Naultinus flavirictus, Hitchmough & Nielsen & Lysaght & Bauer, 2021
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4915.3.7 |
|
publication LSID |
lsid:zoobank.org:pub:4B0B7EDD-7BED-4C23-948A-7C9467C52C65 |
|
DOI |
https://doi.org/10.5281/zenodo.4495410 |
|
persistent identifier |
https://treatment.plazi.org/id/52E975BD-A361-4B5D-B247-0958768B48F4 |
|
taxon LSID |
lsid:zoobank.org:act:52E975BD-A361-4B5D-B247-0958768B48F4 |
|
treatment provided by |
Plazi |
|
scientific name |
Naultinus flavirictus |
| status |
sp. nov. |
Naultinus flavirictus sp. nov.
( Fig. 1 View FIGURE 1 )
Naultinus grayi : (part) Robb & Hitchmough (1980).
Naultinus “North Cape”: Hitchmough et al. (2007).
Holotype. Adult female holotype MONZ RE003322, original field number G.789 ( Fig. 1 View FIGURE 1 ), collected from Te Paki, Northland, New Zealand, collected by A. H. Whitaker in May 1972.
Paratypes. Two adults, one male, MONZ RE.005611 (original field number RAH195 ), collected from the Te Kao District , Northland, New Zealand, by Conrad Hepers in August 1986 , and one female, MONZ RE.005609 (original field number RAH254 ; captive offspring of a female collected from Te Kao, Northland, New Zealand, by Conrad Hepers in August 1986) .
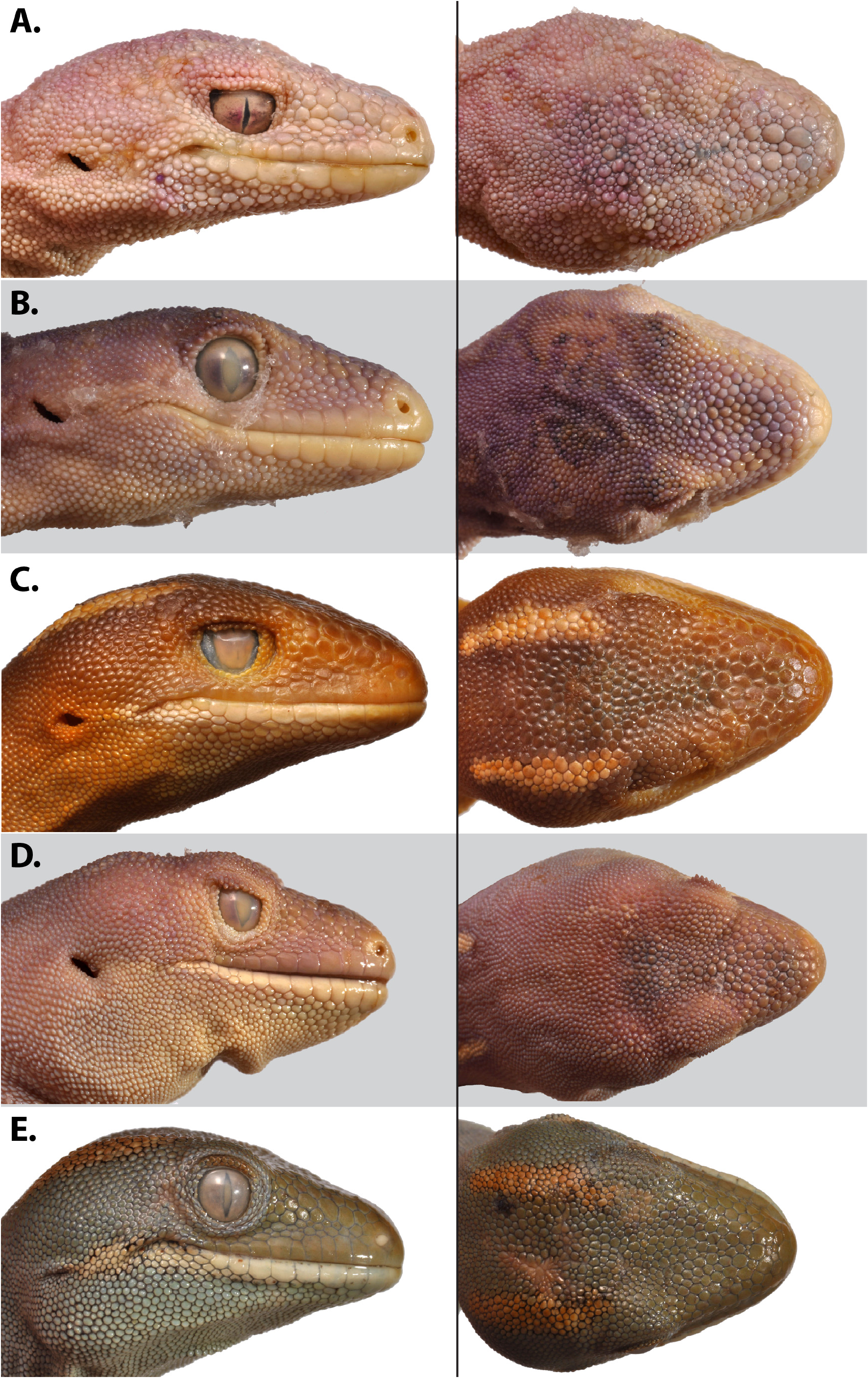
Etymology. Naultinus flavirictus sp. nov. (formed from the Lain flavus = yellow + rictus = gaping of the mouth) is named for the continuation of the yellow-orange colouring of the jaws and tongue onto the external corners of the mouth, which is diagnostic for the species. The epithet is formed as a noun in apposition.
Diagnosis. Naultinus flavirictus sp. nov. is a small Naultinus (maximum SVL ~ 70 mm), with a short, deep snout, fine granular body scales, flattened scales on the dorsum of the snout, rostral scale divided by a median crease, pale lavender mouth lining, and yellow-orange jaws, tongue and external corners of the mouth.
It may be distinguished from all other North Island Naultinus species by the pale lavender mouth lining, with yellow-orange jaws and tongue, and by the colour of the jaws extending to the external corners of the mouth, with similar pigment present around the nostrils. A central crease dividing rostral in two is typical in this species, but rare in N. grayii and N. elegans , and partial or absent in other Naultinus . It is distinguished from N. grayii and N. punctatus by much smaller adult size (to ~ 70 mm vs ~ 95 mm SVL), and by secondary bluish colouration of adult males including the entire ventral surface rather than being confined to a lower lateral band. It is further distinguished from N. grayii by its proportionally shorter, deeper snout ( Fig. 2 View FIGURE 2 ). Like N. grayii , but unlike all other Naultinus , the scales on the dorsal surface of the snout are flattened ( Fig. 2 View FIGURE 2 ).
The new species may be distinguished from most South Island Naultinus by its fine, granular dorsal body scales (coarser and more conical in South Island species). Among its South Island congeners it is readily distinguished from N. rudis (Fischer) , N. tuberculatus and Otago / Southland populations of N. gemmeus by the pale lavender and orange mouth lining. It differs from N. manukanus and N. rudis in the absence of any greatly enlarged dorsal body and head scales (although there is a north–south cline in the number and prominence of these enlarged scales within N. manukanus , and they may be few in number and easily missed in the northernmost population on Stephens Island; this population is morphologically similar to north-western-most populations of N. stellatus ) ( Bull & Whitaker 1975). It may be distinguished from Canterbury populations of N. gemmeus by its smaller adult size, and by males having bluish belly colouration rather than having the green colouration progressively replaced by brown or grey during growth to maturity. It is most similar morphologically to north-western-most populations of N. stellatus , but distinguished by more subimbricate, slightly flattened granular (vs strongly domed, bead-like, tightly juxtaposed) dorsal body scales, less domed canthal scales ( Fig. 2 View FIGURE 2 ), presence of an enlarged patch of supratemporal scales, and rows of enlarged, equal-sized scales each side of the canthal row in N. flavirictus sp. nov. (vs irregular-sized scales bordering enlarged canthal row in N. stellatus ). Naultinus flavirictus sp. nov. is distinguished from other populations of N. stellatus by its uniform green background colour, with or without simple, discrete, irregular-shaped spotted or striped markings rather than large, circular blotches with concentric rings of colour.
Description of the holotype. Body moderately slender, not depressed; 67.1 mm SVL. HeadL 16.6 mm (25% SVL), HeadW 12.1 mm (73% HeadL) and HeadH 8.8 mm (53% HL). Head subtriangular with a blunt, rounded snout, clearly demarcated from neck; interorbital/frontal region neither concave nor convex, nasal region without indentation; canthus not inflated. SnEye 7.5 mm (45% HeadL), OrbD 3.4 mm (45% SnEye). Scales on dorsum of anterior snout approximately 3-4 times the diameter of those on occipital region, loreal scales similar, abruptly decreasing in size at level of anterior edge of orbit; patches of enlarged supratemporal scales (smaller than dorsal snout and loreal scales but distinctly larger than occipital granules). OrbD 20% HeadL; pupil oval, margins smooth. Ear opening very small (1.1 mm), oval, obliquely oriented; eye to ear distance greater than diameter of eyes (EyeEar 5.6 mm (165% OrbD). Rostral almost rectangular, much broader (3.0 mm) than high (1.3 mm) completely divided by a strong rostral crease, contacted dorsally by one large round internasal and two large supranasals, each similar in size to the internasal, contacted laterally by first supralabial, excluded from nostril. Nostrils rounded, laterally oriented, and surrounded by two postnasals on right hand side, three on left, first supralabial and first supranasal. Mental subtriangular, broader (2.8 mm) than tall (1.6 mm). First infralabials separated by three postmental, the central one largest and oval, longer than wide, the others wider than long. Anterior infralabials and postmental bordered posteriorly by 3–4 series of enlarged hexagonal chin shields, ¼ to ½ the size of central postmental chin shield, changing abruptly to small, rounded scales beneath throat. Scale rows ventral to posterior infralabials on lateral surface of jaw slightly enlarged and elongated relative to ventral throat scales. Eleven (right) and 10 (left) enlarged infralabial scales, posterior most about 4 times size of rictal scales, 10 (left)–12 (right) enlarged supralabial scales, 7 (left) or 10 (right) supralabials to midpoint of orbit; 36 scale rows between supraciliaries (24 between supraocular skin folds), 11 scale rows across frontal bones at midpoint of orbit. Supraciliaries rounded to somewhat conical posteriorly.
Dorsal scales small, mostly uniform in size with scattered much smaller scales interspersed, homogenous in shape, bead-like, somewhat flattened, granular. Ventral scales roughly twice diameter of dorsals, smooth, flattened, subimbricate, slightly enlarged in precloacal region. Approximately 99 scale rows around mid-body. No ventrolateral skin folds. Scales on dorsal surface of upper forelimb roughly equal in size to dorsum; on forearm slightly smaller. Scales on preaxial surface of thigh similar to those on dorsum, gradually decreasing in size posteriorly to smaller granules on post-axial surface. Scales on palms and soles smooth, flattened and subimbricate. Fore- and hindlimbs short and moderately robust (Forearm 8.3 mm; ForeaL/SVL 0.12; Crus 11.1 mm; CrusL/SVL 0.17). Digits relatively elongate, all bearing claws. Claws short and moderately robust basally, recurved to a fine point. Basal portion of digits relatively narrow, approximately twice width of distal phalanges. Distal portion of toe strongly compressed, lamellae continuing in uninterrupted series to claw. Apical pads absent. Relative length of digits of manus: I<II<III<V<IV, and of pes: I<II<V<III<IV; no webbing between digits. Lamellae straight or very weakly bowed, unpaired. Lamellar counts (including all scales> twice size of scales at base of digits) from right (and left) sides 7-10-13-14-11 (6-9-14-14-11) manus and 7-13-14-16-15 (7-13-15-17-15) pes.
Only scattered pitted scales present in precloacal area (c. 5 rows). Small, weakly developed cloacal spurs with 3 enlarged scales on each side just posterior to vent. Tail original but broken (9.5 +79.7 = 89.2 mm); 133% snoutvent length, muscular, tapering, round in cross-section. Dorsal caudal scales medium, flat, juxtaposed to weakly subimbricate, roughly circular, arranged irregularly. Surface of tail not segmented. Ventral scales slightly larger than dorsal, smooth, somewhat irregular in shape, imbricate.
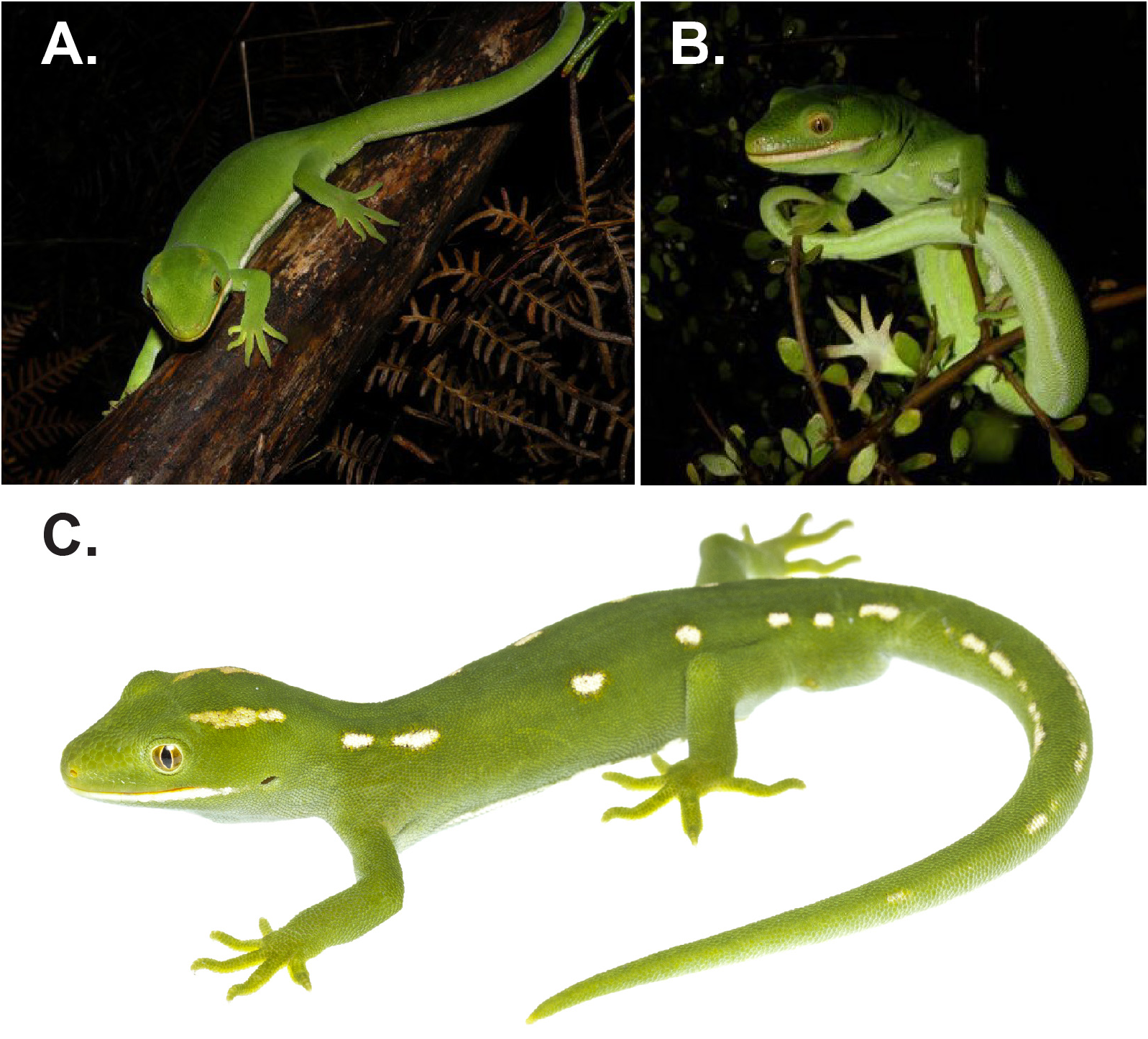
Colour in preservative. Dorsum uniform dull dark green with dorso-lateral rows of irregularly shaped pale cream spots, comprising paired longitudinal streaks behind the eyes, seven spots on each side between the neck and the pelvic region; spots continue along the tail where they become elongated into streaks. Uniform whitish ventro-lateral stripes between insertions of fore and hind limbs separating dark green lateral from pale green ventral surface. Small white blotch of c. 7 scales on dorsum of left thigh. Lower lip with strong white marking extending almost to earhole ( Figs. 1 View FIGURE 1 , 2 View FIGURE 2 ). Faint yellow colouration around nostrils. Undersides of feet and toes similar in colouring to venter.
Colour in life. (from paratypes, supplemented by non-vouchered photographs of other wild individuals – Fig. 3 View FIGURE 3 ). As in preservative, except that the basic colour is very bright green rather than dull dark green. Pattern of spots or stripes is variable among individuals, or can be absent. There is sexual dichromatism, with adult and subadult males having the ventral surface and lower lateral surface very pale blue; these areas are light green in females. In a few individuals the background bright green colouration is replaced by bright yellow (P. Miller pers. comm.; van Winkel et al. 2018). Pale lavender mouth lining, with yellow-orange jaws and tongue, with the colour of the jaws extending to the external corners of the mouth, and with similar pigment present around the nostrils.
Paratype variation. Both MONZ RE.005611 and MONZ RE.005609 are very similar in appearance to the holotype. They differ in the extent of pale dorsal markings, and presence of mid-lateral markings in MONZ RE.005611), number and arrangement of internasals and postmentals, and have minor differences in numbers of infralabials and supralabials, scale rows between supraciliaries, scale rows across frontal bones at midpoint of orbit, lamellae counts and numbers of precloacal pores (which are more strongly developed in the male paratype).
MONZ RE.005611 has a small, diamond-shaped internasal, posterior to which are a pair of considerably larger, hexagonal internasals, which exclude the small central one from contact with the supranasals. Four postnasals on left, three on right. Two hexagonal postmentals. Chin shields ¼ to same size as postmentals. 11 (left), 10 (right) infralabials. 13 (left), 11 (right) supralabials. 9th (left), 8th (right) in mid-orbital position. 39 scale rows between supraciliaries (29 between supraocular skin folds), 10 scale rows across frontal bones at midpoint of orbit. Dorsal and particularly lateral scales weakly subimbricate. Lamellar counts (including all scales> twice size of scales at base of digits) from right (and left) sides 5-12-13-14-10 (6-11-13-14-12) manus and 7-13-15-14-15 (7-13-14-14-13) pes. Precloacal pores 112 in total, in 5 rows, 3 extending onto thigh, 2 reaching mid-point of thigh. Scale row immediately posterior to precloacal spores includes 5 pitted scales – not included in count. Four left and three right, enlarged, raised and sharply pointed cloacal spurs. Colour in preservative deep lavender dorsally, becoming paler on flanks and limbs, body with paired, mostly symmetrical series of cream-coloured markings, fragmented into several elongate lines on left side, series of more variable lines and blotches on right side, extending onto tail as two broken lines; head with a pair of crescentic supratemporal markings, a large marking extending between left and right antorbital positions, narrowing in the interorbital region and expanding again into a blunted arrowhead shape on the parietals. All except last marking outlined by a thin, more or less continuous black line. Narrow cream line extending from infralabials almost to ear opening. Ventrolateral marking as in holotype. Small scattered white patches on posterior flanks (one on left, two on right). Limbs without markings. Venter immaculate. Dorsum and venter of digits pale straw colour.
MONZ RE.005609 has a single hexagonal internasal and three postnasals on both left and right sides. Two irregular postmentals, right three times size of left. Chin shields 1/3 to same size as postmentals. 11 (left), 12 (right) infralabials. 13 (left), 14 (right) supralabials. 10th (left), 11th (right) in mid-orbital position. 37 scale rows between supraciliaries (27 between supraocular skin folds), 11 scale rows across frontal bones at midpoint of orbit. Dorsal and particularly lateral scales weakly subimbricate. Lamellar counts (including all scales> twice size of scales at base of digits) from right (and left) sides 5 (other digits damaged) (5-9-10-11-10) manus and 5-12- damaged-damaged-14 (6-damaged-damaged-14-damaged) pes. Six rows of pitted scales (c. 75 total) only one extending onto base of thigh. Four left and three right, enlarged, raised and sharply pointed cloacal spurs. Colour in preservative pale lavender, only visible dorsal markings are faded, slightly bowed, whitish supratemporal markings.
Geographic distribution. The northern half of the Aupōuri Peninsula, the peninsula at the northern extremity of the North Island of New Zealand. Most records are from the Te Paki area in the far north (a general distribution map is provided by Nielsen et al. 2011). The gecko is found on the northern half of the peninsula, from about Houhora northwards. Exact locality details are not provided to discourage illegal collection, which is a well-documented threat to New Zealand’s native herpetofauna ( Nelson et al. 2014; Hitchmough et al. 2016) and globally (Bryan et al. 2006).
Biogeography. The paleo-islands at the northern end of the peninsula are formed of Cretaceous ophiolitic (basalt, gabbro and serpentinite) and Lower Miocene marine sedimentary rocks ( Brook & Thrasher 1991). These paleo-islands were probably emergent above sea-level from Late Miocene or Early Pliocene time. During Early to Mid Pleistocene time extensive deposits of marine and aeolian sand accumulated north of Kaitaia; paleo-islands probably existed in the Reinga-North Cape and Houhora areas during interglacial periods (i.e., high sea levels), but a proto-Aupouri Peninsula would have existed during glacial low stands. Dune buildup during Late Pleistocene time formed the present land connection along the Peninsula. Thus, the far northern islands were probably isolated throughout the Pliocene, and were intermittently connected with the rest of Northland during the Pleistocene, with the connection becoming permanent in the Late Pleistocene ( Brook & Thrasher 1991).
Although we did not perform specific dating analyses per se, if we assume a mutation rate for ND2 of 0.65% per million years (as proposed by Macey et al. 1998), the ~4% mitochondrial distance separating N. flavirictus sp. nov. from its closest kin (Table S01) correlates to ~2.5 million years. It is therefore reasonable to infer that the ancestors of Naultinus flavirictus sp. nov. reached the Te Paki area while it was an island or series of islands and evolved there in isolation until the formation of the Aupōuri tombolo enabled it to come back into contact with N. grayii during the Pleistocene. Contact zones with N. grayii may have repeatedly formed and then been removed by inter-glacial sea-level rise, and the current contact is likely to have formed in the Late Pleistocene.
Reproduction. Animals collected from Te Kao in August 1986 then held in captivity in Auckland gave birth to twins in late January 1987 (C. Hepers, pers. comm.). This is almost two months earlier than births of N. grayii , both in the field and in captivity in Auckland ( Robb & Hitchmough 1980).
Conservation status. This species is listed by Hitchmough et al. (2016) under the New Zealand Threat Classification System as “At Risk – Declining” on the basis of criterion C (2/1) (total area of occupancy> 100 km 2, predicted decline 10–70%), and with the Qualifiers Data Poor and One Location. The One Location qualifier means that the species occupies a single area of continuous habitat, so a new threat could very quickly spread to affect the entire population.
Under the IUCN Redlist system, we suggest the species is Endangered under the criteria B1a,b(v), B2a,b(v): Geographic range in the form of (B1) extent of occurrence estimated to be (1) less than 5,000 km 2, and (a) estimates indicating severely fragmented or known to exist at no more than five locations and (b) continuing decline, observed, inferred or projected, in (v) number of mature individuals. Geographic range in the form of (B2) area of occupancy estimated to be (1) less than 500 km 2, and estimates indicating (a) severely fragmented or known to exist at no more than five locations and (b) continuing decline, observed, inferred or projected, in (v) number of mature individuals.
The presumed original habitat of N. flavirictus sp. nov., tall subtropical rainforest, has been almost entirely removed since human arrival in the 13 th Century AD, and now only exists in a handful of tiny remnant patches (3.2% of the Ecological District; Lux et al. 2009). Fires set by Māori removed most of the original forest on the Aupōuri Peninsula soon after their arrival in New Zealand in about the 12 th Century CE; initially it was replaced by extensive areas of bracken fern ( Pteridium esculentum (G. Forster)) ( Elliot et al. 1995) . The Te Paki area is now largely covered in fire-maintained secondary shrublands dominated by Kunzea Reichenbach and Leptospermum J.R. Forster & G. Forster. However , this shrubland is readily occupied by Naultinus , so the total area of habitat has probably changed relatively little. Only parts of the available habitat are known to be occupied, probably as a result of fire history.
With fire now better controlled, threats are almost entirely from invasive species. Almost the full suite of small predatory introduced mammals which have decimated native lizard populations across mainland New Zealand is present and common: cats ( Felis catus Linnaeus ), hedgehogs ( Erinaceus europaeus Linnaeus ), brush-tailed possums ( Trichosurus vulpecula (Kerr)) , stoats ( Mustela erminea Linnaeus ), weasels ( Mustela nivalis Linnaeus ), mice ( Mus musculus Linnaeus ), and rats (both Rattus rattus (Linnaeus) and R. norvegicus (Berkenhout)) . Feral pigs (Sus scrofa Linnaeus), which have also recently been demonstrated to eat large numbers of native lizards (J. Reardon pers. comm.), are widespread and abundant. Te Paki was one of the last areas of the country reached by possums, which have become abundant only in the last 20 years. In 1985 they were still recorded as absent but were spreading rapidly northward up the Aupōuri Peninsula, where they had reached about 10 km south of Waitiki Landing ( Mitchell 1985). The relatively abundant (compared to neighbouring mainland areas) Naultinus elegans populations on Waiheke Island, where possums are absent but most other invasive small mammals are abundant, suggests that arboreal possums may be particularly significant predators of arboreal Naultinus .
In addition to the threats from mammalian predators, social insects are an emerging threat. Introduced yellowjacket ( Vespula Thomson spp.) and paper wasps ( Polistes Latreille spp.) are now very abundant in Te Paki and prey on the same insect species as Naultinus ( Dymock 2000) . Argentine ants ( Linepithema humile (Mayr)) (both a competitor and a predator) are beginning to invade the area (https://www.doc.govt.nz/news/media-releases/2011/ doc-in-far-north-winning-battle-against-ruthless-ecological-invader/). The invasive Australian plague skink ( Lampropholis delicata (De Vis)) is spreading rapidly up the Aupōuri Peninsula (Kamera Raharaha, (dec.) pers. comm. to RAH) and is likely to invade Te Paki within the next few years. This species reaches enormous population densities, particularly near its invasion front ( van Winkel et al. 2018), and is likely to be a serious competitor for Naultinus and other native lizards in the area, despite being mainly terrestrial.
Comments. When Robb & Hitchmough (1980) resurrected N. grayii from synonymy with N. elegans , they included MONZ RE003322, the holotype of N. flavirictus sp. nov., among the material examined. Its mouth colour was not visible, but the smaller size, smaller snout scales and blunter snout were all noted; however,the small size of the specimen was interpreted as an indication that it was immature or subadult, and this was thought to explain these differences.
| MONZ |
Museum of New Zealand Te Papa Tongarewa - Entomology |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.