Macrobiotus halophilus, Fontoura, Paulo, Rubal, Marcos & Veiga, Puri, 2017
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4263.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:093CCBCF-E709-4C9D-8697-BF18BFC635F1 |
|
DOI |
https://doi.org/10.5281/zenodo.6015871 |
|
persistent identifier |
https://treatment.plazi.org/id/03BF87BA-0C55-756D-B7C9-A8A43219FDD7 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus halophilus |
| status |
sp. nov. |
Macrobiotus halophilus View in CoL sp. nov.
( Figures 2 View FIGURE 2 C, D, 5, 6, 7; Tables 3, 4)
Material examined. Holotype (slide C. VII-54); 29 paratypes (18 animals, slides C. VII-45 to C. VII-53, and 11 eggs, with two embryonate, slides C. VII-54 to C. VII-59).
Type repository. Holotype and paratypes are deposited in the Department of Biology of the Faculty of Sciences, University of Porto, Portugal (collection P. Fontoura).
Type locality. 38°41'31.79"N; 9°26'8.75"W, Cascais , Boca do Inferno, Portugal .
Specific diagnosis. Macrobiotus of the M. hufelandi -group with eyes. Egg processes in the shape of inverted chalices with dentate distal disc. Egg shell slightly wrinkled ( M. persimilis subgroup). Cuticle smooth with small and sparse circular pores. Pharynx with two macroplacoids (1>2) and microplacoid. First macroplacoid with a deep central constriction. Mouth cavity with an almost imperceptible anterior band of very small teeth; a posterior band of teeth (ventral teeth, larger than dorsal), and three dorsal and three ventral transversal ridge-shaped teeth. Claws of moderate size with accessory points. Smooth lunules on legs I–III and dentate on hind legs. All legs with granulation. Mature males with two lateral gibbosities on hind legs.
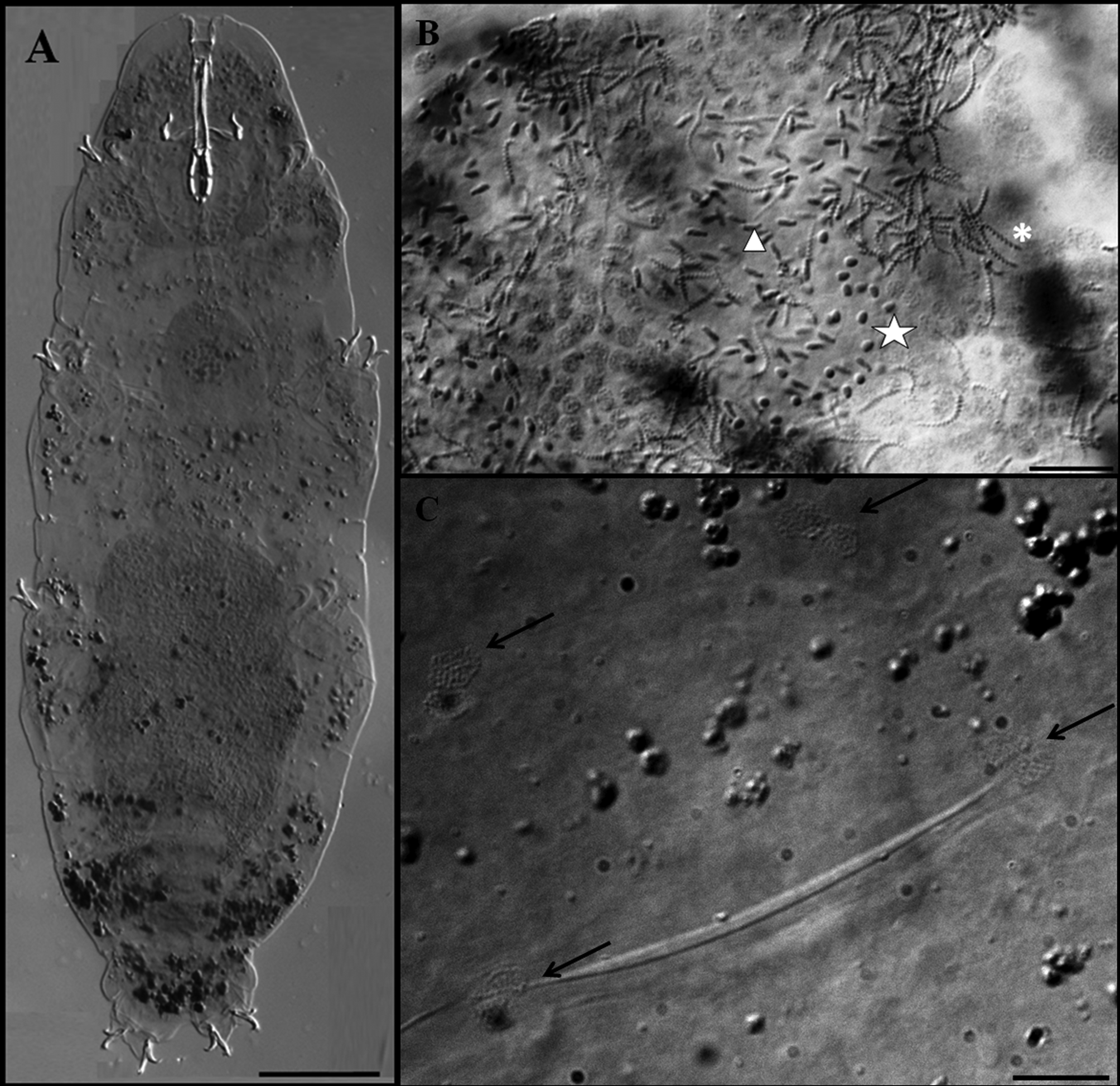
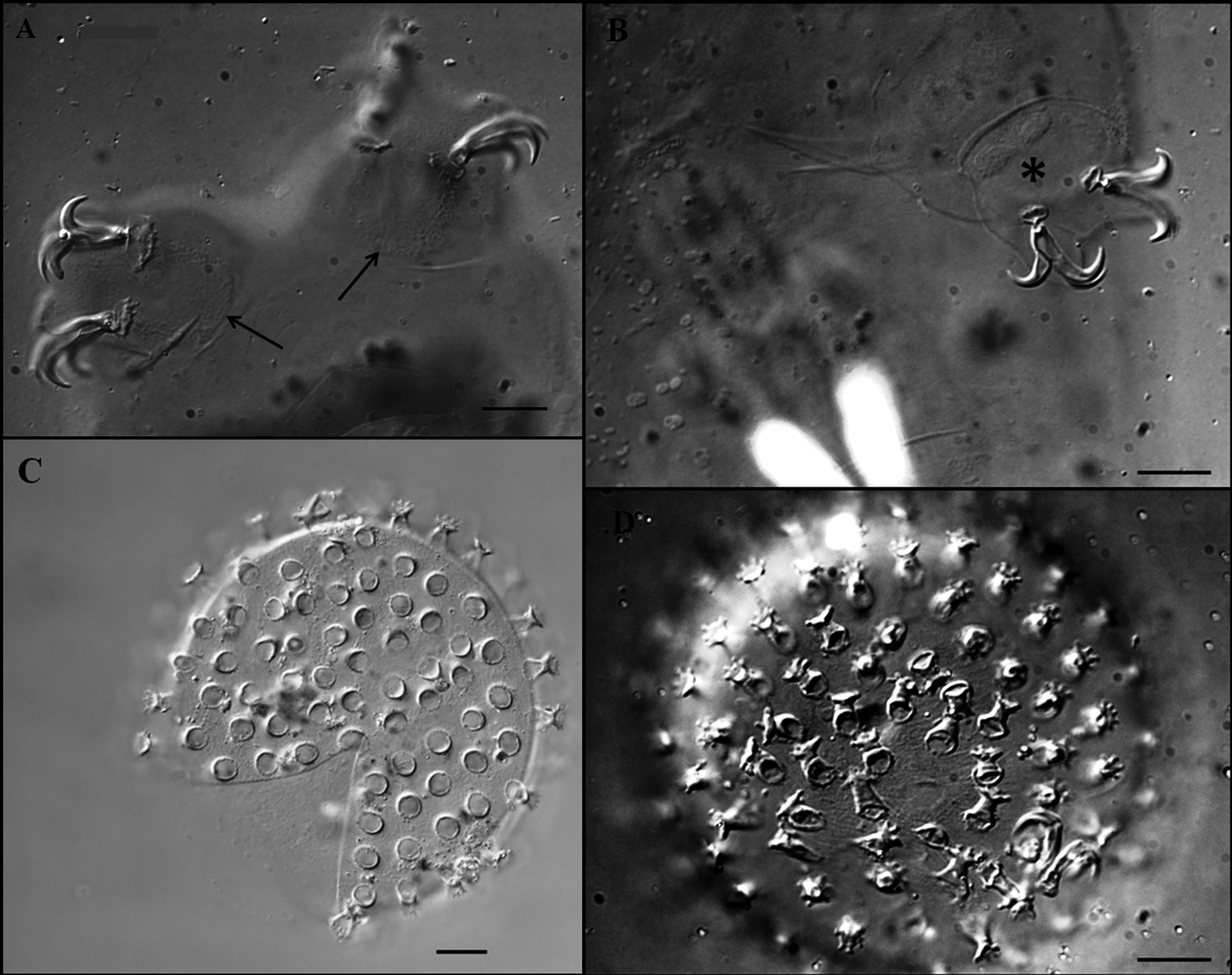
Description of the holotype. Colourless male with a body length of 470 µm (table 3). Eyes present. Cuticle smooth except for tiny granulation on all legs. Small and very sparse circular pores (diameter 0.5–0.8 µm) are present. Two lateral gibbosities present on hind legs ( Figs 5 View FIGURE 5 A; 6A, arrows). Granular nodes ( Fig. 5 View FIGURE 5 C, arrows) corresponding to muscle attachment points are only clearly visible under DIC, arranged in a distinct pattern organised into eleven ventral and fifteen dorsal rows ( Figs 2 View FIGURE 2 C–D). Bucco-pharyngeal apparatus of the Macrobiotus type. Mouth terminal surrounded by ten peribuccal lamellae. Buccal armature consists of three bands of teeth. The first band, in an anterior position, is almost imperceptible and consists of several rows of very small teeth. The second band comprises 3–4 irregular rows of granular teeth, and the third band of three almost fused dorsal and three ventral transverse, ridge-shaped teeth ( Figs 6 View FIGURE 6 C, D arrows). Ventral teeth of the second band are larger than the dorsal teeth ( Figs 6 View FIGURE 6 C, D arrows). In the third band, the ventro-median ridge-shaped tooth is shorter than the dorsomedian, which is slightly ragged, giving the impression that it has been combined from several smaller, fused teeth. Buccal tube 39.3 µm long and 6.0 µm wide ( Fig. 6 View FIGURE 6 C). Stylet supports inserted on the buccal tube at 79.4 % of its length. Ovoid pharyngeal bulb with grain-like apophyses, two rod-shaped macroplacoids and microplacoid. The first macroplacoid is much longer than the second (1>2) and has a very deep central constriction ( Fig. 6 View FIGURE 6 C, asterisk). The caudal part of the second macroplacoid has a constriction ending by a sub-spherical swelling. A small microplacoid present ( Fig. 6 View FIGURE 6 C).
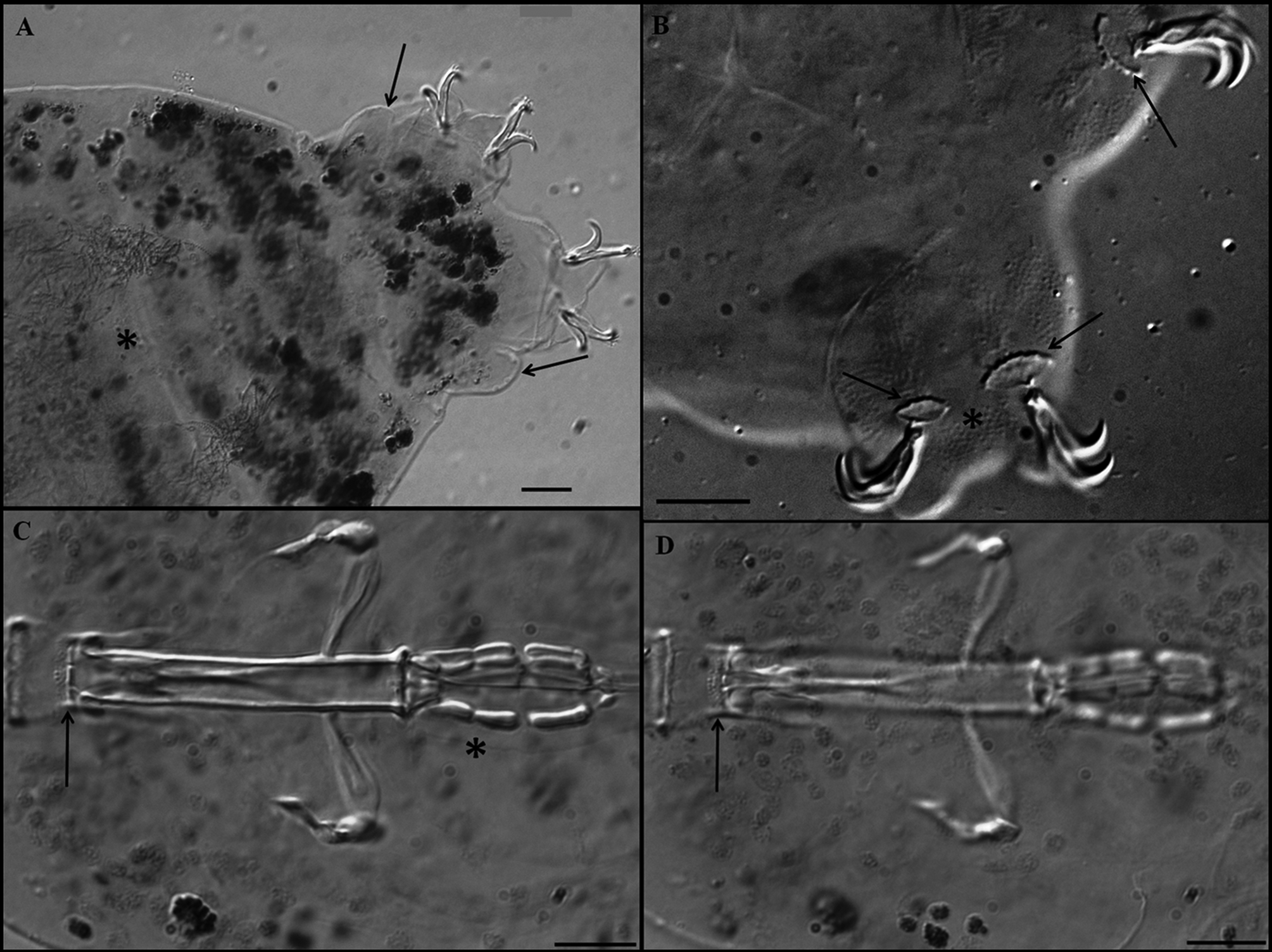
Claws robust of the hufelandi - type, with well-developed accessory points on main branches ( Figs 7 View FIGURE 7 A, B). External and internal claws of similar size slightly increasing in length from the first to the fourth pair of legs. Smooth lunules are present at the base of claws on legs I–III ( Fig. 7 View FIGURE 7 B). On legs IV lunules are larger and with dentate margins ( Figs 6 View FIGURE 6 B, arrows, 7A), and are connected by a U-shaped granular patch ( Fig. 7 View FIGURE 7 A, arrows). Faintly sclerotised and slightly protruded areas on legs I–III are present ( Fig. 7 View FIGURE 7 B, asterisk) (only under DIC).
Eggs ( Figs 7 View FIGURE 7 C, D; table 4): free laid, whitish, spherical with a diameter of 66.1–86.0 µm without processes and 75.7–95.0 µm including. Egg processes in the shape of an inverted chalice with a base diameter 4.1–6.3 µm, and 3.9–5.9 µm long. The distal discs are clearly dentate (each disc with 6–12 teeth which are 0.9–1.4 µm long). The diameter of distal discs, 3.7–5.6 µm, is slightly smaller than the base diameter. There are 22–36 processes on the egg circumference. The inter-process egg surface is slightly wrinkled (only under DIC).
Remarks. Measurements and statistics of structures obtained for specimens of the new species are provided in table 3, and raw data as Supplementary Files.
Acetic-orcein stained specimens showed that the presence of gibbosities on legs IV was restricted to mature males with testis having spermatogonia, spermatids and spermatozoa ( Figs 5 View FIGURE 5 B, 6A). Thus, in accordance to the observations of Rebecchi & Bertolani (1994) on Macrobiotidae , M. halophilus can be considered a dioecious species, and the presence of gibbosities on hind legs a secondary sexual character.
In the same sample, M. halophilus sp. nov. was found jointly with Milnesium tardigradum .
Etymology. The name halophilus , from the Greek halo = salt + philia (a Latin transliteration of the Greek, meaning “friendship”) is attributed to the organisms that exhibit tolerance to high concentrations of salt and can grow under saline conditions, as it seems to be the case of the new species.
Differential diagnosis. Within the Macrobiotus hufelandi group, the M. persimilis subgroup are species that lay free eggs with processes in the shape of an inverted chalice that have smooth or wrinkled (but without pores or dots) chorion surface. Six species, excluding the new species, can be attributed to the M. persimilis subgroup: M. persimilis Binda & Pilato, 1972 ; M. patagonicus Maucci, 1988 ; M. polonicus ; M. marlenae Kaczmarek & Michalczyk, 2004 ; M. kazmierskii Kaczmarek & Michalczyk, 2009 and M. anemone Meyer, Domingue & Hinton, 2014 .
The dentate lunules on hind legs make M. halophilus sp. nov. most similar to M. persimilis , M. polonicus and M. marlenae .
Characteristics of the eggs Mean ± SD (Range; N) Diameter without processes 76.6± 5.5 (66.1–86.0; 11) Diameter with processes 87.7 ± 5.4 (75.7–95.0; 11) Number processes on circumference 28.5 ± 4,5 (22–36; 11) Processes base 4,9± 0.6 (4.1–6,3; 29)
Processes length 4,8± 0.5 (3.9–5.9; 29)
Diameter of processes distal disc 4.5 ± 0.5 (3.7–5.6; 31) Distance between processes 3.8 ± 0.8 (2.0–5.7; 30) Number of teeth on the process disc 9.4± 1.5 (6–12; 19) The new species clearly differs from M. marlenae by: 1. the presence of a longer first macroplacoid (pt 23.4–31.3 in the new species; pt 17.1–22.2 in M. marlenae ); 2. laying eggs with more numerous egg processes (22 – 36 processes in the circumference in the new species; c. 16 in M. marlenae ), smaller (process base 4.1–6.3 µm and process length 3.9–5.9 µm in M. halophilus sp. nov., and respectively 6.5–6.9 and 8.4–8.8 µm in M. marlenae ); 3. the egg processes having smooth surfaces in the new species, (with a series of undulations beneath the terminal discs in M. marlenae ); and 4. smaller terminal discs (3.7–5.6 µm in diameter in the new species; 9.5–11.4 µm in M. marlenae ).
While the eggs of M. halophilus sp. nov. are very similar to the eggs of M. persimilis , adult specimens differ by the narrower buccal tube of the new species (pt 9.6–12.5 in the new species; pt 13.1–20.3 in M. persimilis ), granulation on legs I–III (lacking in M. persimilis ), and much smaller and less evident cuticular pores.
Macrobiotus polonicus is the most similar species and shares with M. halophilus sp. nov. the presence of two lateral gibbosities on the hind legs. In M. halophilus sp. nov. these gibbosities are only exhibited by mature males, representing a secondary sexual character (Rebecchi & Bertolani 1994). Thus, the new species can be considered amphimictic. According to the description of M. polonicus these gibbosities were present in all examined specimens, which would suggested a parthenogenetic mode of reproduction; unless all the examined specimens of M. polonicus were males. The aspect this character, therefore, needs to be confirmed for M. polonicus . The two species can be distinguished by: 1. the presence of strongly granulated legs I–III in the new species (cuticle on legs I–III in M. polonicus is smooth); 2. having less abundant and smaller circular cuticular pores (0.5–0.8 µm in diameter in the new species; circular or elliptical, 1.0x2.0 µm, in M. polonicus ); and 3. via morphometric differences - despite the small sample size, after normalising for buccal tube length, the buccal tube is significantly wider in the new species; claws are consistently longer, and placoids are slightly shorter (see table 5).
Lichens are very common habitats for tardigrades in terrestrial environments ( Kinchin 1994, Nelson et al. 2015) but, the strong influence of marine environment on the supralittoral certainly transform them into a very distinct transitional habitat that may require specific adaptations for organisms to survive (see Møbjerg et al. 2011, Glime 2013).
Milnesium tardigradum , also recorded from the supralittoral zone ( King et al. 1981), and Macrobiotus hufelandi View in CoL (both considered as senso lato) are eurytopic species (Nelson et al. 2015) and had previously been considered cosmopolitan ( Ramazzotti & Maucci 1983, McInnes 1994, Pilato & Binda, 2001). However, with the advances in tardigrade taxonomy, those species have been split into many similar species ( Bertolani & Rebecchi 1993, Tumanov 2006, Michalczyk et al. 2012). In this study, we found a Milnesium species for which the morphological attributes perfectly match those of M. tardigradum (senso stricto, as re-described by Michalczyk et al. 2012). Our new species, Macrobiotus halophilus View in CoL sp. nov., is the seventh species to be described as belonging to the M. persimilis View in CoL subgroup within the M. hufelandi View in CoL group.
Ramazzottius oberhaeuseri View in CoL has also been reported from the supralittoral zone ( King et al. 1981) and has a broad geographical distribution ( Ramazzotti & Maucci 1983, McInnes 1994, Biserov 1997 /98). This is a xerophilous species that is of small size, has a thick tuberculated and pigmented cuticle, and a great ability for tun formation. These are all adaptations that offer UV protection, and provide survival mechanisms for coping with desiccation, and fluctuations in temperature and salinity (see Glime 2013). It is therefore suitably adapted to survive the extreme environmental conditions imposed on the flora and fauna of the supralittoral zone.
Our description of a new Ramazzottius View in CoL species from the supralittoral zone now questions the identification of previous records of R. oberhaeuseri View in CoL from similar habitats and we suggest these records should be re-examined. Ramazzottius littoreus sp. nov. is not the only Ramazzottius View in CoL species from the supralittoral zone. Another coastal species, R. rupeus View in CoL (also similar to R. oberhaeuseri View in CoL ), was collected from lichen on a rock 50 m from the coast of the Barents Sea and 40 m above sea level ( Biserov 1999). These results confirm the reports by several authors that R. oberhaeuseri View in CoL is a complex of species (e.g. Biserov 1997 /98, Kaczmarek et al. 2006, Pilato et al. 2013, Dastych, 2011), each with narrow ecological requirements and strictly adapted to limited environmental conditions.
For taxonomic purposes, special attention to the pattern of muscle arrangement has been given in this study. It has been shown that the muscular architecture and the patterns of muscle arrangement are very similar among all eutardigrades, especially the ventral muscle group anatomy ( Schmidt-Rhaesa & Kulessa 2007; Marchioro et al. 2013). However, according to Marchioro et al. (2013), the dorsal muscle group does show species specific differences, which concurs with the results of this study. This potentially useful taxonomic character of muscle arrangement pattern is not available for most of the known eutardigrade species, and the value of this character should be explored in the future.
Finally, our results indicate that supralittoral lichens are habitats for both marine and limnoterrestrial tardigrades (e.g. Kinchin, 1992; Kaczmarek et al. 2015), and could harbour a very specific tardigrade fauna. However, further studies on the diversity of tardigrades in supralittoral lichens from a variety of regions is required before a more detailed analysis can be made of the habitat type and the uniqueness of the species compositions.
With the description of these two new species, the number of limnoterrestrial tardigrades species from the Iberian Peninsula is raised to 127.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.