Neoseiulus wearnei ( Schicha, 1987 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4500.4.1 |
|
publication LSID |
lsid:zoobank.org:pub:16A34E21-D55D-40E9-BF2D-43D3BD8A6AF2 |
|
persistent identifier |
https://treatment.plazi.org/id/03E987BF-FFE7-FFC2-FF44-F997FAC9E22B |
|
treatment provided by |
Felipe |
|
scientific name |
Neoseiulus wearnei ( Schicha, 1987 ) |
| status |
|
Neoseiulus wearnei ( Schicha, 1987) View in CoL : another name for Nc -AH.
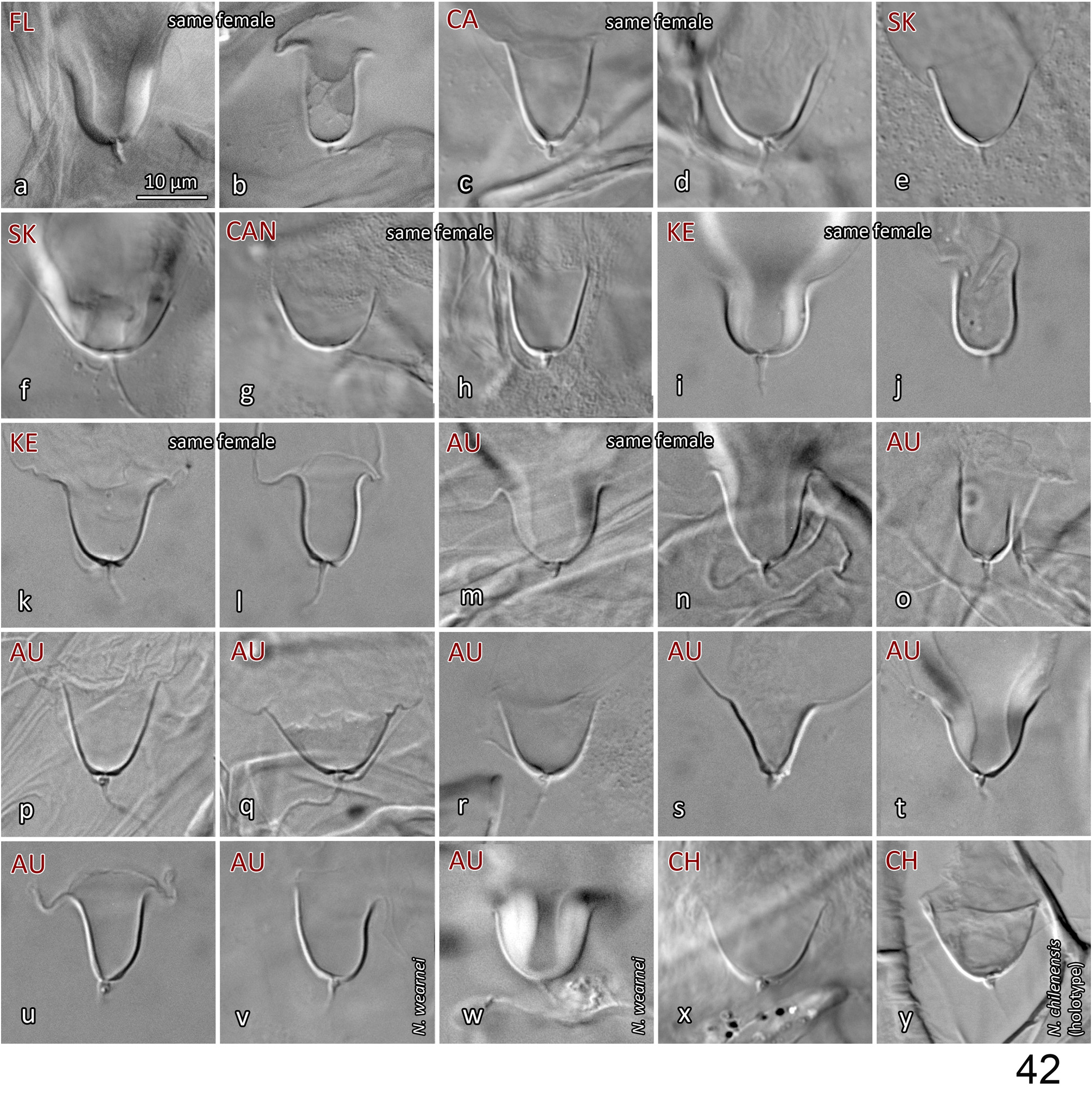
Tixier et al. (2014) originally proposed the synonymy of N. wearnei with N. californicus (sensu Athias-Henriot) . That N. wearnei and Nc -AH represent the same species concept was also supported by Griffiths (2015). The data we collected from our examination of specimens of N. wearnei , including one male with exactly the same collection data (slide lot #40) as the holotype and female paratypes ( Schicha, 1987), further supports their conspecificity with populations of Nc -AH. We found no marked differences in morphometrics for females and males (Table 3), nor in structures, shield ornamentation, pore-like structures, or spermathecal apparatus (see Fig. 42v, w View FIGURE 42 ). The female specimens of N. wearnei exhibit the lowest extreme in the length of dorsal setae across all populations studied, but this is largely due to one individual. Moreover, the lowest values are all within the range of values presented in Tixier et al. (2008) and Okassa et al. (2011) for Nc -AH.
4 This includes measurements made directly on a female paratype (57/35615-3) and measurements made on photos of the holotype (57/35615-1).
Neoseiulus chilenensis (Dosse, 1958) as the valid name of N. californicus sensu Athias-Henriot (although see below).
The holotype and paratypes of N. chilenensis are held in Görlitz, at the Senckenberg Museum of Natural History ( SMNG; Axel Christian, pers. comm. Dec. 2015). We borrowed and studied a female and a male paratype, and attained most measurements for the holotype based on photos that were provided by the SMNG (lot #43, Tables 1, 3; images courtesy of Axel Christian; Fig. 42y View FIGURE 42 ; available at http://cms.virmisco.org/index.php/search.html) .
The calyces of the spermathecal apparatus of the N. chilenensis holotype and paratypes are typical for, and fall within, the range of shapes observed for Nc -AH (as clearly indicated by published illustrations; Gonzalez & Schuster, 1962; Schicha, 1987; Tixier et al., 2008; Papadoulis et al., 2009; Xu et al., 2013), and this calyx shape was present in at least some specimens of all populations we studied ( Figs 42d, e, g, k, m, r, w, x, y View FIGURE 42 ). As pointed out by Griffiths (2015), the calyx illustrated by Dosse (1958a, b) is somewhat distinct, with almost parallel walls; however, this probably represents an inaccuracy in the illustration itself given that the calyces of the holotype, and of at least two paratypes, are all of a shape typical for Nc -AH, with slightly diverging walls. Note that some specimens of Nc -AH also possess calyces with almost parallel walls (on either or both calyces) ( Fig. 42b, j, l, o, v View FIGURE 42 ).
All the morphometrics taken for the female and male paratypes of N. chilenensis fall within the overall range of those of the Nc -AH specimens examined. In turn, all morphometrics of Nc -AH specimens that we studied fall within the range of values shown in Tixier et al. (2008) and Okassa et al. (2011), except for the dorsal shield width (176–216 here vs 130–189 µm). Their width measurements differ from ours because they measured the shield width at or near level of seta s4, whereas we measured the shield width where it is broadest, at a level just anterior to S2. Our measurements for shield width at s4 level (137–188; n=17) are similar to their measurements. Note that several dorsal setae of the N. chilenensis female paratype (and holotype, based on photos) are slightly longer than those of specimens from certain samples (from Florida, Canada, South Korea, Australia), but similar to those of other samples (California, Kenya; Table 3). That is congruent with what was observed by Tixier et al. (2008) and Okassa et al. (2011), with specimens from Chile having longer dorsal setae on average than specimens from samples collected elsewhere, especially from South Korea, Italy (Tuscany) and France (Marsillargues). Nevertheless, the measurements from the Chilean specimens overlap with the range of setal lengths of all populations from other locations. Furthermore, the genetic analysis of Okassa et al. (2011) showed minimal differences (averages of 0–0.1% between populations) in mitochondrial (12S rRNA, CytB) and nuclear (ITS) markers between 15 populations of Nc -AH, including specimens from Chile (except for two discrete populations from France, for mitochondrial markers only). Although Xu et al. (2013) noted variation in a few morphological characters, putatively intraspecific, between specimens from southern China vs. from elsewhere, they did not appear to have observed significant differences between specimens from Chile and non-Chinese specimens. Molecular analyses (Lv et al., 2016) further supported conspecificity between the Nc -AH population from China and populations from around the world.
In the original description of the N. chilenensis female ( Dosse, 1958b), setae j1 are more distant from each other (ratio of distance j1–1 / j1 length = 0.71–0.73) than those of the Nc -AH examined (0.19–0.49, n=63) or described in the literature (e.g. Athias-Henriot, 1977; McMurtry, 1977; Tixier et al., 2008; Papadoulis et al., 2009; Xu et al., 2013). This is also the case for N. chilenensis illustrated by Gonzalez & Schuster (1962) (and for Nc -AH in Çakmak & Çobanoğlu (2006) and Denmark & Evans (2011)). However, the male N. chilenensis illustrated in Dosse (1958b) has j1 setae much closer together, with j1–j1 / j1 ratio similar to the Nc -AH we examined, suggesting that the more distantly separated j1 setae for the female in Dosse (1958b) are inaccurate, and this is confirmed by our examination of the holotype (female) and paratypes (both sexes), which have j1 setae close to each other (j1–j1 = 8–9 µm; j1–1 / j1 ratio = 0.35–0.44). The more separated j1 setae shown in Gonzalez & Shuster (1962) are probably also an inaccuracy, since we have examined two females previously identified by R.H. Gonzalez, one of which was mentioned as material examined in Gonzalez & Schuster (1962) (slide lots #44–45) and it has j1 setae close to each other (j1–j1 = 7–9 µm). Another discrepancy is the set of measurements for the dorsal setae of N. chilenensis in Ehara (1964), which indicates that the dorsal setae are shorter than those in other descriptions. However, these measurements remain within the range of lengths given for dorsal setae in Tixier et al. (2008); moreover, the illustration in Ehara (1964) shows setae that are similar in length to those of N. chilenensis and Nc -AH described elsewhere.
Beside the aforementioned exceptions, the original description ( Dosse, 1958b) and redescriptions from Chile and Japan ( Gonzalez & Schuster, 1962; Ehara, 1964; Athias-Henriot, 1966; see also Hirschmann, 1962) of N. chilenensis show high morphological similarity to the specimens we examined and to descriptions of Nc -AH from various regions of the world, including Chile ( Athias-Henriot, 1977; McMurtry, 1977; Beglyarov, 1981; Jung et al., 2006; Guanilo et al., 2008a; Lofego et al., 2009; Papadoulis et al., 2009; Kade et al., 2011; Abo-Shnaf & de Moraes, 2014). Note that several morphological studies ( Gonzalez & Schuster, 1962; Tixier et al, 2008; Xu et al., 2013) and a molecular study ( Okassa et al., 2011) were based on specimens collected from the same region (Valparaíso Region, Chile) of the type locality of N. chilenensis (Valparaíso, presumably the city). It seems also that Athias-Henriot had examined at least one female specimen from that region (“Olme, Valparaíso ”), as indicated in Xu et al. (2013); it is also probable that the female described as N. californicus from “Valgo” (sic), Chile, in Athias-Henriot (1977), is the same as the one described as N. chilenensis from the same locality in Athias-Henriot (1966) (note that, as pointed out by Griffiths (2015), “Valgo” is a typographical error for “Valpo”, a diminutive for Valparaíso city).
Further evidence supporting N. chilenensis being conspecific with Nc -AH populations comes from the crossbreeding experiments of McMurtry & Badii (1989), which indicated that morphologically indistinguishable populations originating from California (on strawberry), Peru (on avocado) and Teno, Chile (citrus) were reproductively compatible, at least for producing viable eggs and adults of the F1 progeny. Because Teno is approximately 200 km from Valparaíso, the type locality of N. chilenensis , it is, again, likely that they were dealing with the same species as the one that Dosse (1958b) described. Gonzalez & Schuster’s study (1962) suggested that N. chilenensis is widespread in the region, from Valparaíso (north) to Talca (south), including Curicó (20 km from Teno).
Overall, the available evidence points toward the conspecificity of Nc -AH populations and N. chilenensis . Neoseiulus chilenensis was originally collected from water hyacinth growing in a greenhouse (along with Phytoseiulus riegeli Dosse , now a junior synonym of P. persimilis Athias-Henriot ) ( Dosse, 1958b: 48). This is compatible with most collection records of Nc -AH, which are predominantly from greenhouse and field-grown agricultural hosts, and plants associated with disturbed habitats including naturalised plants (e.g. Ragusa & Vargas, 2002; Guanilo et al., 2008a; Faraji et al., 2011, Xu et al., 2013; Griffiths, 2015; Seyedizadeh et al., 2017).
| SMNG |
Senckenberg Museum fuer Naturkunde Goerlitz |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |