Ephedrocephalus microcephalus Diesing, 1850
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4370.4.3 |
|
publication LSID |
lsid:zoobank.org:pub:C61BC695-51BB-4088-B2D4-64D2C8F7A673 |
|
DOI |
https://doi.org/10.5281/zenodo.5962970 |
|
persistent identifier |
https://treatment.plazi.org/id/03FD87AC-FF8C-FFDB-E6BA-FC2E14330F6E |
|
treatment provided by |
Plazi |
|
scientific name |
Ephedrocephalus microcephalus Diesing, 1850 |
| status |
|
Ephedrocephalus microcephalus Diesing, 1850
( FIgS. 1–18 View FIGURES1–9 View FIGURES10–12 View FIGURES13–18 )
SYN. Rudolphiella microcephalus ( DIeSINg, 1850) BROOKS, 1995
Type and only known host. Phractocephalus hemioliopterus (BlOch & SchNEIdER) ( SIlURIfORmES : PImElOdIdaE ).
Site of infection. POSTERIOR INTESTINE.
Type locality. SOUThERN AmaZON RIVER baSIN, MaTO GROSSO STaTE (fORmER caPTaINcY Of MaTO GROSSO), BRaZIl— SPEcIfIc lOcalITY UNKNOWN. MaTERIal cOllEcTEd bY JOhaNN NaTTERER IN 1828 ( SEE DIESINg 1855).
Additional localities. AmaZON RIVER, NEaR ITacOaTIaRa, AmaZONaS STaTE, BRaZIl, 3°09’S, 58°26’W; AmaZON RIVER, IqUITOS, REgION Of LORETO, PERU (dE ChambRIER et al. 2015b).
Prevalence of infection. INfEcTEd 5 Of 29 P. hemioliopterus EXamINEd, I.E., 17%, fROm ITacOaTIaRa, BRaZIl.
Representative DNA sequences. 5.8S-ITS2-28S RDNA gENES ( AY551143 View Materials ), 18S RDNA gENE ( AY551108 View Materials , KC786007 View Materials ), 28S RDNA gENE ( AJ388605 View Materials , KC786017 View Materials ), 16S RDNA gENE ( AJ389509 View Materials , KC785994 View Materials ), cox 1 mTDNA gENE ( AJ275056 View Materials , KC785982 View Materials ) (ZEhNdER & MaRIaUX 1999; HYPša et al. 2005; SchOlZ et al. 2013).
Morphological description. DIESINg (1850, 1855), MOla (1906), WOOdlaNd (1933), FURhmaNN (1934), REgO (1984), PRESENT STUdY.
Material studied. SYNTYPE—MHNG-PLAT-40806, WhOlE-mOUNTEd SPEcImEN (1 SlIdE), STaINEd fRagmENTS (3 SlIdES) aNd 34 SlIdES Of SERIal cROSS-SEcTIONS. AddITIONal SPEcImENS—CHIOC 38886, MHNG-PLAT-21851, 21853, 21899, 21909, 21910, 5 WhOlE-mOUNTEd SPEcImENS (7 SlIdES) aNd 7 SlIdES Of SERIal cROSS-SEcTIONS, cOllEcTEd ON 5.X.1995, hOST fIEld NOS. BR 539b-E, 540a,b; MHNG-PLAT-22009, WhOlE-mOUNTEd SPEcImEN (1 SlIdE), cOllEcTEd ON 9.X.1995, hOST fIEld NO. BR 649E; MHNG-PLAT-22371, 28296, 2 WhOlE-mOUNTEd SPEcImENS (4 SlIdES) aNd 8 SlIdES Of SagITTal SEcTIONS Of ScOlEX, cOllEcTEd ON 10.X.1995, hOST fIEld NO. BR 652a,b; all SPEcImENS fROm ITacOaTIaRa, AmaZONaS, BRaZIl, cOllEcTEd bY A. A. REgO aNd A. dE ChambRIER.
Redescription (baSEd ON 5 WhOlE-mOUNTEd WORmS; 12 SlIdES WITh SERIal cROSS-SEcTIONS Of maTURE PROglOTTIdS aNd 8 SlIdES WITh SagITTal SEcTIONS Of 1 ScOlEX; 1 ScOlEX aNd PROglOTTId STUdIEd USINg SEM). PROTEOcEPhalIdaE , fORmER SUbfamIlY EPhEdROcEPhalINaE . TESTES aNd VITEllINE fOllIclES cORTIcal; OVaRY aNd UTERUS mEdUllaRY; mEdIUm- SIZEd WORmS. TOTal bOdY lENgTh 45–95 mm (N = 4), maXImUm WIdTh UP TO 3.3 mm (N = 4). STRObIla acRaSPEdOTE, aNaPOlYTIc, ThIcK-WallEd, WITh lONgITUdINal aNd TRaNSVERSE gROOVES, cONSISTINg Of abOUT 105–145 PROglOTTIdS: 50–65 ImmaTURE, 15–20 maTURE, 30–40 PREgRaVId aNd 10–20 gRaVId. ImmaTURE, maTURE aNd PREgRaVId PROglOTTIdS maRKEdlY WIdER ThaN lONg (lENgTh: WIdTh RaTIO 0.05–0.25), gRaVId PROglOTTIdS WIdER ThaN lONg (lENgTh: WIdTh RaTIO 0.60–0.80).
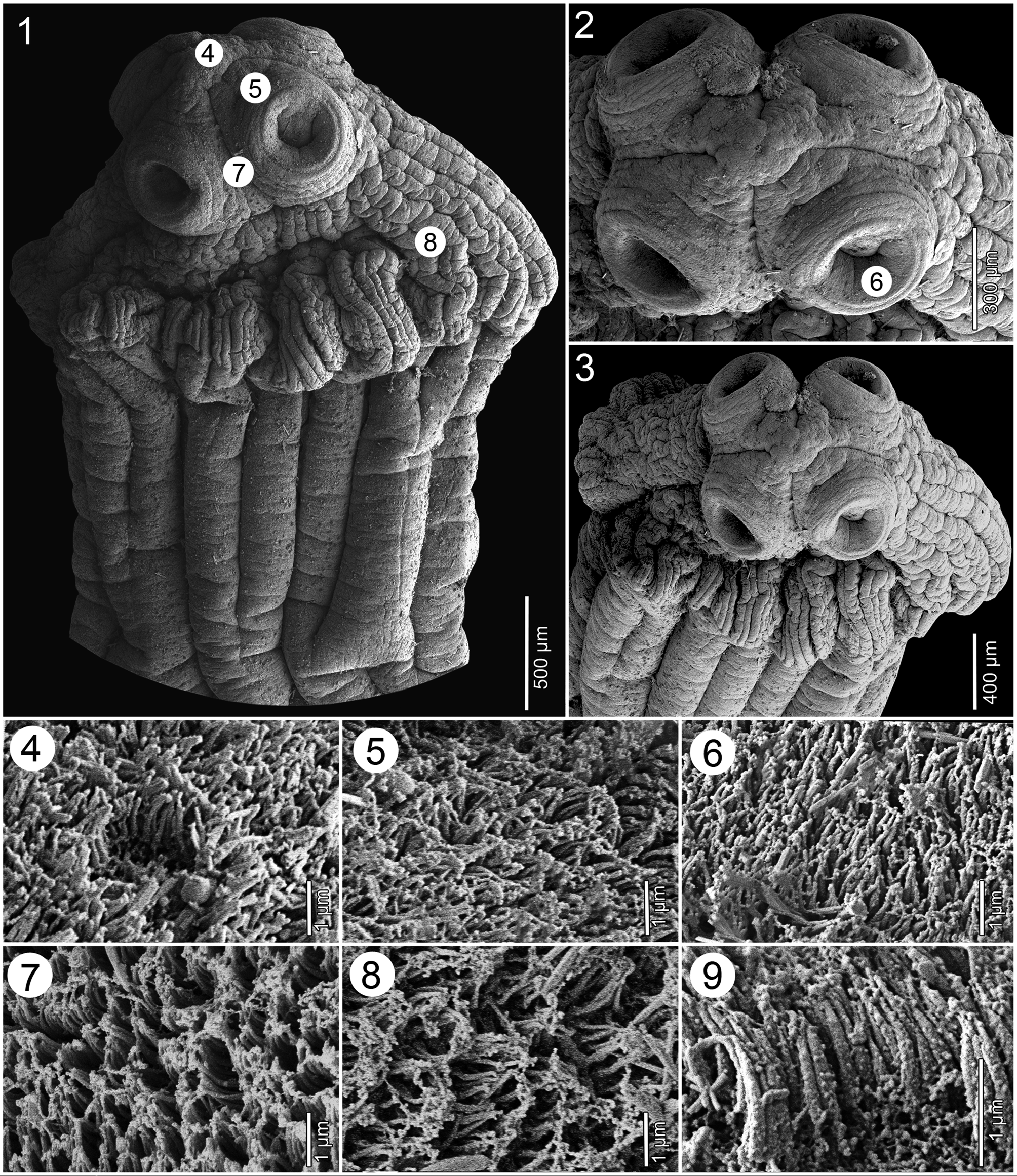
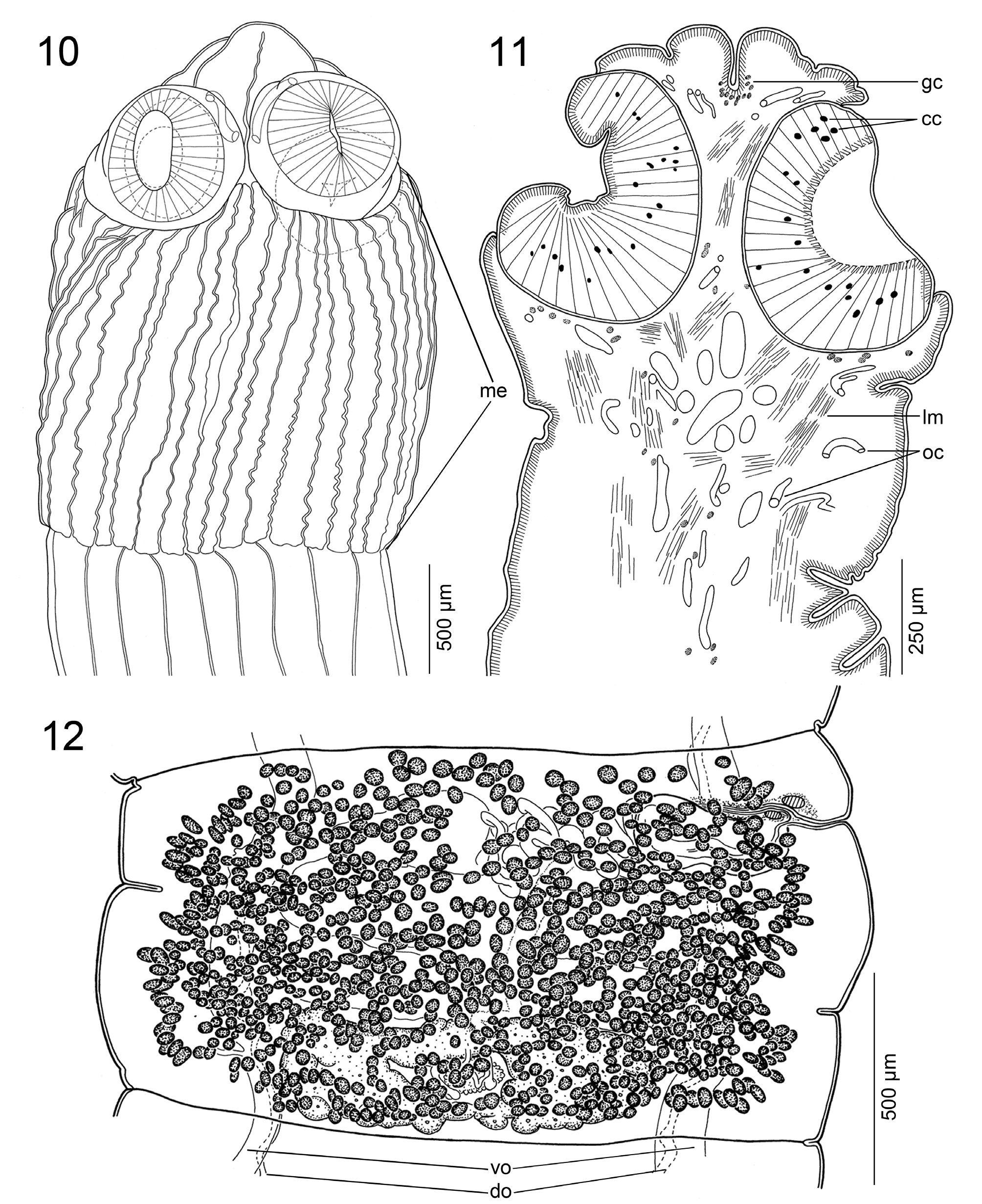
ScOlEX 2.5–2.8 × 2.4–2.6 mm (N = 3), WIdER ThaN NEcK (PROlIfERaTION ZONE), 1.6–2.0 × 1.2–1.8 mm, bEaRINg 4 UNIlOcUlaTE SUcKERS aNd mETaScOlEX fORmEd bY ENlaRgEmENT Of NEcK, WITh NUmEROUS (TENS) dEEP lONgITUdINal WRINKlES, gROUPS Of Small glaNd cEllS aNd SPaRSElY dISTRIbUTEd NETWORK Of OSmOREgUlaTORY caNalS ( FIgS. 1–3 View FIGURES1–9 , 10, 11 View FIGURES10–12 ). SUcKERS SPhERIcal, STRONglY mUScUlaR, 570–605 IN dIamETER (N = 12). APEX cONIcal, lacKINg aPIcal ORgaN, WITh fEW glaNd cEllS ( FIgS. 2, 3 View FIGURES1–9 , 10, 11 View FIGURES10–12 ). APEX Of ScOlEX, EXTERNal aNd INTERNal RIm Of SUcKERS, SURfacE bETWEEN SUcKERS, mETaScOlEX aNd STRObIla cOVEREd WITh caPIllIfORm fIlITRIchES Of SImIlaR aPPEaRaNcE aNd dENSITY ( FIgS. 4–9 View FIGURES1–9 ).
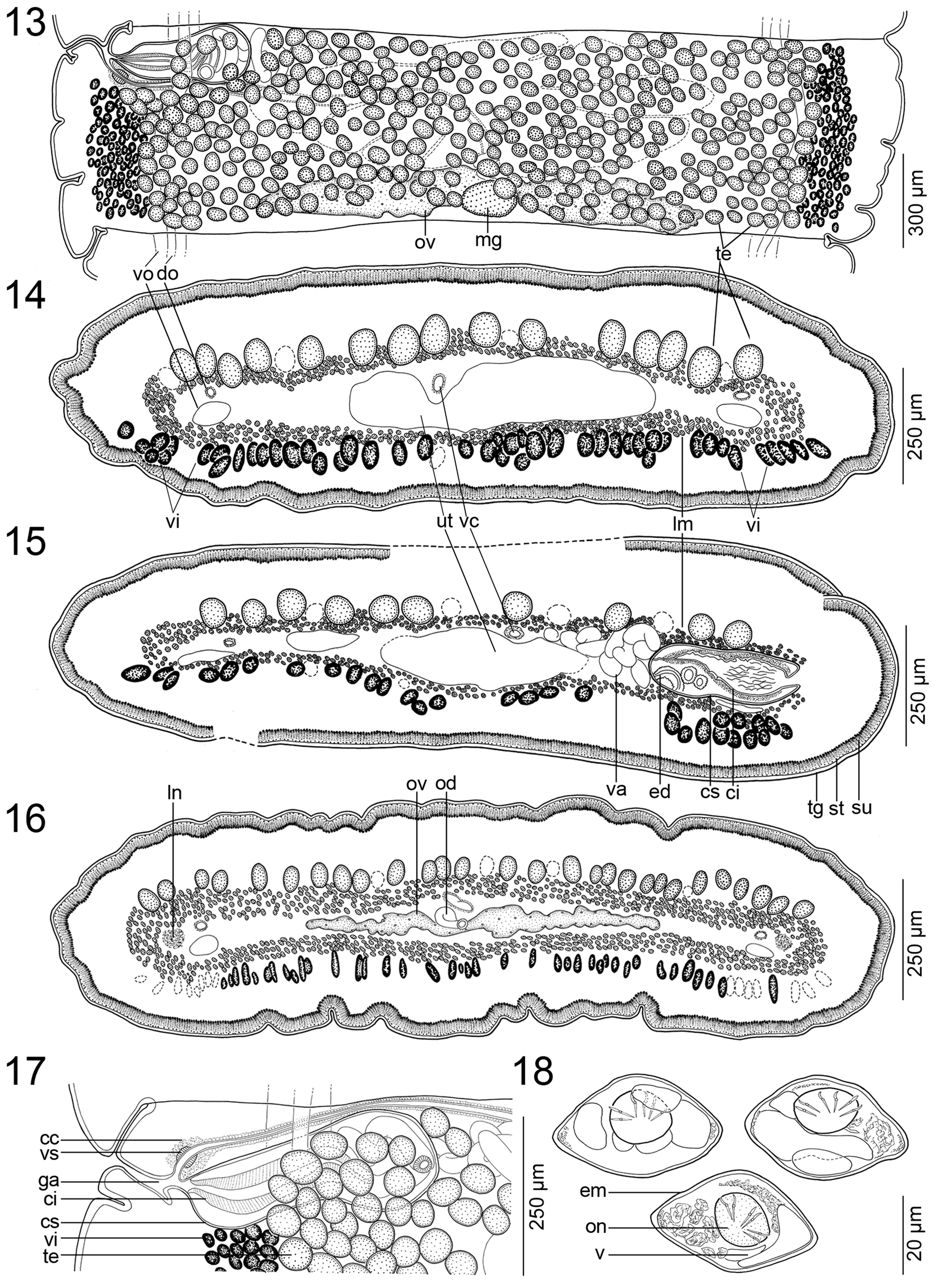
INNER lONgITUdINal mUScUlaTURE WEll-dEVElOPEd, fORmEd bY 3–5 ROWS Of NUmEROUS, Small bUNdlES Of mUSclE fIbERS ( FIgS. 14–16 View FIGURES13–18 ). OSmOREgUlaTORY caNalS SITUaTEd aT SamE lEVEl, mEdIaN TO laTERalmOST TESTES aNd VITEllINE fOllIclES, SlIghTlY SINUOUS; VENTRal OSmOREgUlaTORY caNal cONSIdERablY WIdER ThaN dORSal ONE ( FIgS. 12–17 View FIGURES10–12 View FIGURES13–18 ).
TESTES NUmEROUS, cORTIcal, SPhERIcal, 40–65 IN dIamETER, IN ONE laYER, 200–340 (X = 270; N = 8) PER maTURE PROglOTTId ( FIgS. 13–16 View FIGURES13–18 ). TESTES fORm 1 SlIghTlY IRREgUlaR fIEld ON dORSal SIdE, laTERallY SURPaSSINg OSmOREgUlaTORY caNalS, dORSallY OVERlaPPINg cRRUS-Sac aNd OVaRY ( FIgS. 13–16 View FIGURES13–18 ). TESTES PRESENT alSO IN gRaVId PROglOTTIdS.
VaS dEfERENS cOIlEd, WITh lOOPS fORmINg ElONgaTE fIEld REachINg TO, bUT NOT cROSSINg, mEdIaN lINE Of PROglOTTId ( FIg. 12 View FIGURES10–12 ). CIRRUS-Sac ElONgaTE TO PEaR-ShaPEd, ThIN-WallEd ( FIgS. 12 View FIGURES10–12 , 13, 15, 17 View FIGURES13–18 ), 345–500 × 130–235 (N = 12), ITS lENgTh REPRESENTINg 15–30% (X = 21; N = 12) Of PROglOTTId WIdTh. SPERm dUcT (INTERNal VaS dEfERENS) SINUOUS ( FIgS. 13, 15, 17 View FIGURES13–18 ). CIRRUS UNaRmEd, mUScUlaR, REachINg UP TO 60% (N = 12) Of cIRRUS-Sac lENgTh. COmmON gENITal aTRIUm NaRROW, dEEP ( FIgS. 12 View FIGURES10–12 , 13, 17 View FIGURES13–18 ). GENITal PORES alTERNaTINg IRREgUlaRlY, maRKEdlY PRE-EqUaTORIal, SITUaTEd aT 2–9% (X = 6; N = 12) Of PROglOTTId lENgTh fROm aNTERIOR maRgIN ( FIgS. 12 View FIGURES10–12 , 13, 17 View FIGURES13–18 ).
OVaRY mEdUllaRY, bIlObEd, cOmPacT, OccUPYINg 45–55% (X = 52%; N = 12) Of PROglOTTId WIdTh, ITS lENgTh REPRESENTINg 25–40% (X = 30%; N = 12) Of PROglOTTId lENgTh ( FIgS. 12 View FIGURES10–12 , 13, 16 View FIGURES13–18 ). MEhlIS’ glaNd abOUT 97–135 IN dIamETER, REPRESENTINg 4–9% Of PROglOTTId WIdTh (N = 7). RElaTIVE OVaRIaN SIZE, I.E., PERcENTagE Of OVaRY SURfacE TO TOTal SURfacE Of maTURE OR PREgRaVId PROglOTTIdS (SEE dE ChambRIER et al. 2012), 10–15% (X = 13%; N = 8).
VagINal caNal SlIghTlY SINUOUS, SURROUNdEd bY chROmOPhIlIc cEllS, WIdER IN TERmINal PaRT (pars copulatrix vaginae); VagINal SPhINcTER SPhERIcal, NEaR OPENINg Of VagINa TO gENITal aTRIUm ( FIgS. 12 View FIGURES10–12 , 13, 17 View FIGURES13–18 ). VagINa aNTERIOR (35%) OR POSTERIOR (65%) TO cIRRUS-Sac (N = 20). VITEllINE fOllIclES cORTIcal, ScaTTEREd ThROUghOUT all PROglOTTId, fORmINg ONE VENTRal fIEld, WITh IRREgUlaR dISTRIbUTION ( FIgS. 12–16 View FIGURES10–12 View FIGURES13–18 ).
UTERUS mEdUllaRY, WITh dEVElOPmENT Of TYPE 1 (SEE dE ChambRIER et al. 2004a, 2015a); UTERUS WITh 2–5 laTERal dIVERTIcUla ON Each SIdE ( FIg. 13 View FIGURES13–18 ). EggS lEmON-ShaPEd, EmbRYOPhORE WITh VacUOlES 41–47 × 25–27, ONcOSPhERE 14–16 × 13–14 (N = 6), EmbRYONIc hOOKS 5–6 lONg (N = 6) ( FIg. 18 View FIGURES13–18 ).
Remarks. ThE SPEcIES WaS bRIEflY dEScRIbEd bY DIESINg (1850); ThE SamE aUThOR ( DIESINg 1855) PROVIdEd IllUSTRaTIONS Of ThE ScOlEX aNd STRObIla. MOla (1906) REdEScRIbEd ThE SPEcIES baSEd ON ITS TYPE maTERIal dEPOSITEd IN ThE NaTURal HISTORY MUSEUm, VIENNa; SUbSEqUENT mORPhOlOgIcal accOUNTS dId NOT ImPROVE SIgNIfIcaNTlY mORPhOlOgIcal chaRacTERIZaTION Of ThE SPEcIES (SEE WOOdlaNd 1933; FUhRmaNN 1934; REgO 1984). LaTER, ThE SamE aUThOR (MOla 1929) EREcTEd a NEW, mONOTYPIc SUbfamIlY, EPhEdROcEPhalINaE , TO accOmmOdaTE E. microcephalus, WhIch WaS WIdElY accEPTEd (SEE REgO 1994).
ThE ScOlEX Of E. microcephalus haS bEEN REPEaTEdlY REPORTEd aNd IllUSTRaTEd TO bEaR a cOllaR-lIKE mETaScOlEX, WIdER laTERallY ThaN dORSOVENTRallY (SEE FIgS. 2–5 View FIGURES1–9 IN DIESINg 1855, FIgS. 1–3 View FIGURES1–9 IN MOla 1906 aNd FIgS. 1A–C View FIGURES1–9 IN FURhmaNN 1934), bUT ITS mORPhOlOgY dEPENdS ON ThE mEThOd Of fIXaTION. ThE TYPE maTERIal WaS mOST lIKElY fIXEd IN UNhEaTEd fIXaTIVE, WhIch maY haVE caUSEd ITS UNNaTURal cONTRacTION (SEE alSO WOOdlaNd 1933).
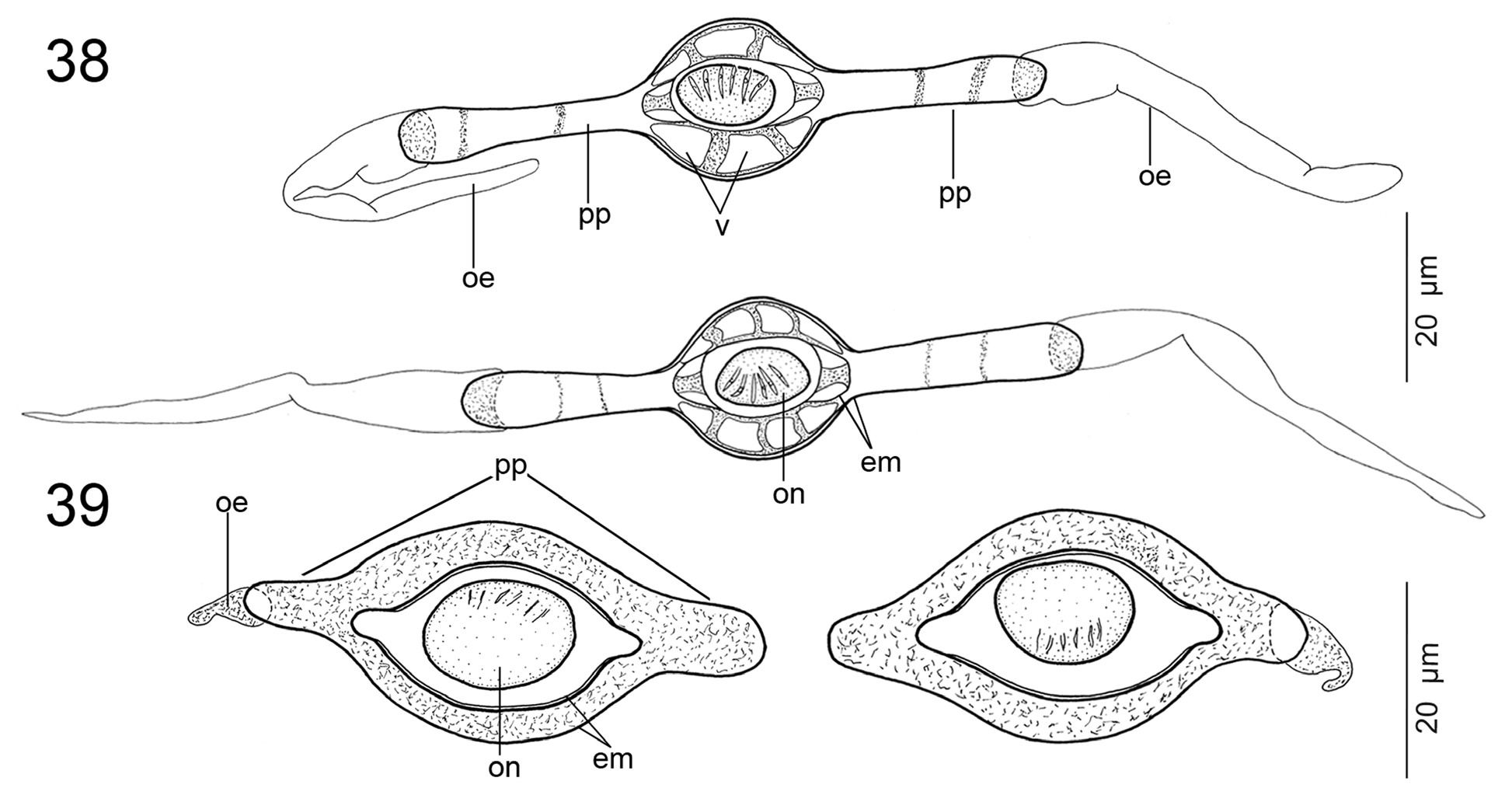
WOOdlaNd (1933) REPORTEd OPERcUlaTE EggS IN E. microcephalus , a fEaTURE ThaT WaS alSO INclUdEd IN ThE gENERIc dIagNOSIS Of Ephedrocephalus bY FREZE (1965) aNd REgO et al. (1999), aPPaRENTlY WIThOUT EXamININg SPEcImENS. If TRUE, ThIS WOUld bE ThE fIRST caSE Of ShEllEd EggS WITh aN OPERcUlUm amONg all PROTEOcEPhalIdS (aNd all acETabUlaTE TaPEWORmS; SEE CaIRa & JENSEN 2017). ThE PRESENT STUdY PROVIdES EVIdENcE ThaT ThIS ObSERVaTION Of WOOdlaNd (1933) WaS ERRONEOUS, bEcaUSE ThE EggS Of E. microcephalus aRE NOT ShEllEd, I.E., cOVEREd WITh a TaNNEd caPSUlE PRESENT IN POlYlEcIThal EggS Of EaRlIER dIVERgEd TaPEWORmS (SEE CONN & SWIdERSKI 2008), aNd dO NOT POSSESS aNY OPERcUlUm (SEE FIg. 18 View FIGURES13–18 IN ThE PRESENT STUdY). IN ITS mORPhOlOgY, ThE EggS Of E. microcephalus mOST RESEmblE ThOSE Of SPEcIES Of Rudolphiella FUhRmaNN, 1916, Brooksiella praeputialis (REgO, dOS SaNTOS & SIlVa, 1974) aNd Cangatiella macdonaghi (SZIdaT & NaNI, 1951) (SEE GIl dE PERTIERRa & VIOZZI 1999; GIl dE PERTIERRa & dE ChambRIER 2000; dE ChambRIER et al. 2004b; PRESENT STUdY—FIg. 39).
A cOmPaRaTIVE STUdY Of ThE EggS Of bOTh SPEcIES Of Cangatiella REVEalEd ThaT C. arandasi PaVaNEllI & dOS SaNTOS, 1991 IN facT POSSESSES lONg POlaR PROjEcTIONS ( FIg. 38 View FIGURES 38, 39 ), NOT POlaR fIlamENTS aS STaTEd bY REgO (1994). IT IS alSO WORTh NOTINg ThaT ThE UTERUS Of C. macdonaghi cONTaINS bOTh ImmaTURE EggS TOgEThER WITh fUllY dEVElOPEd ONES, I.E., ThOSE cONTaININg SIX-hOOKEd ONcOSPhERES. ThIS fEaTURE IS RaRE IN PROTEOcEPhalIdS, haVINg bEEN ObSERVEd ONlY IN a fEW SPEcIES, E.g., Goezeella siluri FURhmaNN, 1916 aNd G. mariae AlVES, dE ChambRIER, LUqUE & SchOlZ, 2017 (UNPUbl. daTa).
ThE PRESENcE Of VacUOlES IN ThE EggS Of E. microcephalus IS alSO UNcOmmON IN PROTEOcEPhalIdS. TO ThE bEST Of OUR KNOWlEdgE, ONlY ThE EggS Of Travassiella jandia (WOOdlaNd, 1934), Zygobothrium megacephalum aNd C. arandasi , all PaRaSITES Of NEOTROPIcal caTfIShES, haVE ThE EggS WITh VacUOlES (SEE FIg. 8 View FIGURES1–9 IN dE ChambRIER & GIl dE PERTIERRa 2002 aNd FIgS. 37 View FIGURES 34–37 , 38 View FIGURES 38, 39 IN ThE PRESENT STUdY).
EVEN ThOUgh SOmE PROTEOcEPhalIdS haVE ThE VITEllINE fOllIclES IN ThE VENTRal cORTEX (SEE AlVES et al. 2017a aNd REfERENcES ThEREIN), NONE Of ThEm POSSESS ThE fOllIclES OccUPYINg ThE ENTIRE VENTRal aREa Of PROglOTTIdS aS ObSERVEd IN E. microcephalus bUT INSTEad ThE VITEllINE fOllIclES aRE dISTRIbUTEd IN TWO laTEROVENTRal baNdS.
ThE PhYlOgENETIc POSITION Of E. microcephalus REmaINS UNRESOlVEd, EVEN ThOUgh IT lIKElY REPRESENTS a mORE REcENTlY dIVERgEd TaXON cOmPaREd TO Z. megacephalum (SEE bElOW) aS IT aPPEaRS aS a SEPaRaTE lINEagE IN a bIg POlYTOmY cOmPOSEd Of cOSmOPOlITaN REPTIlIaN PROTEOcEPhalIdS, a SPEcIES fROm ThE cOmmON OPOSSUm (MammalIa) aNd a fEW REPRESENTaTIVES fROm NEOTROPIcal caTfIShES, INclUdINg TWO SPEcIES Of Pseudocrepidobothrium REgO & IVaNOV, 2001, I.E., Ps. eirasi (REgO & dE ChambRIER, 1995) aNd Ps. ludovici RUEdI & dE ChambRIER, 2012, WhIch alSO OccUR IN Ph. hemioliopterus (SEE dE ChambRIER et al. 2015a).
| PERU |
Universit� di Perugia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |