Lepidocyrtus chorus Mateos & Lukić, 2019
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4550.2.4 |
|
publication LSID |
lsid:zoobank.org:pub:C8D2B580-C156-4075-8FD1-E4A111DAC33F |
|
DOI |
https://doi.org/10.5281/zenodo.5935988 |
|
persistent identifier |
https://treatment.plazi.org/id/3B52475B-12DD-42F3-9B6E-5E65DE58F6A4 |
|
taxon LSID |
lsid:zoobank.org:act:3B52475B-12DD-42F3-9B6E-5E65DE58F6A4 |
|
treatment provided by |
Plazi |
|
scientific name |
Lepidocyrtus chorus Mateos & Lukić |
| status |
sp. nov. |
Lepidocyrtus chorus Mateos & Lukić View in CoL sp. nov.
Figs 1–31 View FIGURES 1–4 View FIGURES 5–8 View FIGURES 9–13 View FIGURE 14 View FIGURE 15 View FIGURES 16–18 View FIGURES 19–20 View FIGURE 21 View FIGURES 22–28 View FIGURES 29–31 , Table 1
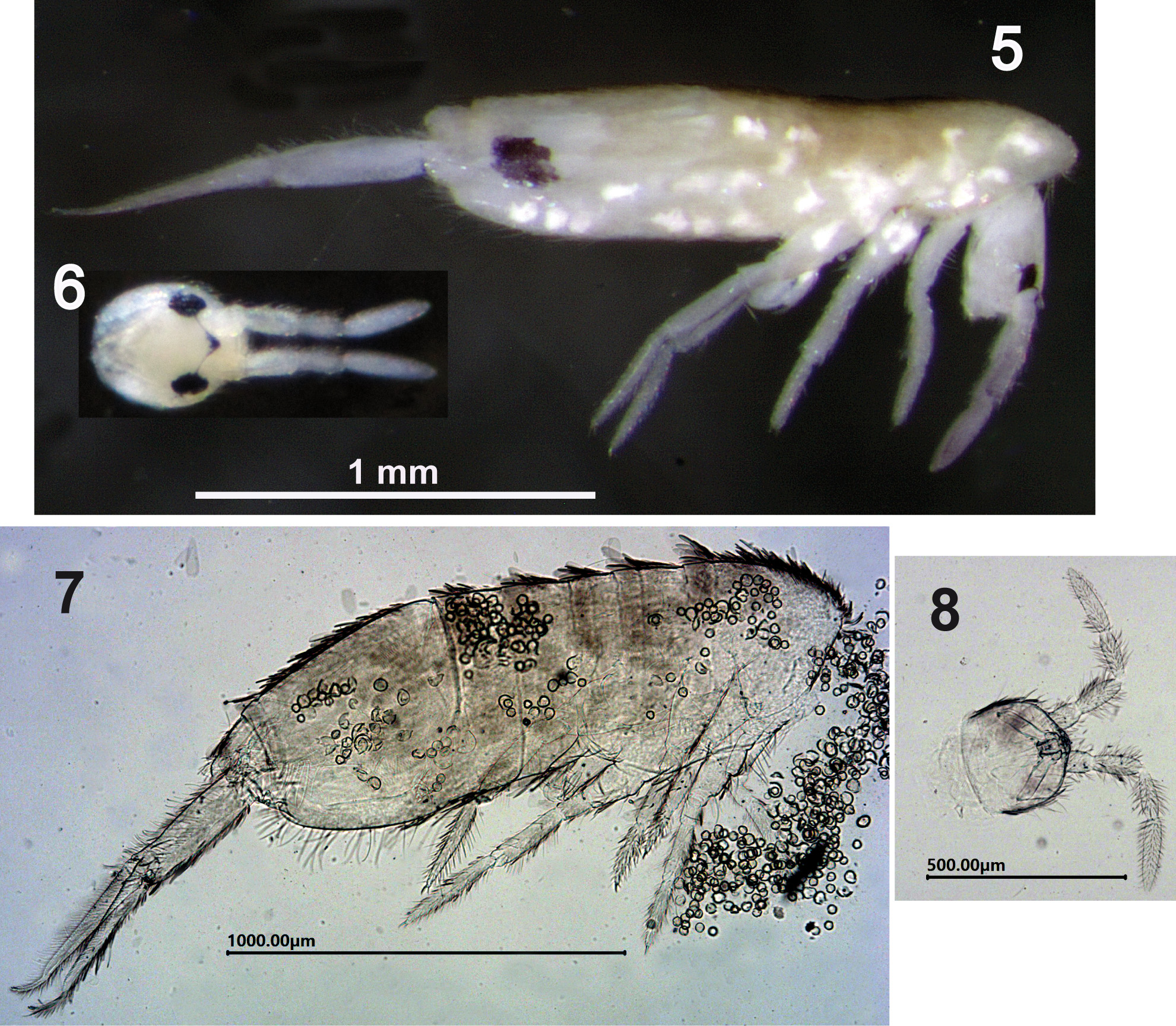
Type material. Holotype: female on slide (slide code: CRBA-77842), Krka National Park , village Oklaj , Town Šibenik, hidropowerplant Miljacka, Croatia, 95 m above sea level, N44°00’04.0” E16°01’05.9”, on stone steps ( Figs 1–2 View FIGURES 1–4 ), hand collecting, 29.iv.2015, leg. M. Lukić. GoogleMaps Paratypes: 16 females on slides (slide codes LP383-1 to LP383-09 , CRBA-77843, and six slides with code CLL4558 ), and 18 specimens (of unknown sex) in a vial with absolute ethanol (vial code LP383 (3 specimens) and CLL4558 (15 specimens )); same data as holotype GoogleMaps . 4 females on slides (code: CLL5100 ) and 1 specimen (of unknown sex) in a vial with absolute ethanol (vial code CLL5100 ), 25.ix.2018, leg. N. Sudar; same data as holotype GoogleMaps .
The holotype and paratype CRBA-77843 are deposited at the Centre de Recursos de Biodiversitat Animal, Facultat de Biologia, Universitat de Barcelona, Barcelona, Spain (http://www.ub.edu/crba/). Paratypes LP383-1 to LP383-09 on slides and three specimens in absolute ethanol (vial code LP383 ) deposited at the E. Mateos collection, Departament de Biologia Evolutiva , Ecologia i Ciències Ambientals, Facultat de Biologia, Universitat de Barcelona, Barcelona, Spain . 10 paratypes ( CLL4558 , six specimens; CLL5100 , four specimens) on slides and 16 specimens in absolute ethanol ( CLL4558 , 15 specimens; CLL5100 , 1 specimen) deposited at the Croatian Biospeleological Society Collection , Zagreb, Croatia .
Diagnosis. Trunk uniformly white, with one lateral dark-violet spot on each side of abd.IV. Th.II projecting over head. Ant.I-II, legs, ventral tube and posterior region of manubrium with scales. Apical bulb on ant.IV absent. Labial chaetae M 1 M 2 REL 1 L 2 in “p row” well developed and ciliated, R shorter. Dorsal cephalic and body macrochaetae formula as A 0 [A 2a]A 2 A 3 Pa 5 /00/0101+3. Without chaeta s on abd.IV.
Molecular diagnosis. This species includes all populations that cluster with COXII and EF-1α sequences of the individuals LP383-1 to LP383-4 ( Table 1, see also Mateos et al. 2018), with significant support in an adequate molecular delimitation model.
Etymology. The specific name refers to the dance-like behaviour observed in situ. In latin “ chorus ” means “a dancer”.
Description. Holotype body length 1.6 mm (without head nor furca), paratypes 1.4–1.7 mm. Live specimens of silver color (due to coating of scales, Figs 3–4 View FIGURES 1–4 ), specimens in alcohol white with a dark-violet lateral spot on abd.IV ( Fig. 5 View FIGURES 5–8 ). Th.II projecting over head ( Fig. 5 View FIGURES 5–8 ). Head with a dark-violet apical triangular spot between antennae bases ( Fig. 6 View FIGURES 5–8 ). Ant.II-III-IV slightly violet. Scales densely covering dorsal and ventral surfaces of head, trunk, legs, ventral tube and manubrium, dorsal surface of ant.I-II, and anterior surface of dens ( Figs 7–8 View FIGURES 5–8 ).
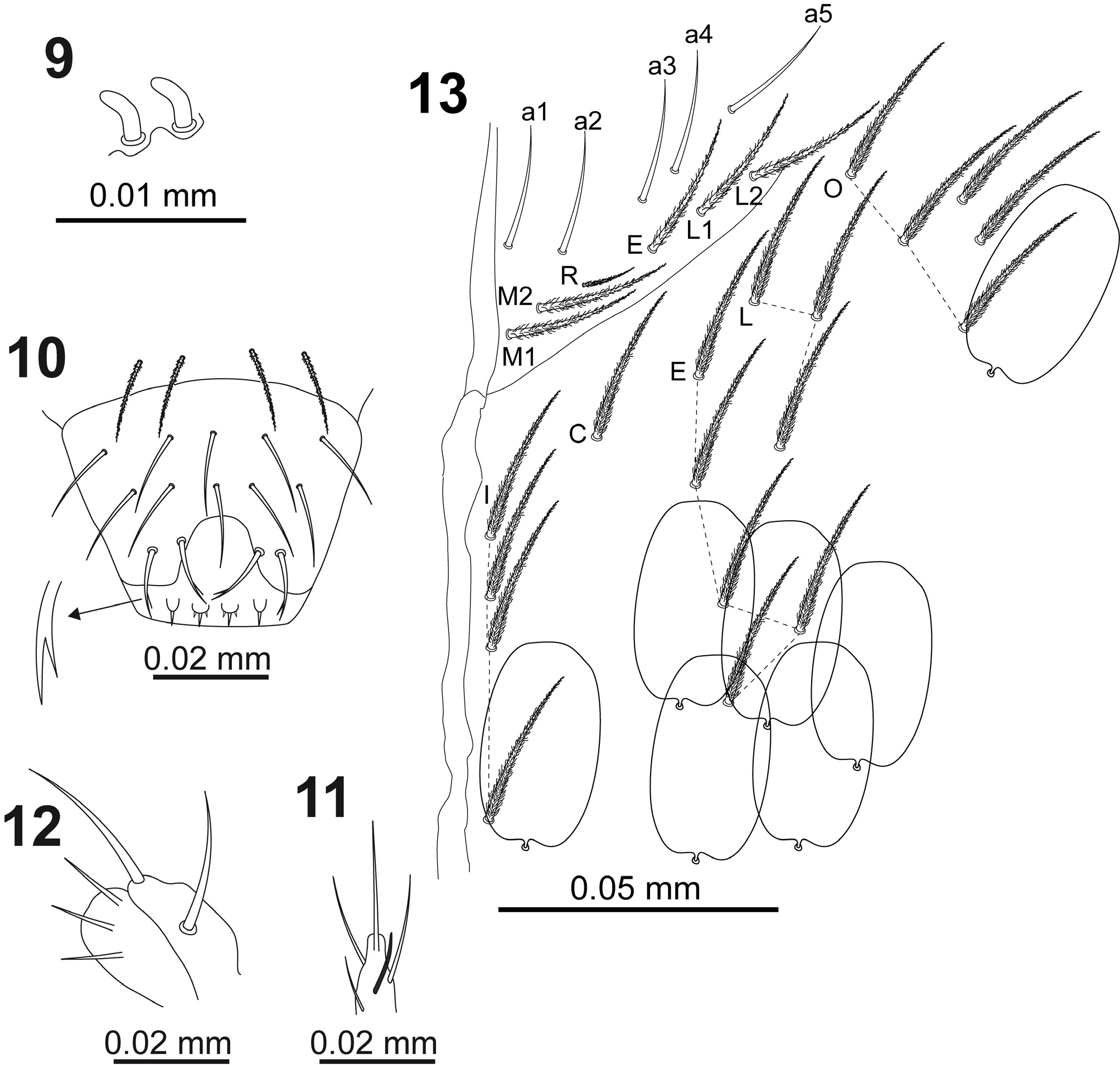
Antennal length to head diagonal length ratio (head diagonal measured from cervical edge to apex of mouth part) 1.5–1.7, holotype 1.7. Relation of antennal joints I–IV as 1:1.7–1.9:1.4–1.7:2.7–3.4, in holotype as 1:1.7:1.7:2.7. Ant.I with three dorsolateral small acute chaetae in a triangle (antennal-I-organ sensu Huther, 1986). Ant. III sense organ composed of two bent sensory rods partially behind a cuticular fold ( Fig. 9 View FIGURES 9–13 ). Ant. IV without apical bulb.
Clypeus with twelve ciliated chaetae (3 in row pf, 5 in row f, and 2– 2 in rows L 1 –L 2, respectively). Arrangement of chaetae on labrum 4/554, prelabral chaetae ciliated, first and second row of labral chaetae smooth, apical row branched ( Fig. 10 View FIGURES 9–13 ). Labrum intrusion inverted U-shaped, labral edge with four minute rounded labral papillae with one-pointed end (the lateral papillae) and three-pointed end (the central papillae) ( Fig. 10 View FIGURES 9–13 ). Labial palp with lateral process on papilla E slightly curved, with rounded apical end and reaching apex of papilla ( Fig. 11 View FIGURES 9–13 ). Outer maxillary palp with two smooth chaetae and three smooth sublobal chaetae ( Fig. 12 View FIGURES 9–13 ).
Labium chaetotaxy formed by 5 smooth chaetae (a1–a5) in anterior row; basal row with ciliated chaetae M 1 M 2 REL 1 L 2 ( Fig. 13 View FIGURES 9–13 ) with R smaller than other chaetae (ratio of R/M ~0.4). Postlabial chaetotaxy ( Fig. 13 View FIGURES 9–13 ) with 4+4 ciliated chaetae along ventral cephalic groove.
Dorsal cephalic macrochaetae formula A 0 A 2 A 3 Pa 5 ( Fig. 14 View FIGURE 14 ), but also with pair of smaller supplementary macrochaetae A 2a between A 0 and A 2; maximum number of macrochaetae An on head 13+13.
Eye patches dark blue. Diameters of eyes A–F about the same. Eyes G and H slightly smaller (A:G; A:H = 1.5). Interocular chaetotaxy ( Fig. 14 View FIGURE 14 ) with s, t, q chaetae and 2–3 intraocular scales.
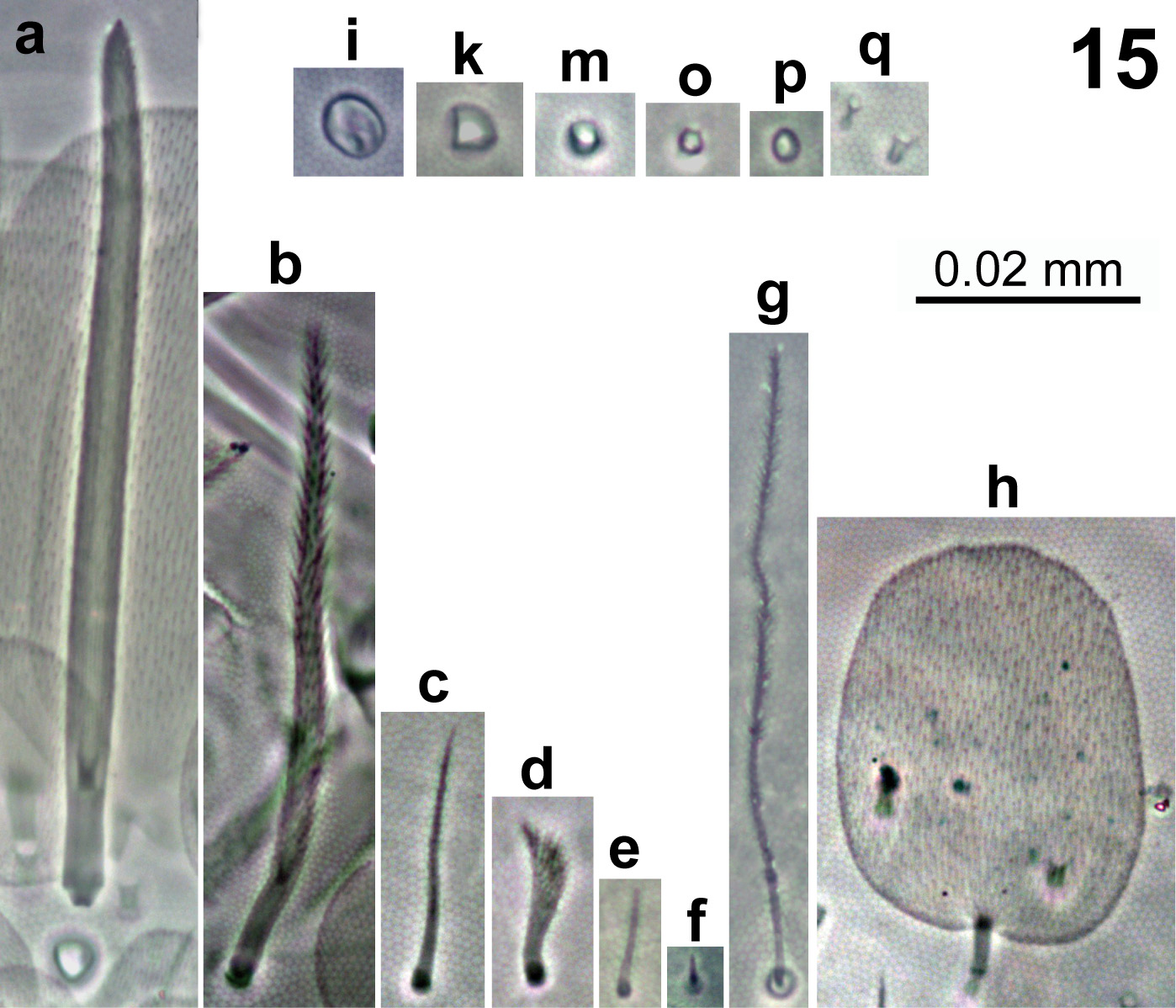
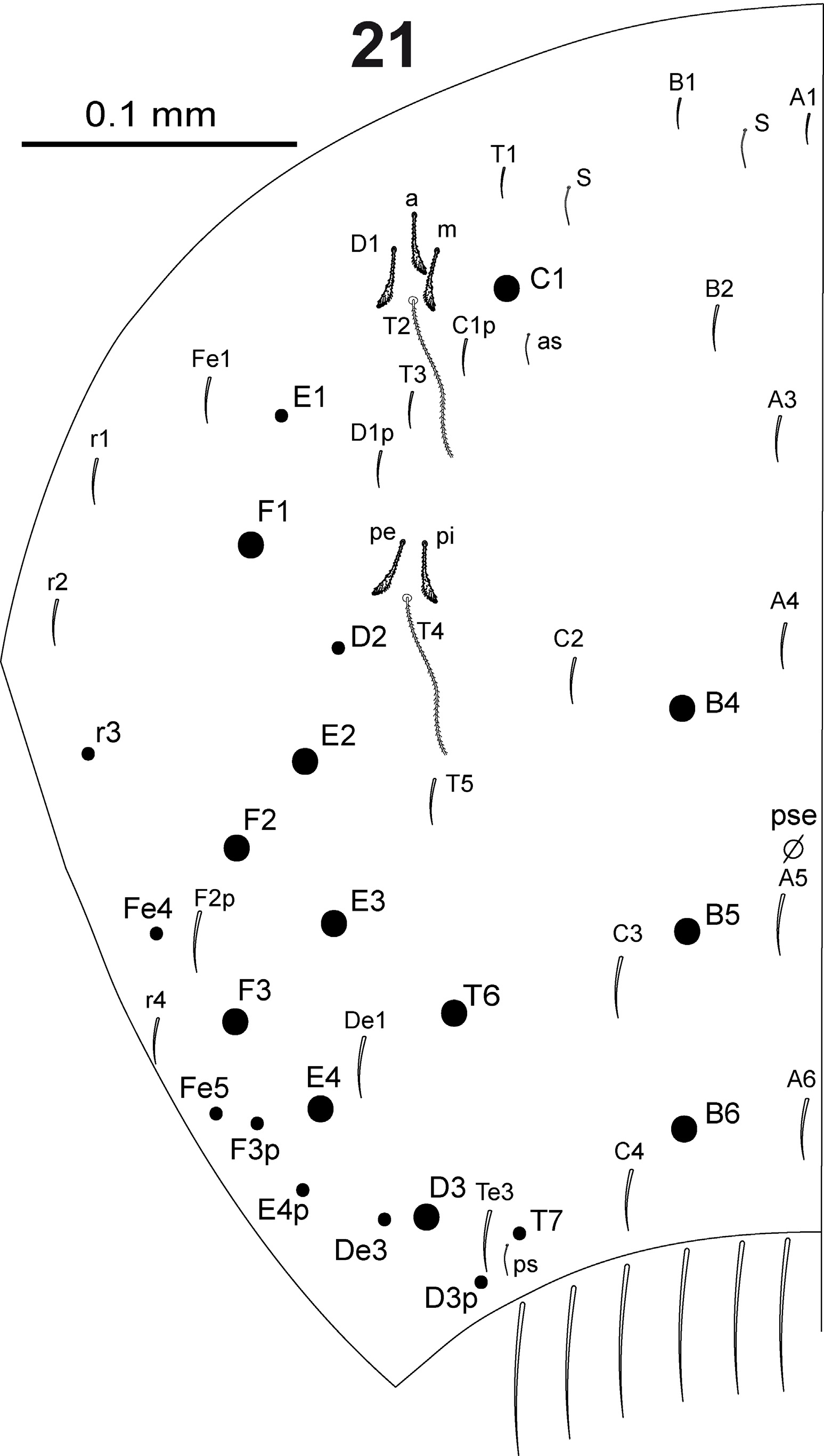
In Fig. 15 View FIGURE 15 are represented all the elements composing dorsal body chaetotaxy. Dorsal body macrochaetae formula 00/0101+3 (m3 on abd.II, C1+B4, B5, B6 on abd.IV). Dorsal chaetotaxy of th.II–III and abd.I as in Figs 16–18 View FIGURES 16–18 . Th.II with 2 lateral S-chaetae (al and ms) and without macrochaetae in dorsal position. Th.III with a lateral sensillum (al) between two ciliated chaetae. Abd.I with a lateral S-microchaeta (ms) external to a6. Chaetotaxy of abd.II–III as in Figs 19–20 View FIGURES 19–20 . Abd.II chaetotaxy between the two dorso-medial trichobothria with mesochaetae a2, a3, a2p, m3e, p4, sens as and macrochaeta m3; macrochaeta m5 with socket of similar size of macrochaeta m3. Abd.III with S-chaetae as and ms; chaeta d3 present. Chaetae associated with trichobotria on abd.II–III fan-shaped. Chaetotaxy of abd.IV as in Fig. 21 View FIGURE 21 ; macrochaetae B4, B5, B6, C1, D3, E2, E3, E4, F1, F2, F3 and T6 broader with broad socket (bcM in Fig. 15-a View FIGURE 15 ), while D2, De3, D3p, E1, E4p, F3p, Fe4, Fe5, r3 and T7 thinner with smaller socket (tcM in Fig. 15-b View FIGURE 15 ); macrochaeta F2 above macrochaeta E3; the ratio of distances between macrochaetae C1–B4 / B4–B6 1.1–1.3; accessory chaeta s associated with trichobotrium T2 absent; all chaetae associated with trichobotria on abd.IV (D1, a, m, pe, pi) fan-shaped; sens chaetotaxy composed by 2 anterior dorsomedial elongate S-chaetae (S in Fig. 21 View FIGURE 21 and Fig. 15-e View FIGURE 15 ), as and ps. Dorsal chaetotaxy of abd.V as in Fig. 22 View FIGURES 22–28 ; with S-chaetae as, acc.p4 and acc.p5.
Trochanteral organ with 12–14 smooth spiny chaetae forming a V shape pattern ( Fig. 23 View FIGURES 22–28 ). Ungues ( Fig. 24 View FIGURES 22–28 ) with paired basal teeth at 54% from inner edge, one sub-equal median tooth at 70%, and a tiny apical tooth at 86% from inner edge, respectively; three external teeth, 2 paired laterally and 1 unpaired basally, also present; unguiculi lanceolate, with denticles along outer edge, some specimens with unguiculi II-III smooth or with tiny denticles; tibiotarsal tenent hair spatulate, smooth and as long as claw; ratio of supraempodial chaeta (smooth chaeta on tibiotarsus III opposite to tenent hair) / unguiculus is around 0.7.
Ventral tube with a maximum of 5+5 ciliated chaetae on anterior side ( Fig. 25 View FIGURES 22–28 ) and 15 weakly ciliated chaetae on posterior side; lateral flap with a maximum of 18 laterodistal chaetae (6 ciliated and 12 smooth, Fig. 26 View FIGURES 22–28 ).
Manubrium with 2+2 ciliated apical chaetae on anterior side; manubrial plate with 3–4 inner chaetae and 6–9 chaetae outer to the 2 pseudopores ( Fig. 27 View FIGURES 22–28 ). Dental tubercle absent. Mucro without spinelet on basal spine ( Fig. 28 View FIGURES 22–28 ). Ratio manubrium/dens/mucro as 17.5:21.5:1.
Ecology and distribution. The specimens of L. chorus sp. nov. were found on old stone steps in the backyard of the hydropower plant ( Figs 1–2 View FIGURES 1–4 ). Limestone steps are situated at 95 m above sea level, at the bottom of a 100 m deep canyon of the river Krka, partially covered by leaf litter and fine gravel, and overgrown by biofilm. The region is characterized by warm Mediterranean climate, type Cfsa according to Köppen climate classification ( Milković & Trninić 2007), with mean air temperature when species was observed being 12.1°C for April 2015 and 13.5°C for October 2016 (data for the nearby meteorological station Knin; time period 1949–2017) (data available at http://www.meteo.hr).
All specimens mounted on slides (17 specimens) collected on 29.iv.2015 had the gut completely and exclusively full of tricolpate pollen grains possibly of Brassica L. species. This pollen was clearly visible inside the gut due to the clearing procedure with Nesbitt fluid applied to the specimens. The gut of the four mounted specimens collected on 25.ix.2018 contained plant material, fungal hyphae, conidia, pollen grains and brown amorphous material. Pollen grains of several plants were recorded in three out of four specimens but were not the dominant type of food in the gut.
This species is known only from its type locality.
Dancing behaviour. Video is available on YouTube (https://youtu.be/8pspWYQroEI) and deposited on Figshare (https://doi.org/10.6084/m9.figshare.7380836. v1). Peculiar dance-like behaviour of the L. chorus sp. nov. was observed during the sampling on 29.iv.2015 and 25.ix.2018. Two types of dance could be identified: ‘grazing dance’ and ‘walking dance’. During grazing dance individuals retain their head position while feeding and at the same time make rapid circular clockwise and counter clockwise movement of the abdomen ( Figs. 29–30 View FIGURES 29–31 , for example see video at 00:37). When finished with grazing individuals continue with walking dance where they progress with the movement in a certain direction and at the same time continue with the rapid circular movement of the abdomen ( Fig. 31 View FIGURES 29–31 , for example see video at 00:30 and 01:13). Individuals were usually few centimetres apart from each other and there was no obvious interaction between them. When coming in closer contact, they did not change their behaviour and continue with the dance, usually moving away from each other. On 07.x.2016, additional footage of this dance-like behaviour was captured with macro lenses that revealed more details. The behaviour of the specimens on this occasion was similar, although the circular movement of the abdomen was less conspicuous (for example see video at 02:22). Individuals were also observed grazing without circular movement of the abdomen. On this occasion two specimens were photographed in close interaction while grazing ( Figs 3–4 View FIGURES 1–4 ).
Discussion. Morphological characters clearly assign Lepidocyrtus chorus sp. nov. to the Lepidocyrtus lignorum -group (sensu Mateos 2011). Also, genetic sequences done by Mateos et al. (2018) clearly place the new species in this European species group. Currently L. lignorum -group is composed by 12 species (13 with the inclusion of L. chorus sp. nov.), namely L. barbulus Mateos, 2011 , L. instratus Handschin, 1924 , L. intermedius Mateos, Escuer & Álvarez-Presas, 2018 (in Mateos et al. 2018), L. juliae Mateos, 2011 , L. lignorum ( Fabricius, 1793) , L. peisonis Traser & Christian, 1992 , L. ruber Schött, 1902 , L. tellecheae Arbea & Jordana, 1990 , L. traseri Winkler, 2016 , L. uzeli Rusek, 1985 , L. vexillosus Loksa & Bogojević, 1967 and L. violaceus Lubbock, 1873 . All of them have trunk macrochaetotaxy formula 00/0101+3. The dorsal head macrochaetotaxy formula A 0 A 2 A 3 Pa 5 is also shared by all species of the group (and also by L. chorus sp. nov.) except L. intermedius (A 0 A 2 Pa 5), L. ruber (A 0 A 2 A 3) and L. vexillosus (A 0 A 2 Pa 5)
Lepidocyrtus chorus sp. nov. shares the body colour pattern with L. vexillosus (one dark spot on each side of abd.IV), but can be clearly differentiated from it because L. vexillosus lack dorsal cephalic macrochaeta A3 and, in the foot complex, has the unguis paired basal teeth in more apical position (76% from the inner edge), very reduced unguis apical tooth, and unguiculus without denticles (see Loksa & Bogojević, 1967). By the characteristic body colour pattern L. chorus sp. nov. clearly differs from all the other species of the L. lignorum -group; further differences include the morphology of abd.IV chaetae r3 (thin ciliated macrochaeta in L. chorus sp. nov. and smooth mesochaeta in all the other species) and T6 (broad ciliated macrochaeta in L. chorus sp. nov. and thin ciliated macrochaeta in all the other species). Other particular differences of L. chorus sp. nov. with each above mentioned species include: two M chaetae in labial chaetotaxy (more than two in L. barbulus ), mesothorax slightly projecting over the head (strongly projecting in L. instratus ), without ocular chaeta q and lateral pseudopores on abd.IV (present in L. juliae , unpublished data), unguiculus acuminate (truncate in L. peisonis , L. ruber and L. uzeli ), apex of third row of labral chaetae branched (simple in L. tellecheae ), abd.IV chaeta B6 broad ciliated macrochaeta (thin ciliated macrochaeta in L. traseri ), ratio of distances between macrochaetae C1–B4 / B4–B6 1.1– 1.3 (0.53–0.75 in L. lignorum and 0.61–0.72 in L. violaceus ). Also L. chorus sp. nov. clearly differs from L. barbulus , L. juliae , L. intermedius , L. lignorum , L. tellecheae and L. violaceus in the phylogenetic analyses of genes CoxII and EF-1α done by Mateos et al. (2018).
Analyses of gut content revealed definite preference of L. chorus sp. nov. for feeding on pollen of just one plant species (possibly of a Brassica species) at the time of the sampling on 29.iv.2015, while on 25.ix.2018 the gut was filled with plant material, fungal hyphae, conidia, brown amorphous material but also with pollen of several plant species. Unfortunately, filmed specimens on 07.x.2016 are not available for the study of gut content. In the detailed overview of Collembola as pollen feeders, Kevan & Kevan (1970) gave several dozen of Collembola species, among them at least three Lepidocyrtus species, that have been recorded ingesting pollen either directly by visiting flowers or probably feeding on wind-borne pollen. Pollen wall characteristics (porosity, thickness, and composition) are responsible for differences in digestibility among pollen types by animals ( Roulston & Cane 2000). Some Collembola species, like Onychiurus pseudofimetarius Folsom , can digest the pollen wall ( Scott & Stojanovich 1963), but not all species possess this digestive ability. To crack open the pollen wall mechanically is another method used to extract pollen content ( Roulston & Cane 2000), and the mandibular molar plate of Lepidocyrtus could be useful for this method of digesting pollen grains. Some pollen grains in the gut of L. chorus sp. nov. had the wall broken meaning that their content was digested. With the data available we can hypothesize that L. chorus sp. nov. feeds exclusively on pollen grains during bloom of certain plants and favors this type of food but also feed oportunistically during other seasons.
Feeding preference and occasional grazing during dancing of L. chorus sp. nov. suggest that such behaviour is probably related to feeding and searching for the wind-borne pollen deposited on the stone surface and possibly other type of food. In support comes the fact that during dancing no courtship or spermatophore transfer has been observed and that subadults and juvenile specimens have also been observed dancing, leading to conclusions that it is not related to mating. Somewhat similar dance related to search for food is described for the blowfly Phormia regina (Meigen) , where specimens perform a series of loops and spirals rather than straight line movement when they come in contact with the sugar (Dethier l957, Nelson 1977).
To our knowledge, this is the first record of peculiar dancing behaviour related to feeding among Collembola . Laboratory experiments on L. chorus sp. nov. is needed to confirm that the dance is triggered by the presence of certain type of food and exclusively related to feeding.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lepidocyrtus chorus Mateos & Lukić
| Mateos, Eduardo & Lukić, Marko 2019 |
Lepidocyrtus chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. chorus
| Mateos & Lukić 2019 |
L. intermedius
| Mateos, Escuer & Alvarez-Presas 2018 |
L. traseri
| Winkler 2016 |
L. barbulus
| Mateos 2011 |
L. juliae
| Mateos 2011 |
L. barbulus
| Mateos 2011 |
L. juliae
| Mateos 2011 |
L. peisonis
| Traser & Christian 1992 |
L. tellecheae
| Arbea & Jordana 1990 |
L. tellecheae
| Arbea & Jordana 1990 |
L. uzeli
| Rusek 1985 |
L. vexillosus
| Loksa & Bogojevic 1967 |
L. vexillosus
| Loksa & Bogojevic 1967 |
L. instratus
| Handschin 1924 |
Onychiurus pseudofimetarius
| Folsom 1917 |
L. ruber
| Schott 1902 |
L. violaceus
| Lubbock 1873 |
L. violaceus
| Lubbock 1873 |
Lepidocyrtus
| Bourlet 1839 |
Lepidocyrtus
| Bourlet 1839 |