Phallobrycon adenacanthus, Menezes, Naércio A., Ferreira, Katiane M. & Netto-Ferreira, André Luiz, 2009
|
publication ID |
https://doi.org/ 10.5281/zenodo.275063 |
|
DOI |
https://doi.org/10.5281/zenodo.6216288 |
|
persistent identifier |
https://treatment.plazi.org/id/B62D87F1-FFAA-0156-FF46-829BFE725671 |
|
treatment provided by |
Plazi |
|
scientific name |
Phallobrycon adenacanthus |
| status |
sp. nov. |
Phallobrycon adenacanthus View in CoL , new species
Figs. 1 View FIGURE 1 and 2 View FIGURE 2
Holotype. MZUSP 98892, 37 mm SL, mature male, Brazil, Mato Grosso: Campinápolis, rapids in rio Culuene, rio Xingu basin, 13º49’S, 53º15’W, F.C.T. Lima, F.A. Machado, C.A. Figueiredo & J.L. Birindelli, May 2007.
Paratypes. MZUSP 98893, 1, 34 mm SL, taken with holotype; MZUSP 98905, 1, 37 mm SL, Brazil, Pará: Altamira, vila Castelo dos Sonhos, rio Curuá, tributary of rio Iriri, rio Xingu basin, 08º19’07”S, 55º05’23”W, J.L. Birindelli, L.M. Sousa, A.L. Netto-Ferreira, M.H. Sabaj-Perez & N.K. Lujan, 20 October 2007. Following lots collected in the rio Xingu basin, Mato Grosso. MZUSP 91367, 2, 38.5 mm SL: Canarana, rio Coronel Vanick, tributary of rio 7 de Setembro, about 58 kilometers from Canarana, road MT- 120, 13º33’32”S, 52º33’51”W, O.T. Oyakawa et al., 18 October 2004; MZUSP 91952, 8, 30–37 mm SL, Paranatinga, rio Culuene, 13º49’S, 53º15’W, J.L. Birindelli, L.M. Sousa, & A. Akama, 21 August 2006; MZUSP 98899, 2, 26 and 39 mm SL, Paranatinga, rio Sucuri, tributary of rio Culuene, 13º55’40”S, 53º17’10”W, J.L. Birindelli & A.Akama, January 2006; MZUSP 98900, 2, 32 and 35 mm SL, Paranatinga, waterfalls of rio Corgão, tributary of rio Culuene, 13º48’18”S, 53º16’04”W, J.L. Birindelli & A. Akama, January 2006; MZUSP 98902, 7, 30–40 mm SL, Paranatinga, rio Culuene, 13º49’S, 53º15’W, L.M. Sousa, A.L. Netto-Ferreira, C.A. Figueiredo & F.A. Machado, 2 July 2007; MZUSP 98903, 13, 28.5–35 mm SL, Campinápolis, rio Couto de Magalhães near vila de São José do rio Couto, 13º50’17”S, 53º03’53”W, F.C.T. Lima, C.R. Moreira, F.A. Machado & A.C. Ribeiro, 6 October 2007; MZUSP 98904, 5, 29.5-35.6 mm SL, (2 cleared and stained), Paranatinga, rio Culuene, 13º49’S, 53º15’W, J.L. Birindelli, L.M. Sousa & A. Akama, 21 August 2006; MZUSP 97031, 24, 13-28.6 mm SL, Campinápolis, rio Couto de Magalhães, mouth of córrego Água Clara, Fazenda Meu Ranchinho, 13º48’02”S, 53º03’43”W, F.C.T. Lima, A.C. Ribeiro, C.M.C. Leite & T. Moraes, 10 October 2007.
Diagnosis. The same as that for Phallobrycon .
Description. Morphometrics of holotype and paratypes are presented in table 1. Meristic and morphometric data based on all the samples available since no differences were found among the material collected from different localities. Body small (SL 13–41 mm), elongate and laterally compressed; greatest body depth at vertical crossing slightly ahead pelvic-fin origin. Dorsal body profile nearly straight to only slightly inclined at anterior part of snout, gently convex from upper part of snout to dorsal-fin origin, slightly depressed at occipital region, straight and posteroventrally inclined from this point to caudal peduncle. Dorsal profile of caudal peduncle slightly concave. Ventral body profile gently convex from tip of lower jaw to analfin origin, nearly straight and dorsally inclined along anal-fin base and slightly concave along ventral margin of caudal peduncle. Lower jaw included in upper jaw when mouth is closed. Posterior tip of maxilla extending slightly beyond vertical crossing anterior border of orbit.
Dorsal-fin rays ii, 8 in all specimens. Posteriormost ray branched. Anal fin unbranched rays iv, branched rays 18–23 (21), 20.1, posteriormost ray branched. Strongly developed anterior anal-fin lobe including anterior unbranched rays and first 6–7 branched rays in both sexes. Anal fin of sexually mature males with bilateral bony hooks on largest unbranched ray and first two branched rays. In a clear and stained male specimen (35.4 mm SL) there are 5 hooks on largest unbranched ray, 5 on posterior branch of first branched ray and 1 on posterior branch of second branched ray and two developed spines on unbranched portions of fifth, sixth, and seventh branched rays located slightly below the middle of each ray ( Fig. 3 View FIGURE 3 ). Pectoral-fin rays i, 11–13 (i, 11) 11.8. Distal tip of longest pectoral-fin ray falling short of pelvic-fin origin even in mature females, but extending slightly beyond origin of that fin in mature males, however no significant statistical differences found in relative lengths of pectoral fins in males and females. Pectoral-fin rays without hooks. Pelvic-fin rays i, 7. No hooks on pelvic fin of sexually mature males. Distal tip of longest pelvic-fin rays extending slightly beyond anal-fin origin. No significant statistical difference in relative lengths of pelvic fins between males and females. Principal caudal-fin ray count 10/ 9 in all specimens.
Scales cycloid. Lateral line complete, perforated scales 38–40 (40), 39. Predorsal scales 12–14 (13), 12.8. Scale rows between dorsal-fin origin and anal-fin origin 9–10 (9), 9.01. Scale rows around caudal peduncle 14–15 (14), 14.3.
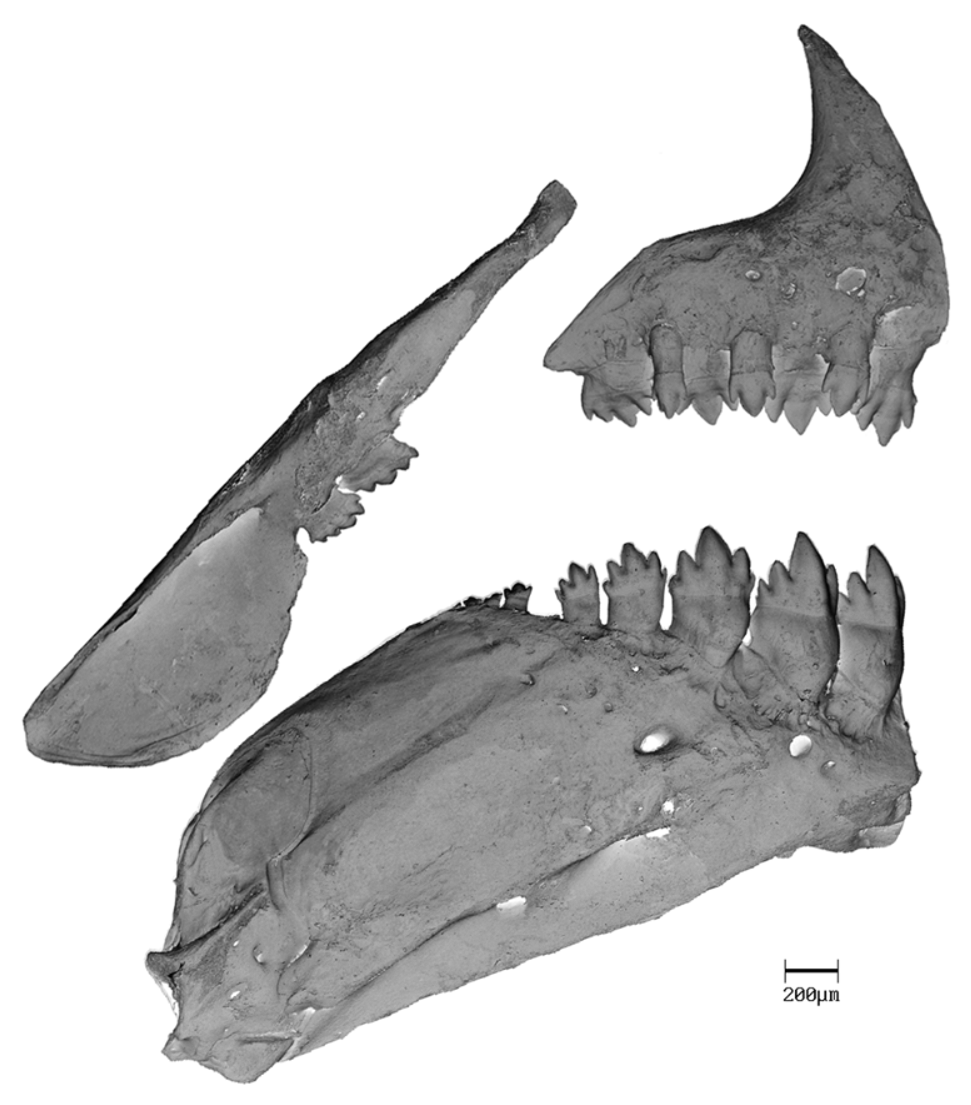
Premaxillary teeth in two rows ( Fig. 9 View FIGURE 9 ); outer row 4 in all specimens. Outer row teeth somewhat cylindrical and elongate, distally conical, with 2 small cusps on each side, slightly shorter than inner row teeth. Inner row teeth 4 in all specimens, compressed, slightly convex on external surface; symphyseal and following 3 teeth pentacuspid in larger specimens, one or two teeth in this series sometimes with 3 or 4 cuspids in smaller specimens; pentacuspid teeth with cusps graduated in size from smallest located laterally with third more medially located usually largest. Maxillary teeth 2 in all specimens ( Fig. 9 View FIGURE 9 ), compressed, with 5 or 6 cusps, middle cusp usually largest. Dentary with 4 anterior large pentacuspid teeth, followed by 2–5 (4), 3.9 smaller tetra or tricuspid teeth. Four large anterior teeth with inner surface concave and external surface convex ( Fig. 9 View FIGURE 9 ).
Vertebrae 37–39 (38), 38. Dorsal limb gill rakers 4–6 (5) 5; ventral limb gill rakers 8–10 (9), 8.5.
Branchiostegal rays 4 in two cleared and stained specimens, 3 rays originating on anterior and l on posterior ceratohyal.
Color in alcohol. Body pale to light yellow. Longitudinal dark stripe on dorsum covering median scale row, extending from occiput to dorsal part of caudal peduncle, separated by one and a half scale rows from lateral body stripe. Dark lateral body stripe extending from upper part of opercle to caudal base where it becomes wider. Stripe anteriorly diffuse, more conspicuous and darker from below dorsal-fin origin to caudal base, its posterior margin covering bases of 3–15 principal caudal-fin rays; medially on caudal fin stripe continues to distal tips of caudal-fin rays 5–9. Vertically elongate dark spot at humeral region. Scattered dark chromatophores on upper sides of head and above and below midlateral dark body stripe. Dorsal part of head dark. Fins hyaline with scattered dark chromatophores.
Sexual dimorphism. The most remarkable sexual difference in Phallobrycon adenacanthus is the presence of a urogenital papilla anterior to the anal fin in the males, already developed in specimens with 18 mm SL ( Fig. 4 View FIGURE 4 ). Another exclusive feature of the males is the presence of spines on the branched rays that are part of the anal-fin anterior lobe where the glandular tissue is confined ( Fig. 3 View FIGURE 3 ).
When expressed as percentages of standard length body depth, caudal peduncle depth and pelvic fin length revealed significant statistical differences between males and females (Table 1) but no differences were found when these morphometric data were considered through linear regression analysis.
Etymology. The name adenacanthus is from the Greek adenos meaning gland and akanthos for spine, with reference to the restriction of the glandular tissue to the area where the anal-fin spines are located.
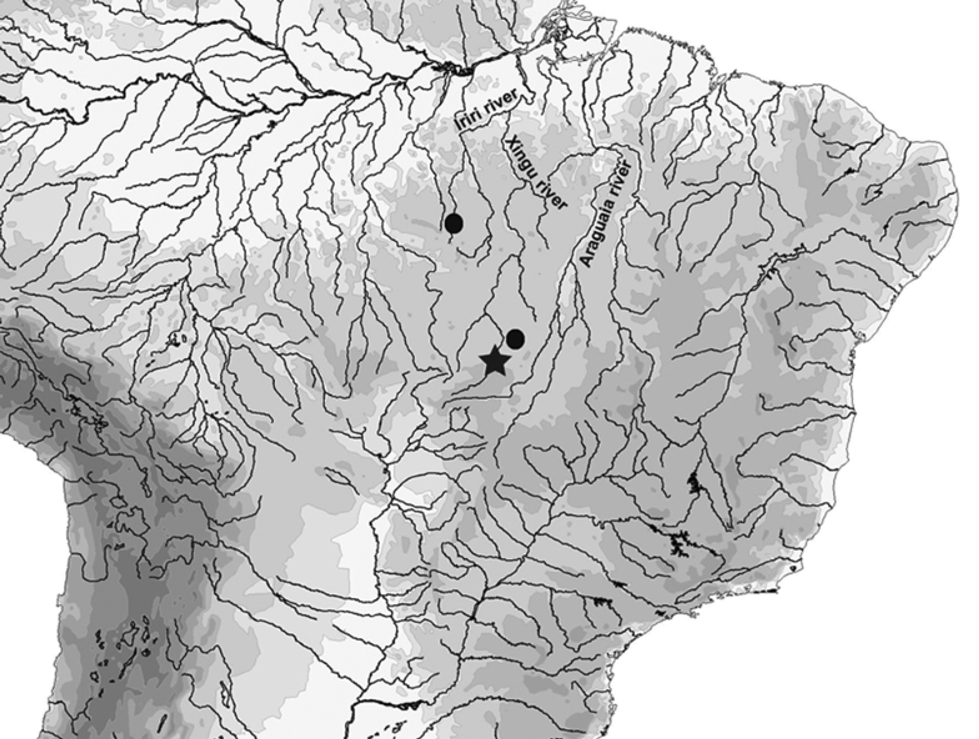
Distribution. This species was collected from tributaries of the upper rio Xingu in the states of Pará and Mato Grosso, Brazil ( Fig. 10 View FIGURE 10 ). One specimen was found in sympatry with Bryconadenos weitzmani Menezes, Netto-Ferreira & Ferreira in rio Curuá, tributary of rio Iriri, rio Xingu basin ( Menezes, Netto-Ferreira & Ferreira, 2009). Such disjunct distribution is possibly due to the lack of knowledge of the fishes of the rio Xingu basin, and it is most likely that Phallobrycon is a group widely distributed along that drainage.
Ecological notes. All specimens of Phallobrycon adenacanthus were collected in the main channel of rivers with fast flowing and rather shallow clear waters, with abundance of rocks and thick sand, an environment similar to that where members of its relative Bryconadenos inhabit. The population on its type locality is severely threatened due to the recent impoundment of the rio Culuene in the exact stretch where the specimens were collected, and other known populations should be preserved.
Phylogenetic relationships of Phallobrycon . Morphological and especially histological studies involving primary and secondary sexual characters intended to analyze the phylogenetic relationships among members of Clade A characids (K.M. Ferreira & N.A. Menezes, in preparation) will provide further information on the relationships of Phyllobrycon. Preliminary results indicate that other than the presence of four inner row premaxillary teeth and ii, 8 dorsal-fin rays no other obvious feature is exclusively shared by Phallobrycon and the other genera belonging to Clade A characids. The peculiar arrangement of the anal-fin spines associated with the glandular tissue (two on some individual branched anal-fin rays) seems to represent a unique feature within the clade. The new genus shares with Bryconadenos the absence of hooks on pelvicfin rays and the presence of intumescent glandular tissue on the anterior portion of the anal fin, and this might suggest some kind of relationship between them. Except for the fusion of distal and medial radials of the anterior five pterygiophores ( Fig. 3 View FIGURE 3 ), none of the distinguishing characters (3 to 7) common to Bryconadenos and Attonitus listed by Weitzman et al (2005: 335) are found in Phallobrycon .
Phallobrycon lacks the derived nature of the characters associated with the caudal organs and modified scales of the Glandulocaudinae and Stevardiinae (see Weitzman et al. 2005: 344), and although having a urogenital papilla, also found in some species of Monotocheirodon , lacks the morphological characters defining this genus, as discussed above.
At this point, we can only suggest that Phallobrycon might be related to the inseminating species of Knodus , but understand that this is just an oversimplification of a very complex problem, as discussed by Weitzman et al. (2005).
| MZUSP |
Museu de Zoologia da Universidade de Sao Paulo |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.