Phyllomedusa vaillantii
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5223.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:2AF3B77E-408A-4104-A058-108101993EBC |
|
persistent identifier |
https://treatment.plazi.org/id/B31987BB-FFA8-FF60-E0D0-56378E2AFC15 |
|
treatment provided by |
Plazi |
|
scientific name |
Phyllomedusa vaillantii |
| status |
|
Phyllomedusa vaillantii View in CoL View at ENA
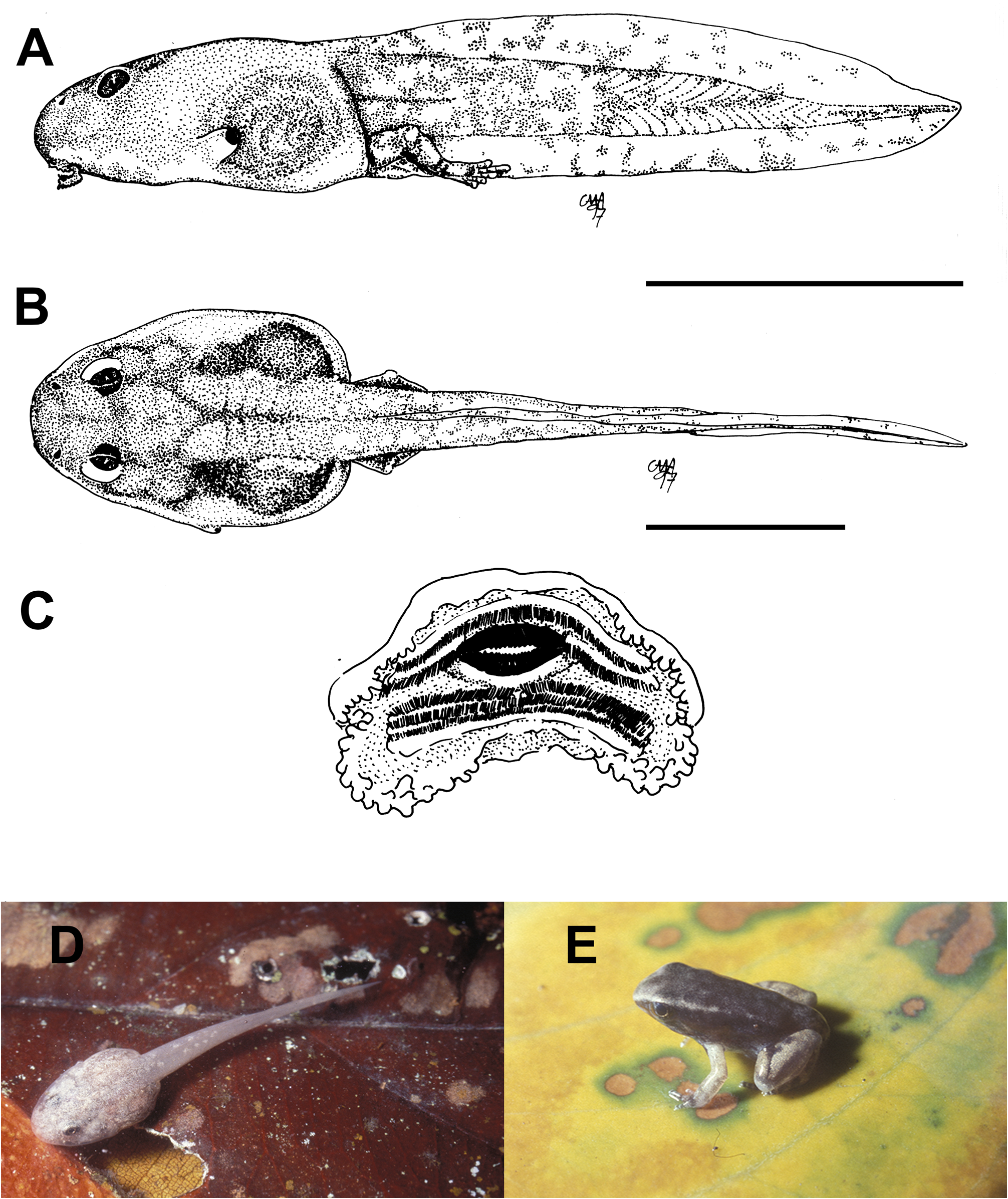
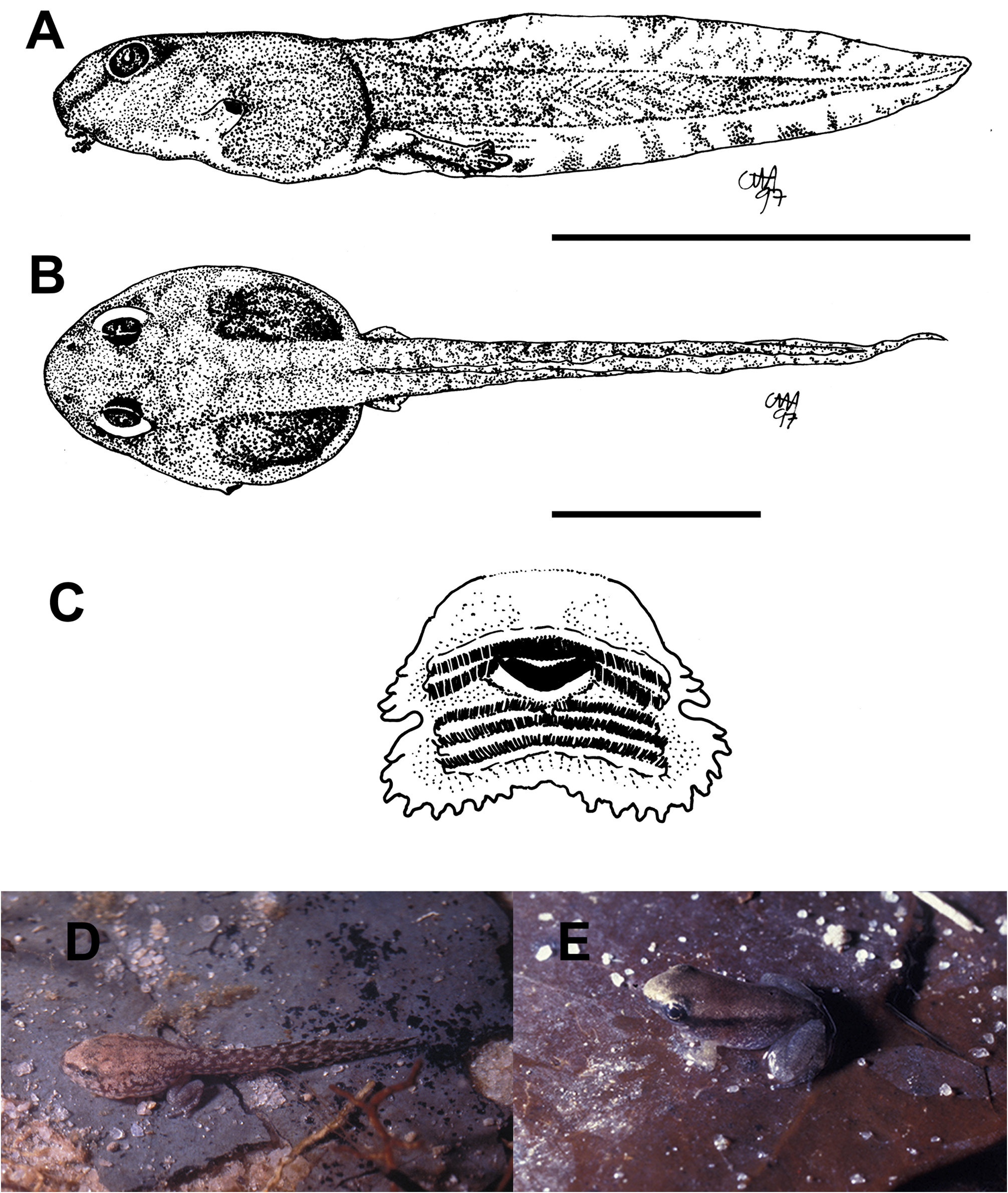
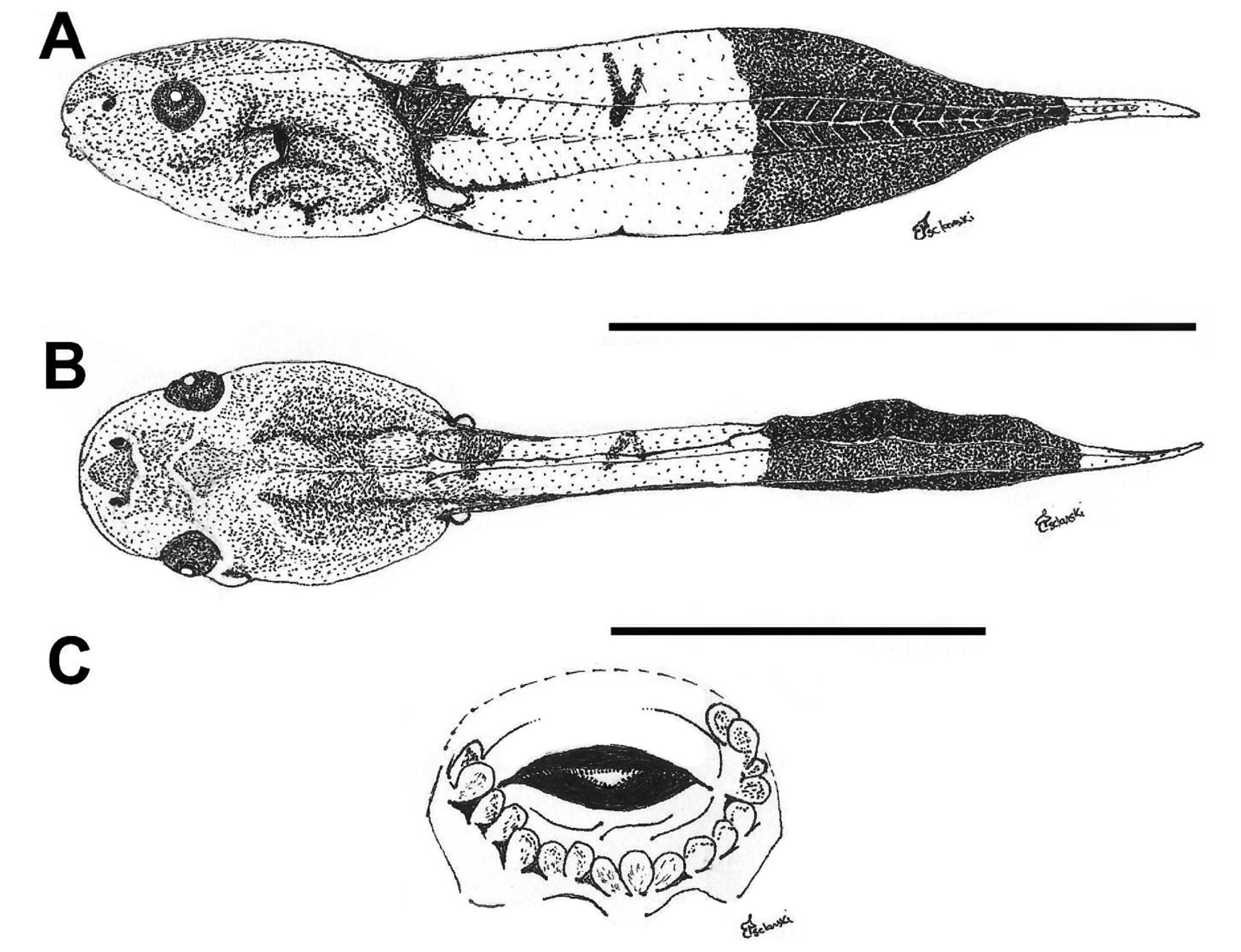
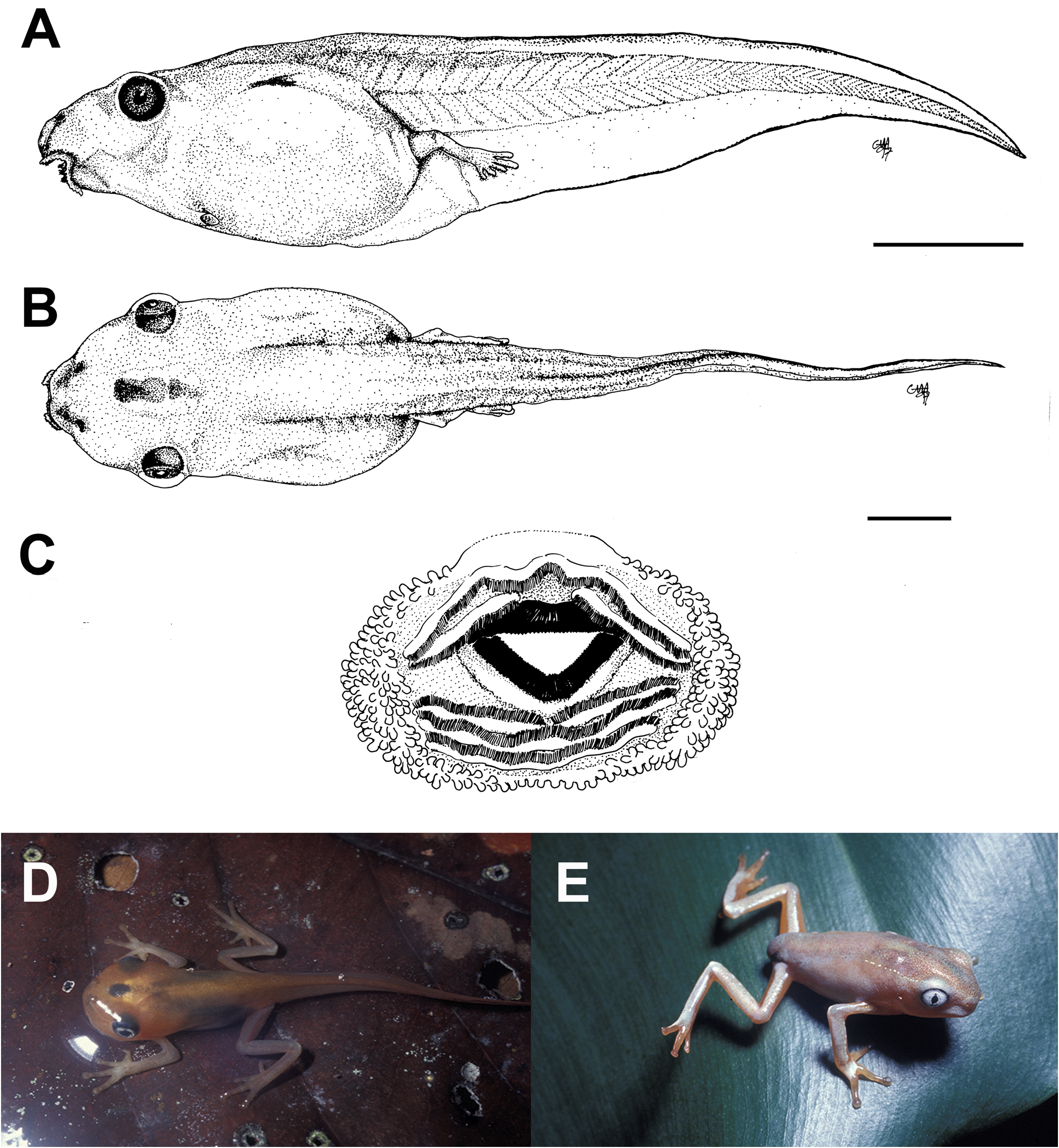
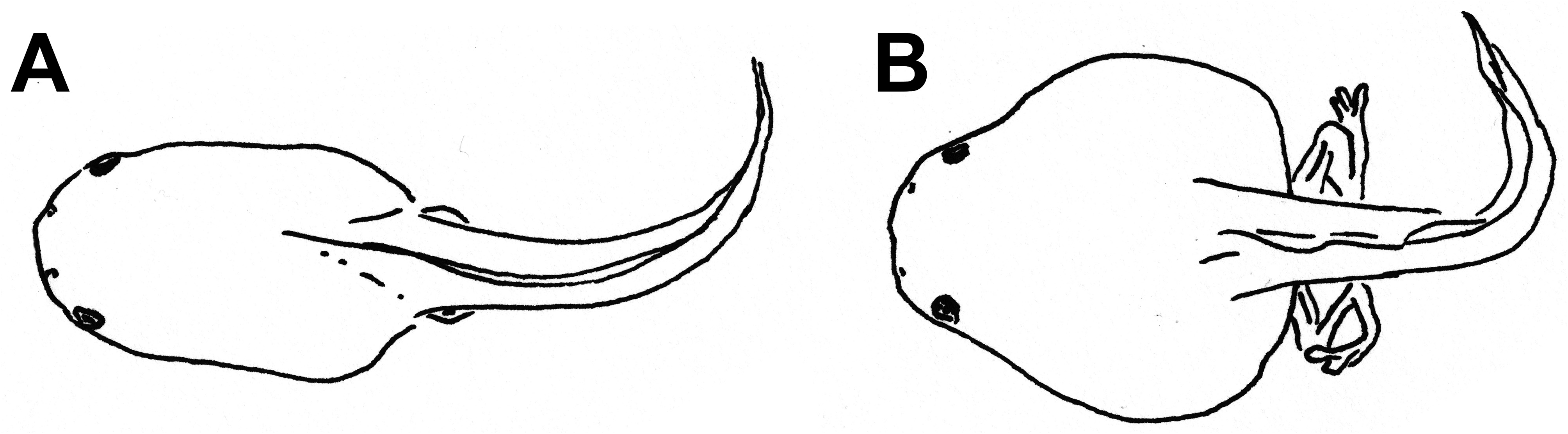
External morphology. Description based on six tadpoles at Stage 37 (LCS 557). Total length 53.2 ± 2.0 mm (N = 6). Body elongate oval in dorsal view and triangular in lateral view ( Fig. 71A, B View FIGURE 71 ). Snout truncate in dorsal and lateral views. Eyes small, positioned and directed laterally. Nostrils small, oval, laterally positioned near to snout, with opening anterolaterally directed, without a projection on the marginal rim. Oral disc ( Fig. 71C View FIGURE 71 ) anteroventral, not emarginate; marginal papillae conical, uniseriate, with a dorsal gap. Submarginal papillae present laterally. LTRF 2(2)/3(1); A1 and A2 of the same length; P1 and P2 of the same length; P3 slightly shorter than P2. Jaw sheaths moderately wide, finely serrated; anterior jaw sheath arch-shaped, posterior jaw sheath V-shaped. Spiracle single, ventrolateral, cylindrical, short and wide, posteriorly directed, with a large opening at the medial third of the body, and with the centripetal wall fused to the body wall and longer than the external wall. Vent tube dextral, fused to the ventral fin, with a dextral opening. Caudal musculature of moderate width; in lateral view gradually tapering to a pointed tip. Dorsal fin originating on the posterior third of the body, shallow throughout its length, highest posteriorly, with a cord, a thickening on the edge of the fin, beginning at posterior third of body and extending until the posterior third of tail; ventral fin of moderate height, convex. Tail tip pointed.
Colour. In preservative body and caudal musculature brownish-light orange; dorsum with a black mark between the eyes; fins translucent. In life body transparent orange with a silver venter; tail transparent orange (Hero 1990); a distinct black mark between the eyes ( Caramaschi & Jim 1983) ( Fig. 71D View FIGURE 71 ).
Metamorphs. At metamorphosis froglets are light grey, nearly white, with orange reflections over dorsum and flanks; venter whitish cream; sides of limbs and toes orange; there is a faint black mark between the eyes and an intermittent glandular dorsolateral line from behind the eyes to the midbody ( Fig. 71E View FIGURE 71 ).
Natural history. Eggs are deposited in a gelatinous mass in leaf nests overhanging isolated ponds in streamside terra-firme forest ponds; upon hatching tadpoles hatch and fall in the water (Neckel-Oliveira 2004; Lima et al. 2012). Gravid females contain from 635 to 1,114 ovarian eggs (Ĥdl 1990; Aichinger 1992). Tadpoles aggregate by size forming polarized schools ( Branch 1983). P. vaillanti was observed surface feeding, suspension feeding and rasping surfaces and had phytoplankton, periphyton and detritus as gut contents ( Branch 1983). In experiments P. vaillanti tadpoles were avoided as prey by all fish species tested ( Hero 1991); the bright orange color is likely aposematic.
Comments. These tadpoles were described by Duellman (1978) from Ecuador, by Caramaschi & Jim (1983) from Southwestern Amazonia, Hero (1990) from Central Amazonia and by Duellman (2005) from Peru. Tadpoles from Central Amazonia (Hero 1990), Ecuador ( Duellman 1978) and Peru ( Duellman 2005) differ from those herein characterized by presenting biseriate marginal papillae row, at least posteriorly on oral disc. Those illustrated by Hero (1990) differ from those herein by presenting LRTF 2(2)/2-3(1), and those from Ecuador ( Duellman 1978) and Peru ( Duellman 2005) differ by presenting the first posterior tooth row undivided [LRTF 2(2)/3]. Tadpoles from southwestern Amazonia differ from those herein characterized by presenting body pyriform in dorsal view, dorsal fin originating at the body-tail junction, and a row of marginal papillae biseriate ( Caramaschi & Jim 1983). Although the authors mentioned the presence of only a dorsal gap, Fig.4 View FIGURE 4 shows an oral disc with a short ventral gap, which differs from all other descriptions, included those herein. Tadpoles from Peru ( Duellman 2005) also differ from those herein characterized by presenting snout rounded and dorsally prominent in lateral view, and dorsal fin originating on proximal caudal musculature. There is no morphological variaton between tadpoles from Colombia illustrated by Lynch & Suárez-Mayorga (2011) and those herein characterized.
Reproductive modes and premetamorphic development
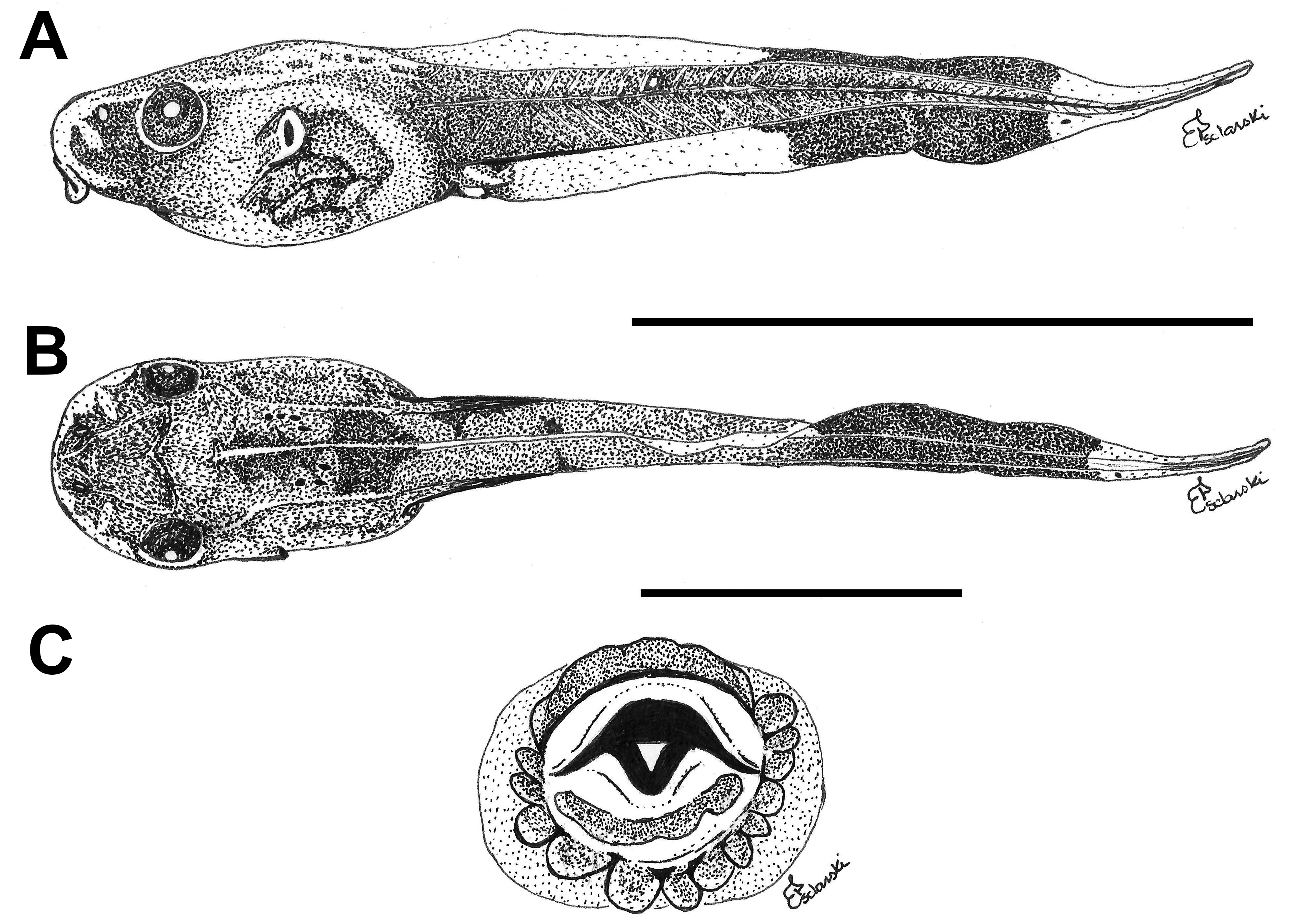
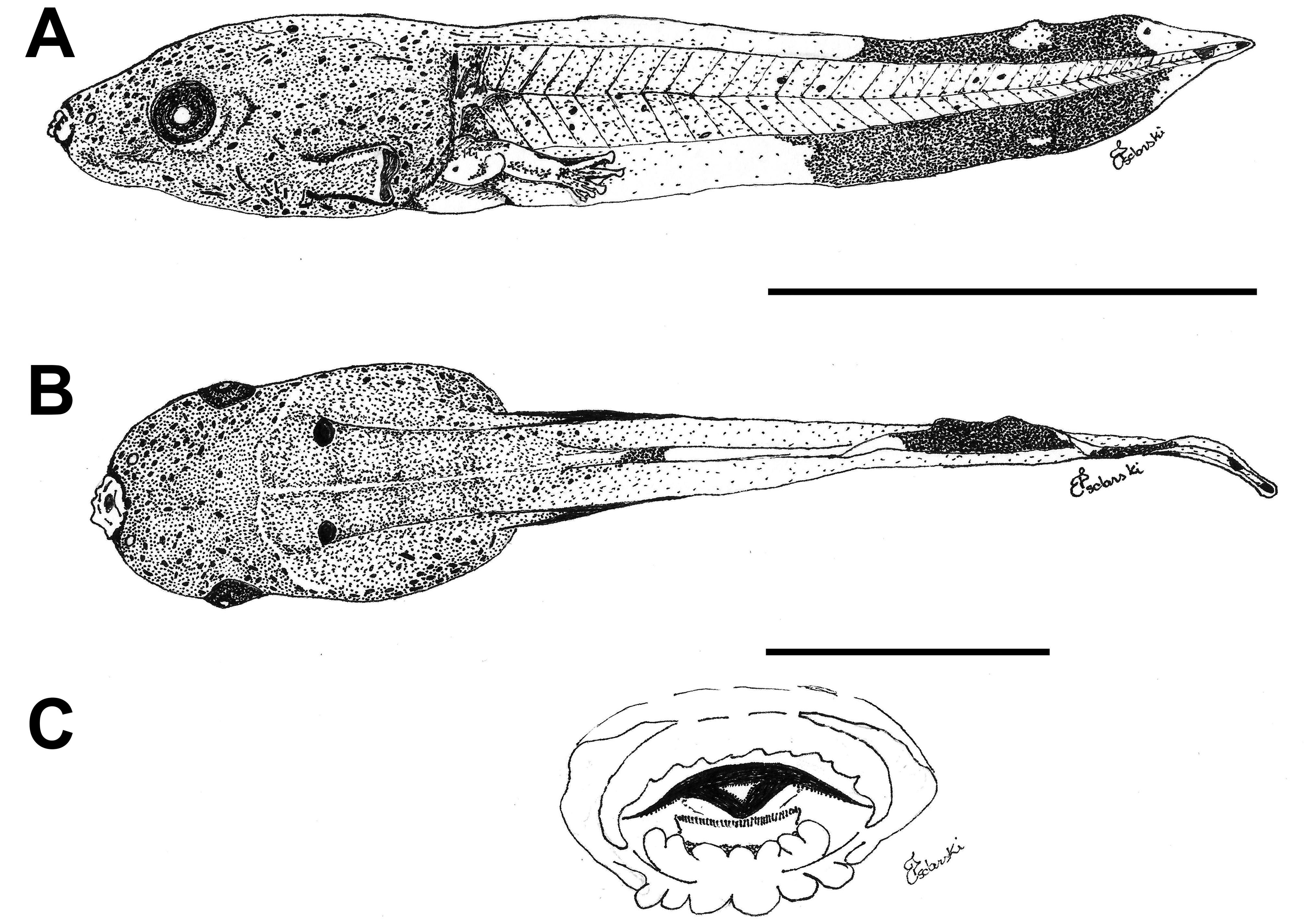
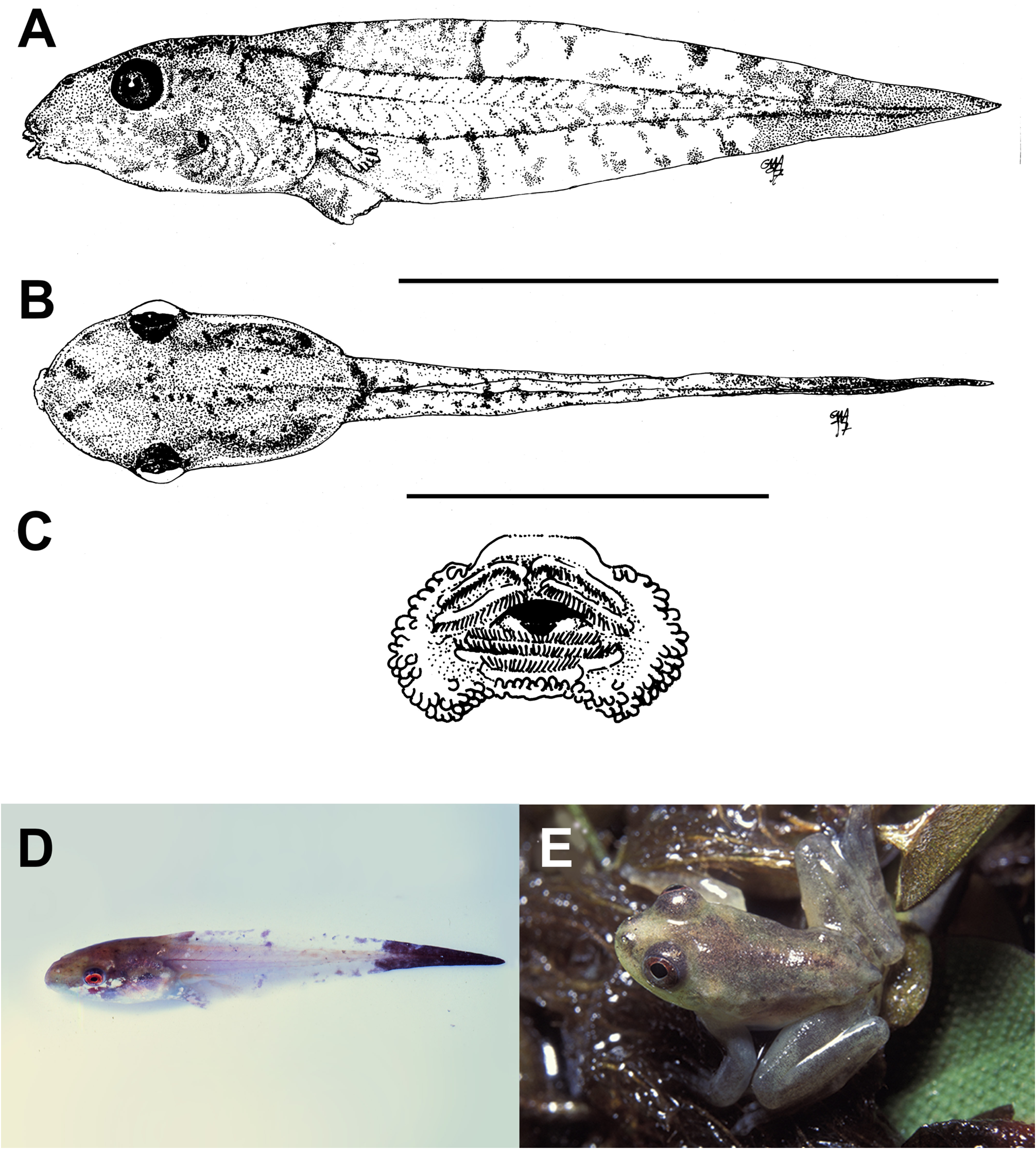
Source of nourishment in premetamorphic development. Altig & Johnston (1989) consider that the source of nutrition for embryos and larvae is a basal dichotomy in anuran biology. Early embryonic development is supported by yolk allocated to the egg during vitellogenesis. In the majority of anuran species, however, the amount of yolk is insufficient to support the entire premetamorphic development. After the yolk is exhausted tadpoles consume a variety of food items taken from the environment, a condition termed exotrophy ( Altig & Johnston 1989; see´Species Interactions/Food’).Among the 99 Central Amazonian anuran species, 85 species (i.e., 86%) are obligate exotrophic (see Table 1; also Fig. 72 View FIGURE 72 for a developmental series of Osteocephalus taurinus , a typical exotrophic larva).
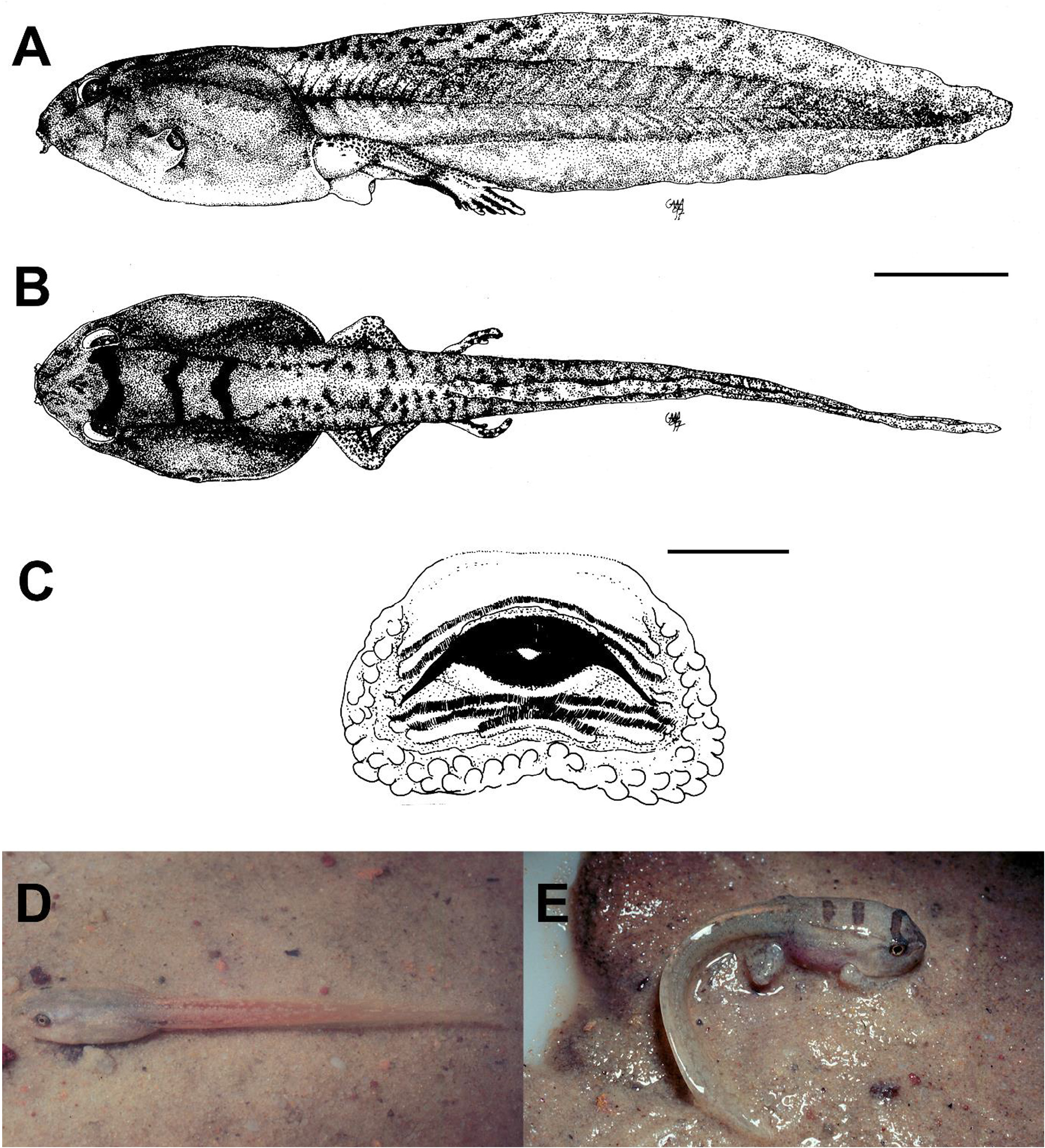
In some cases, however, the entire embryo-larval development is supported by nutrients from parental sources, most often yolk. This category, termed endotrophic ( Altig & Johnston 1989), is observed in species with a range of larval morphotypes, from typical tadpoles to modified forms, and represents, for several lineages, a crucial step for a decreased dependence on the aquatic environment. Among Central Amazonian anurans, 13 species (13%) have complete endotrophic development ( Table 1). Two species ( Pipa pipa , P. arrabali ) are aquatic in the sense that they develop from eggs embedded in the dorsum of the aquatic female, hatching directly into froglets.Another 11 species are fully terrestrial ( Table 1). These terrestrial endotrophic anurans have different degrees of reduction of structures associated with feeding, swimming and respiration. Allobates nidicola , Anomaloglossus stepheni , Adenomera andreae , A. hylaedactyla , Synapturanus ajuricaba and S. mirandaribeiroi retain some vestiges of aquatic larval features. In contrast Pristimantis cf. marmoratus , P. fenestratus , P. ockendeni , P. zimmermanae and Phyzelaphryne sp. all have embryos that develop directly into froglets (see Fig. 72 View FIGURE 72 for a developmental series of P. fenestratus ).
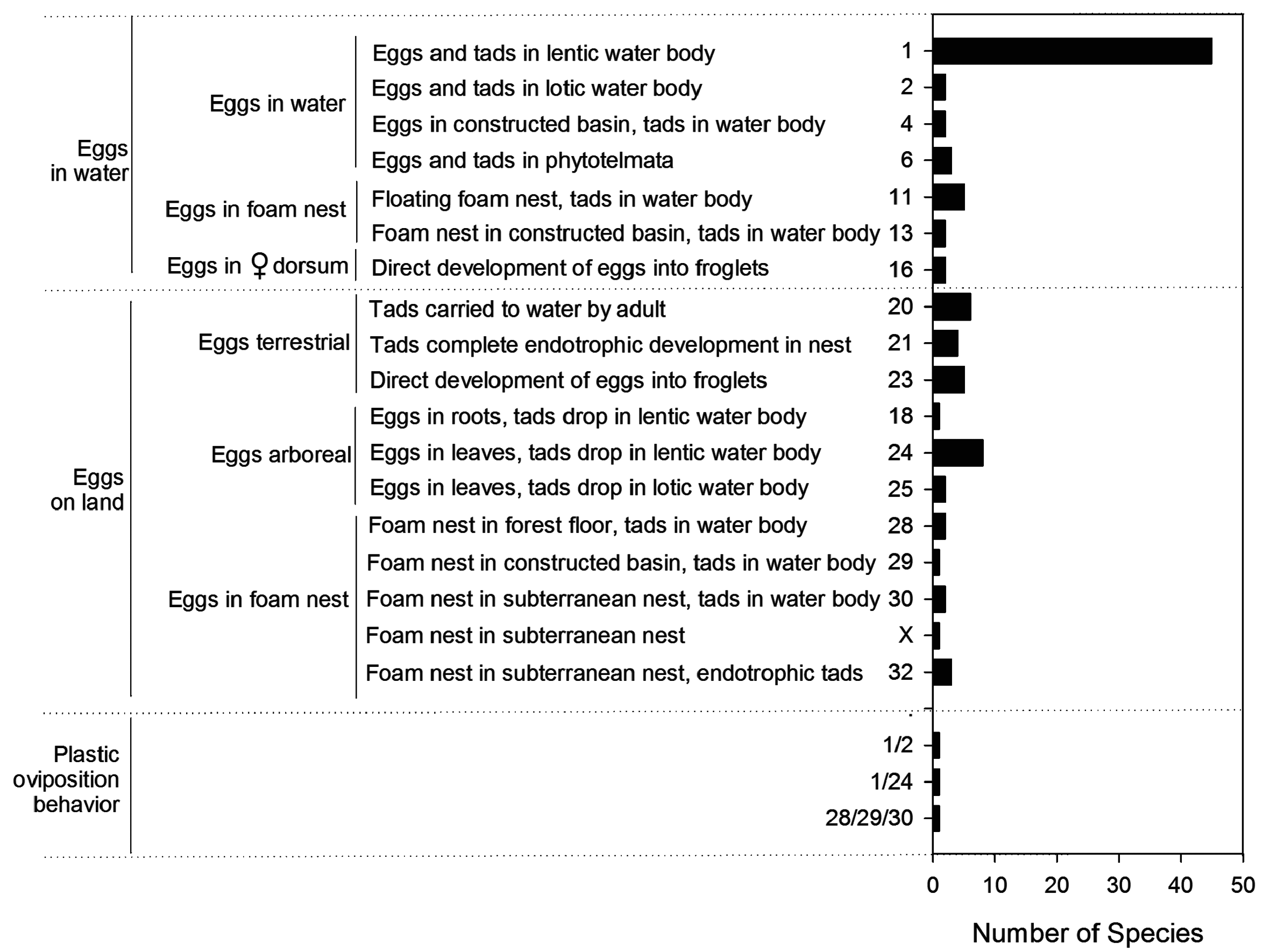
Reproductive modes. Reproductive modes are defined as a combination of traits including oviposition site, ovum and clutch characteristics, rate and duration of development, stage and size of hatchling, and type of parental care (if any). They are important and diverse components of amphibian biology ( Duellman & Trueb 1986; but see Wells 2007 for a critique). There are currently 39 reproductive modes described for anurans ( Haddad & Prado 2005). From these, we recognize 16 reproductive modes in Central Amazonian anurans, corresponding to 73% of all reproductive modes known for Amazonia and 41% of all reproductive modes known for the world ( Haddad & Prado 2005). These reproductive modes, numbered according to Haddad & Prado (2005), are listed in Table 1.
Among the 62 species with fully aquatic premetamorphic development, we recognize seven reproductive modes ( Fig. 73 View FIGURE 73 ). Forty-eight species present the most typical pattern of development comprising aquatic eggs and exotrophic aquatic larvae in surface water bodies—45 of them in lentic water bodies (mode 1), two in lotic water bodies (mode 2), and one ( Boana geographica ) in both lentic and lotic water bodies. Five other species have eggs, embryos and exotrophic tadpoles that develop in lentic water bodies, but either spend their early development in a waterfilled basin constructed by the male adjacent to a larger water body ( Boana boans , B. wavrini ; mode 4) or develop in phytotelmata. These phytotelmata-dependent taxa include Osteocephalus oophagus in water-filled bromeliads, tree holes and buriti palm ( Mauritia flexuosa ) leaf axils; and Trachycephalus cunauaru and T. resinifictrix in spacious waterfilled treeholes; mode 6). Five other species construct foam nests on the water surface ( Leptodactylus leptodactyloides , L. macrosternum , L. riveroi ; L. wagneri , Lithodytes lineatus ; mode 11) or in water accumulated in constructed basins ( Leptodactylus labyrinthicus , L. podicipinus ; mode 13) with subsequent development of exotrophic tadpoles in the adjacent larger water body. Finally, Pipa spp. are fully aquatic (mode 16).
Among the 23 species with semiterrestrial premetamorphic development (i.e., eggs terrestrial, larvae aquatic), we recognize seven reproductive modes. Six species oviposit in the leaf litter and, upon hatching, the tadpoles are transported to a temporary pond on the back of the adult (four species of Allobates and both species of Ameerega ; mode 20). One species lays eggs in roots overhanging lentic water ( Amazophrynella manaos ; mode 18). Eight species lay eggs in leaves overhanging lentic water ( Allophyrne ruthveni ; Dendropsophus cf. brevifrons , D. leucophyllatus , D. triangulum , Callimedusa tomopterna , Phyllomedusa bicolor , P. tarsius and P. vaillanti ; mode 24) and two species lay eggs in leaves overhanging streams ( Hyalinobatrachium iaspidiense and Vitreorana ritae ; mode 25). In all cases, at hatching tadpoles fall in the water, where they complete exotrophic development. Finally, two species construct foam nests on the humid forest floor ( Leptodactlys petersii , L. rhodomystax ; mode 28), one in basins ( Leptodactylus knudseni ; mode 29) and two in burrows ( Leptodactylus fuscus , L. longirostris ; mode 30) adjacent to ponds. Rising water levels following rain flood the nest, and exotrophic tadpoles complete development in the pond. At least one Central Amazonian species with semiterrestrial premetamorphic development displays plastic oviposition behavior; i.e., Leptodactylus mystaceus deposits its eggs in foam nests either on the humid forest floor, in a basin, or in a subterranean constructed nest adjacent to a larger water body (modes 28, 29 and 30).
At last, among the 13 species with fully terrestrial development we recognize three reproductive modes. Two species have foam nests in subterranean constructed chambers with endotrophic tadpoles completing development in the nest. These are Adenomera andreae and A. hylaedactyla (mode 32). Four species lay eggs in the forest floor, either in the leaf litter (i.e., Anomaloglossus stepheni , Allobates nidicola ) or underground (i.e., Synapturanus ajuricaba , S. mirandaribeiroi ), where eggs hatch into endotrophic tadpoles that complete development in the nest (mode 21). Five species have direct development of terrestrial eggs; these are all four species of Pristimantis , and Phyzelaphryne (mode 23; see Fig. 72 View FIGURE 72 ). Leptodactylus pentadactylus in Central Amazonia has a fully terrestrial development in foam nests in subterranean constructed chambers that may (Marcelo Menin pers. obs.) or may not ( Hero & Galatti 1990) contain water. Tadpoles are too large (max. total length 80 mm; Hero & Galatti 1990) to be exclusively sustained by yolk, and they were found to contain eggs in their guts (Marcelo Menin, pers. obs.), and to be voracious egg predators ( Magnusson & Hero 1991). We hypothesize that completion of development requires the regular deposition of eggs by the mother, as recently demonstrated for Leptodactylus fallax in captivity. To the best of our knowledge, a reproductive mode with terrestrial exotrophic tadpoles developing in subterranean constructed chambers has not been described and should be identified as a distinct reproductive mode. Leptodactylus stenodema may present the same reproductive mode of L. pentadactylus . However, despite considerable effort at locating and following burrows occupied by calling Leptodactylus stenodema , we were unable to locate eggs and tadpoles.
Analyzing by macrohabitat, diversity of reproductive modes is far greater in terra-firme forests (16) than in várzea (7) and igapó flooded forests (2). Notable is the scarcity of semiterrestrial and the complete lack of terrestrial modes of reproduction in flooded forests.
Several hypotheses have been postulated for the selective forces shaping the diversity of reproductive modes and, in particular, for the evolution of terrestriality in amphibians ( Wells 2007). These include reduced risk of being carried away by current in swift streams and of dessication in drying ponds, reduced intensity of interspecific competition among tadpoles in the water body, and also the predation pressure on eggs and larvae developing in water bodies. A reduced risk of dessication in drying ponds is an unlikely explanation for a trend towards terrestriality because in humid forested habitats the risk of desiccation is low. Moreover, the diversity and prevalence of semiterrestrial and terrestrial reproductive modes is high, since the most important constraint to terrestrial eggs and larvae, evaporative water loss, is relaxed.
Avoidance of aquatic predators of eggs and larvae has been repeatedly proposed. In an insightful study conduct- ed in the Central Amazon, Magnusson & Hero (1991) found that several species of tadpoles and dytiscid beetles, but not fish, were voracious predators of aquatic amphibian eggs (see´Species Interactions´). They also found that in any given water body the percentage of species with terrestrial oviposition was positively correlated with a general index of egg predation pressure, in turn negatively correlated with fish biomass. The authors interpreted these results as if terrestrial oviposition is maintained, and perhaps evolved, as a strategy to avoid predation, even though not fish predation, in the water body.
Habitat use
Central Amazonian amphibians clearly exhibit differential patterns of space use at the scales of macrohabitat (i.e., among major physiognomies), habitat (i.e., among water bodies) and microhabitat (i.e., within water bodies).
Distributional patterns at macrohabitat scale. Taking into consideration that more research has been devoted to the study of terra-firme forest faunas than várzea and igapó flooded forest faunas, we recognize 67 species in terra-firme forests, 28 in várzea flooded forests and four in igapó flooded forests ( Table 1). Among the 67 species occurring in terra-firme forests, 61 are exclusive to this macrohabitat, six are shared with várzea flooded forests and one with igapó flooded forests. Among the 28 species occurring in várzea flooded forests, eighteen are exclusive to this macrohabitat, four are shared with igapó flooded forests and six with terra-firme forests. Finally, of the four species found in igapó flooded forests, three are shared with terra-firme forests and all four with várzea flooded forests. Few species are wide generalists with respect to macrohabitat use; Rhinella marina and Scinax ruber stand out as occurring from the margins of large rivers to upland forests and both in open areas and closed-canopy forest edge.
At least 21 species are regularly found in forest edge and/or deforested land ( Table 1). Six of them are typically forest species ( A. femoralis , A. sumtuosus , O. taurinus , P. bicolor , P. tarsius , L. knudseni ), 11 are typical of open areas and do not penetrate or are only rarely found inside the forest ( Rhinella merianae , R. marina , Boana lanciformis , Scinax garbei , S. cf. ruber , Trachycephalus typhonius , Leptodactylus fuscus , L. macrosternum , L. longirostris , L. podicipinus , and Elachistocleis helianneae ), and five occur in both ( Dendropsophus minutus , B. boans , B. geographica , S. cruentomma , L. mystaceus ), but are usually more abundant in disturbed habitats.
Distributional patterns at the habitat scale. Terra-firme forests. In terra-firme forests, aquatic exotrophic amphibian larvae clearly segregate habitat among different types of water bodies (Ĥdl 1990; Rodrigues et al. 2010; Table 2). Considering the 53 species occurring in terra-firme forests (i.e., excluding forest edge), 23 species occur in isolated forest ponds, 31 in streamside ponds, nine in forest streams, and five in phytotelmata (numbers do not add up because certain species occur in more than one habitat; Table 2).
The first evident axis of habitat segregation is water flow. Forty-three of 53 (81%) terra-firme species are exclusively lentic. By contrast, only three species (6%) are stream specialists ( Atelopus manauensis , Boana boans and Osteocephalus cabrerai ). One, possibly two, species (2–4%) are primarily stream dwellers occasionally found in streamside ponds (the centrolenids Vitreorana ritae and, by extension, Hyalinobatrachium iaspidiense whose tadpole is unknown; but note that tadpoles are fossorial and therefore avoid the water current). Finally, Boana geographica , B. cinerascens , Osteocephalus taurinus , Phyllomedusa vaillantii and P. bicolor (5 species or 9%) are more typical of lentic water bodies, but may be washed from streamside ponds by heavy rains or colonize lowcurrent stream sections. As pointed out by Gascon (1991), the poorness of the lotic tadpole assemblage in Central Amazonia probably results from the lack of morphological specializations for life in fast-flowing water for most tadpole species, and predation by fish. This is a proximal explanation, though. An ultimate explanation appears to include a strong component of historical biogeography as Southeast Asian amphibian faunas display a contrasting pattern, with about half of the species reproducing in streams ( Inger et al. 1986; Strauss et al. 2010, 2013; Zimmerman & Simberloff 1996). Linked to these historical effects, the mountainous topography provides a variety of lotic flow regimes (e.g., Inger et al. 1986; Strauβ et al. 2013) that may have contributed to a higher prevalence of streamassociated amphibian species in Southeast Asia.
The second axis of habitat segregation is that comprehending the 48 lentic or predominantly lentic species, which segregate habitat between arboreal and surface water bodies, on one hand, and between isolated forest ponds and streamside ponds, on another. In the first group, three species are phytotelmata specialists that are never found in surface water. Osteocephalus oophagus is found in ground or epiphytic bromeliads, burití palm leaf axils ( Mauritia flexuosa ), waterfilled fallen palm bracts or treeholes ( Jungfer & Schiesari 1995). Trachycephalus resinifictrix and T. cunauaru occur in spacious water-filled treeholes in the forest canopy (Schiesari et al. 2003a; Gordo et al. 2013). Allobates femoralis and A. sumtuosus are listed as facultatively phytotelmonous because these species have been found in fallen palm leaves or fallen logs accumulating water. Note that the definition of phytotelm includes any plant or part of plant that holds water, independent of height above ground or volume. The second group comprises the 45 species occurring exclusively or predominantly in surface lentic water bodies. Among the 45 species occurring exclusively or predominantly in ponds, 12 species occur exclusively in isolated forest ponds (here including peccary wallows; Gascon 1991), nineteen species occur exclusively in streamside ponds, and 10 species occur in both habitats ( Table 2). As one might expect, despite the very high diversity observed at the landscape level, the number of species actually found in a water body in any given moment is much lower. For example, mean number of species per water body was 4.7 in isolated forest ponds and peccary wallows, 1.6 in streamside pools and 0.4 species in streams (average across two sampling years; Gascon 1991). A similar pattern of species richness was found in other ecosystems in Brazil. For example, of 20 species in the Atlantic Forest, the mean number of species per water body was 3.1 in isolated ponds and 1.3 in streams ( Jordani et al. 2017).
Large rivers and seasonally flooded forests. Less is known about habitat use in large rivers and seasonally flooded forests. Few species breed and develop in the open waters of large rivers and lakes. Rhinella marina larvae were observed in a lake connected to the Rio Negro in Central Amazonia (LS pers. obs.), and in Eastern Amazonia R. merianae bred more frequently in the Tapajós river, a large clear water river, and associated lakes than in ponds and puddles ( Azevedo-Ramos et al. 1999). Surprisingly, amplectant pairs of R. merianae were found to swim up to 750 m inside the Tapajós river to lay eggs (Magnusson & Windle 1986). Boana wavrini breeds in water-filled nests excavated by the male at the margins of igapó and várzea lakes, rivers and streams; tadpoles swim into the lake water when the nest is flooded by the rising water levels. In eastern Amazonia, B. wavrini tadpoles were found in shallow pools and in the shallow margins of a lake connected to the Trombetas river ( Martins & Moreira 1991).
By contrast, several species breed and develop in protected biotopes of large rivers and lakes. In várzea floodplains, specifically, extensive macrophyte stands form seasonally along the margins and banks of the nutrient-rich, white-water rivers and lakes (known as´floating meadows´; Ĥdl 1977; Junk et al. 1989; B̂ning et al. 2017). Nineteen species of anurans, all of which with aquatic tadpoles, call and breed in the floating meadows. The structurally complex root zone of floating meadows, and the very high primary productivity of aquatic grasses, presumably provide shelter and abundant detritus for the tadpoles ( Schiesari et al. 2003b). Our collections confirmed the occurrence of larvae of Boana raniceps , Lysapsus bolivianus , Dendropsophus haraldschultzi , D. leucophyllatus , D. marmoratus , D. walfordi , Scarthyla goinorum , Sphaenorhynchus carneus , S. dorisae , S. lacteus , and two species of Scinax in the root zone of floating meadows.
Amazonian floodplains present a variety of temporary water bodies. In Alter do Ch„o 600 km east of Manaus, Azevedo-Ramos et al. (1999) sampled 40 water bodies ranging from shallow puddles to lakes connected to the Tapajós river. Thirteen tadpole species were found, 10 of which also occur in Central Amazonia by current taxonomic standards. Five species occurred exclusively in temporary water bodies ( Scinax ruber , Physalaemus ephippifer , Leptodactylus knudseni , L. macrosternum , and ´Hyla sp. 2 ´). Three species occurred exclusively in permanent water bodies ( Boana boans , B. wavrini ,´ Hyla sp. 1´) , and five occurred in both temporary and permanent water bodies ( Rhinella marina , R. major , Boana raniceps , Lysapsus bolivianus , Leptodactylus fuscus ). As in terra-firme forests, alpha diversity was much lower than gamma diversity: even if the 40 water bodies contained altogether 13 species, mean number of species per water body was only 1.6, ranging from 1 to 7 ( Azevedo-Ramos et al. 1999).
Distributional patterns at the microhabitat scale. Clearly differentiating interspecific microhabitat use is challenging due to both variation in individual behavioral responses (i.e., generalism, opportunism, and behavioral plasticity) and variation in habitat structural complexity (i.e., many anuran species occur in small, shallow bodies of water lacking vegetation cover where vertical habitat use differentiation is meaningless). Nevertheless, natural history observations coupled with morphological inferences suggest that there is some level of differential microhabitat use by Central Amazonian tadpoles. These will be dealt with below in´Ecomorphological guilds´.
Phenology
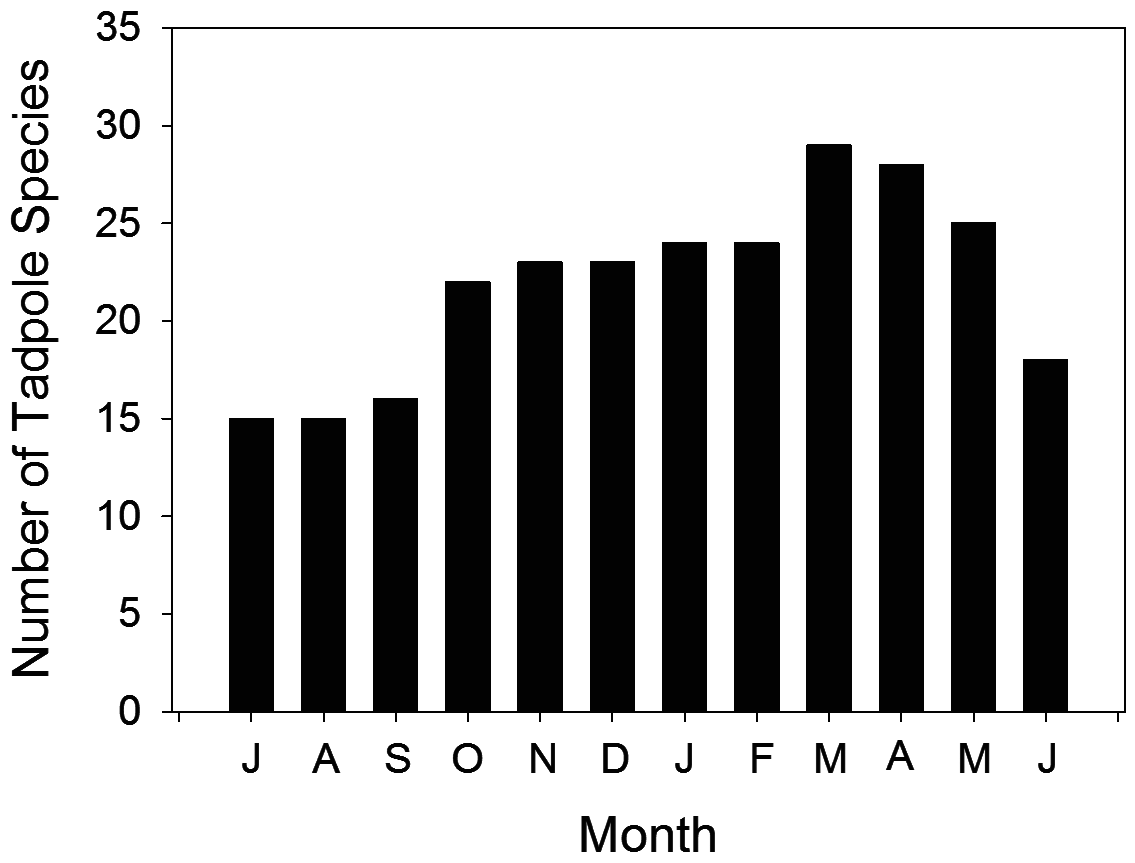
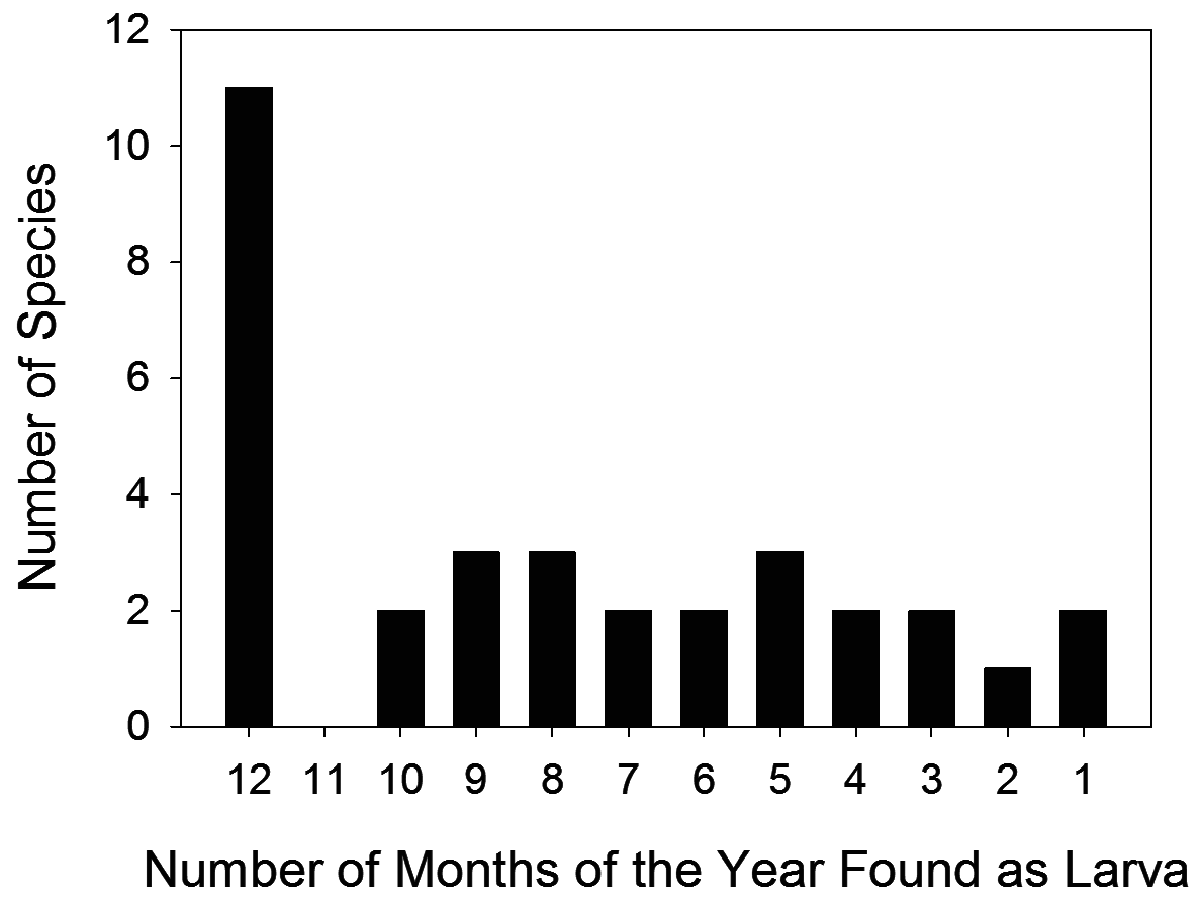
Detailed phenological records of amphibian larvae are available for terra-firme faunas only. A presence-absence analysis of the temporal distribution of anuran eggs, embryos, and larvae in terra-firme forests ( Table 3, Figs. 74 View FIGURE 74 , 75 View FIGURE 75 ) evidences a fairly large number of species in every month of the year but with a clear seasonal trend for higher richness in the wet season. Typically, there are over 24 and up to 30 species in November-May as opposed to 17 species in June-September ( Fig. 74 View FIGURE 74 ). Assuming that a species not found in a given month but found in the previous and following months is a false negative (i.e., would be found as embryo or larva with sufficient sampling effort), no less than 11 of 33 tadpole species (33%) are found in every month of the year ( Fig. 75 View FIGURE 75 ). These include all representatives in genera Allobates , Vitreorana and Phyllomedusa , as well as Boana geographica , B. boans , Osteocephalus taurinus and Leptodactylus knudseni . Another five tadpole species ( Amazophrynella manaos , Atelopus manauensis , Boana cinerascens , Osteocephalus cabrerai , and Chiasmocleis shudikarensis ) are found in more than nine months of the year. The occurrence of 10 species is strongly tied with the rainy season, including Rhinella merianae , R. proboscidea , Scinax ruber , Trachycephalus resinifictrix , Ceratophrys cornuta , Leptodactylus longirostris , L. mystaceus , L. rhodomystax , and Chiasmocleis hudsoni . Three hylids are restricted to the second half of the rainy season ( Dendropsopus minutus , D. sp. of the D. microcephalus group, and D. rossalleni ), as appear to be Dendropsophus cf. parviceps and Trachycephalus coriaceus , recorded in a single month each (sensu Wells 1977; Schiesari & Moreira 1996). Only one species, Rhinella marina , is a clear dry season specialist.
Two life history strategies could account for such prolonged patterns of larval occurrence. One is prolonged breeding; i.e., reproductive events occurring over many months of the year, probably as an opportunistic response to rain fall, low air pressure, and increased humidity. The other is long larval periods, i.e., long times to metamorphosis. For some taxa both strategies may apply. We have limited knowledge on times to metamorphosis for most species in the region, but calling activity patterns indicate that prolonged breeding is a common reproductive strategy among Central Amazonian frog species ( Hero 1991; Gascon 1991). Moreover, long larval periods are unlikely to account for the prolonged patterns of occurrence of 19 species in isolated forest ponds, two species in phytotelmata, and at least part of the 24 species in streamside forest ponds because the water bodies they occur are temporary (cf., Gascon 1991). In conclusion, prolonged patterns of larval occurrence for these 34 species colonizing temporary water bodies likely reflect opportunistic (even if not year-round) breeding.
Among species with terrestrial development, Leptodactylus pentadactylus calling activity and the only three clutches found to date occurred in the end of the dry and onset of the wet season ( Hero & Galatti 1990; Menin et al. 2010; Faria da Costa et al. 2013). Based on calling activity Adenomera andreae , Pristimantis fenestratus and Synapturanus spp. breed during the wet season with their highest probability of breeding in the beginning and middle of the rainy season (Schiesari 1996; Menin et al. 2008; Rojas-Ahumada & Menin 2010).
Only limited information is available regarding the phenology of larval anurans in Amazonian floodplains. Judging from calling sampling surveys conducted by Ĥdl (1977) in the floating meadows of Lake Janauari, prolonged breeding is common in várzea floodplain species as well. Eight of 15 species called in every one of the 8 months in which sampling occurred; two species called in 7 months, one in 6, one in 5, two in 4 and one in 3 months. Gravid females and/or amplectant pairs of several species were found from January to August. Sampling surveys specifically designed for larval amphibians in floating meadows were conducted by us (Marcelo Menin and David O.C. Telles, unpublished data) in Lake Catal„o between January and June 2013; the lake was mostly dry before our sampling started. From a total of 12 tadpole species, seven were found in January, 10 in April and seven in June. Boana raniceps and D. walfordi were common throughout the sampling season, especially in January. Dendropsophus haraldschultzi , D. leucophyllatus , Lysapsus bolivianus , Scinax sp. 1 and Leptodactylus leptodactyloides were found throughout the sampling season as well, but always in lower abundances. Tadpoles of Scarthyla goinorum , Scinax garbei , Sphaenorhynchus dorisae and Trachycephalus typhonius were captured only once.
In conclusion, a variety of reproductive strategies are found among Central Amazonian frogs, from truly prolonged breeders displaying little or only moderate seasonality to explosive breeders for which a single reproductive event in the late rainy season has ever been recorded ( Schiesari & Moreira 1996). Of course, this interpretation should be taken with caution because is based exclusively on presence/absence data. Quantitative data on species abundances will likely reveal interspecific variation in the timing in peaks of abundance and will be essential in furthering our understanding on the importance of species interactions in shaping ecological communities.
Species interactions
Predators. Both observational and experimental data demonstrate that a wide variety of animals prey upon the eggs, embryos, and larvae of Central Amazonian frogs. Considering eggs deposited out of water (i.e., on the forest floor, in leaves overhanging water, or in foam nests in burrows or depressions), recorded predators include spiders, cockroaches, ephydrid and phorid dipteran larvae, staphylinid beetles, ants, wasps, gymnophthalmid and sphaerodactylid lizards, dypsadid snakes, and even capuchin monkeys ( Table 4). There are few records of predators of terrestrial tadpoles undoubtedly due to the difficulty in finding and observing nests of species with terrestrial tadpoles. Their known predators include spiders, cockroaches and wasps. By contrast, many are the predators of aquatic eggs and tadpoles including spiders, aeshnid dragonfly naiads, larval and adult dytiscid beetles, rivulid, lebiasinid, and cichlid fishes, other tadpoles (see´Food´ below), pipid frogs, typhlonectid caecilians, turtles and dipsadid snakes. In addition, hydrophylid beetles and wasps are recorded predators of aquatic eggs, whereas crabs, libellulid dragonfly naiads, and belostomatids are documented predators of aquatic tadpoles ( Table 4).
The intensity of these predator-prey interactions was addressed by only a few, nevertheless insightful, studies (Hero 1990; Magnusson & Hero 1991; Gascon 1992a). These studies found that predation on amphibian eggs ranged from nil to very high. Fishes were experimentally found to be unimportant predators of aquatic amphibian eggs in Central Amazonian terra-firme rainforests ( Hero 1991; Magnusson & Hero 1991). The common pond-dwelling lebiasinid fish Pyrrhulina sp. ate no eggs of Boana boans (a total of 220 eggs offered in eleven 12-hour trials with each individual fish offered 20 eggs), B. geographica (140 eggs offered) and Osteocephalus cabrerai (180 eggs offered). Eggs of O. taurinus were also avoided by Pyrrhulina , considering that only 4 of 120 eggs were consumed. Likewise, the common stream-dwelling cichlid fish Aequidens pallidus ate only 7 eggs of Boana boans (100 eggs offered), 7 of B. geographica (40 eggs offered), and 0 of O. taurinus (100 offered). In strong contrast, Pyrrhulina sp. ate all of the arboreal eggs of Phyllomedusa vaillantii (60 eggs offered; no trials were conducted with A. pallidus ). The fact that Pyrrhulina readily ate 100% of the arboreal eggs, but <1% of the aquatic eggs suggests that aquatic eggs have adaptations to avoid predation, rather than an experimental artifact ( Hero 1991; Magnusson & Hero 1991). This defense mechanism is presumably unpalatability.
The intensity of tadpole predation is also highly variable, and the identity and relative importance of the most important predators of larval amphibians differ from that of eggs. Dragonfly naiads, in particular, are not important egg predators but prey voraciously on tadpoles. Gascon (1992a) found that aeshnid dragonflies were the most effective predators of tadpoles of Osteocephalus taurinus followed by libellulid dragonflies, Pyrrhulina and Rivulus sp. The voracity of aeshnid dragonflies relative to Pyrrhulina over O. taurinus tadpoles was confirmed in a different set of experiments by Gascon (1989b), who also found that predation rates decline with tadpole size as dragonflies are gape limited predators. Considering multiple species, the importance of dragonfly naiads was further reinforced in no-choice experiments in which the aeshnid Gynacanther preyed between 78 and 100% of all tadpoles of 10 species offered ( Boana boans , B. geographicus , Osteocephalus cabrerai , O. taurinus , Phyllomedusa bicolor , P. tarsius , Amazophrynella manaos , Allobates sumtuosus , Leptodactylus knudseni , and Chiasmocleis hudsoni ; Hero 1991).
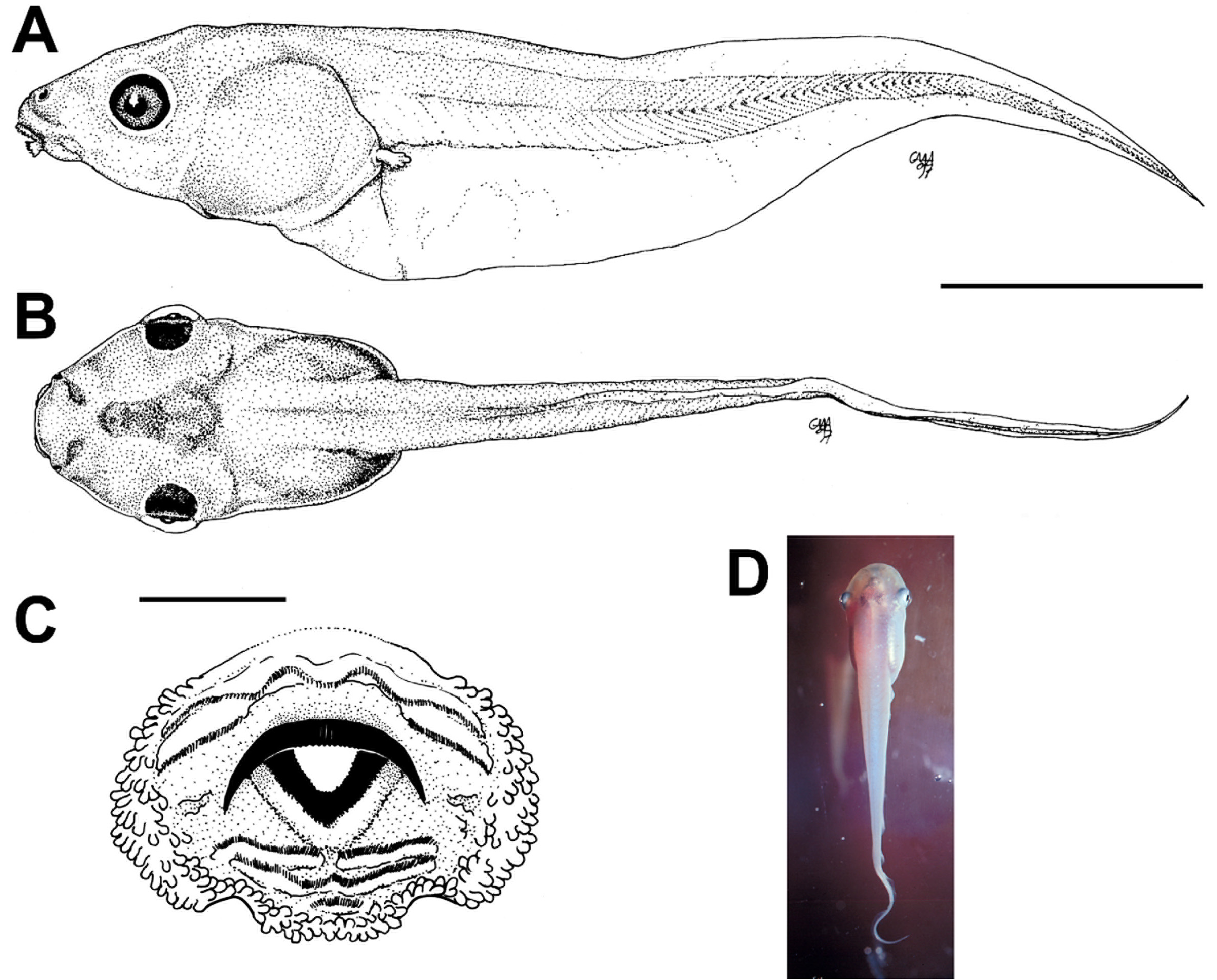
Locomotion patterns appear to be important modulators of predation risk of Amazonian tadpoles against dragonflies. Azevedo-Ramos et al. (1992) recorded distance covered, rapidity and survival times of tadpoles of Boana geographica , Osteocephalus taurinus , Phyllomedusa tarsius and Scinax ruber exposed to Gynacantha membranalis . They found that survival time decreased with distance covered and increased with rapidity (the authors did not measure maneuverability, i.e., angular and linear acceleration, probably more important than velocity at short distances; Hoff & Wassersug 2000). In other words, against these visually-oriented, sit-and-wait dragonfly predators selection appears to favor tadpoles that move infrequently and fast (such as S. ruber ), decreasing the naiads’ efficiency in locating and capturing their prey, rather than tadpoles moving slowly and continuously (such as B. geographica , the only species not co-occuring with G. membranalis ; Azevedo-Ramos et al. 1992). Interestingly, P. tarsius remains most of the time motionless and perpendicular to the water surface by the continuous, independent beating of the tail tip ( Fig. 70D View FIGURE 70 ).
Whereas predation by dragonfly naiads appears to be consistently high (at least until a size-refuge from predation is attained, for species that grow sufficiently large), there is wide interspecific variation in tadpole vulnerability to fish predators.As for eggs, it is likely that unpalatability is an important defensive mechanism for tadpoles, which are, relative to fish, poor swimmers in speed. Following a design similar to that employed for testing egg predation rates, Hero (1991) conducted experiments offering tadpoles of 17 species to the fishes Pyrrhulina sp , Rivulus sp. (a rivulid), Erythrinus erythrinus (an erythrinid), and Aequidens pallidus . Three tadpole species ( Boana boans , B. geographicus , Phyllomedusa vaillantii ) were unpalatable to all fish species tested (that is, less than 20% of tadpoles offered were eaten). Eight were palatable to some fish predators and unpalatable to others ( Amazophrynella manaos , Rhinella merianae , Boana cinerascens , Osteocephalus cabrerai , O. taurinus , Phyllomedusa bicolor , P. tarsius , and Callimedusa tomopterna ) and six were moderately to highly palatable (that is, at least 50% of tadpoles offered were eaten) to all fish species ( Allobates sumtuosus , Leptodactylus knudseni , L. rhodomystax , Scinax ruber , Osteocephalus oophagus and Chiasmocleis hudsoni ). These were no-choice experiments as Hero (1991) offered tadpole species individually. However, predators exhibit preferences. Gascon (1992a) compared the predation rates of tadpoles of Osteocephalus taurinus , Allobates femoralis and Callimedusa tomopterna exposed to aeshnid dragonflies, libellulid dragonflies, and the fishes Pyrrhulina sp. , Rivulus sp. 1 and Rivulus sp. 2 and found that Callimedusa tomopterna was consistently preferred over O. taurinus and A. femoralis .
Compared to naiads and fish, tadpoles are unimportant consumers of other live tadpoles with the possible exceptions of Ceratophrys cornuta , Dendropsophus rossalleni , and Leptodactylus rhodomystax ( Table 4).
As in other animals, in Central Amazonia tadpole unpalatability may be coupled with a presumably aposematic (warning) coloration. Visually-oriented predators eating distasteful tadpoles with conspicuous coloration would associate the color to the bad taste, and would learn to avoid them after one or some experiences. At least three and possibly four species appear to satisfy these conditions. Rhinella marina and Boana geographica are entirely black and unpalatable to all fish species tested (see Wassersug 1973 for R. marina ). Phyllomedusa vaillantii and P. bicolor are bright orange; P. vaillantii is unpalatable to all fish species tested whereas P. bicolo r is unpalatable to some fish species, but not others. The efficiency of a warning coloration is enhanced by the aggregative, schooling behavior of the tadpoles. The large, black tadpoles of Boana geographica form conspicuous schools of up to 3,000 individuals ( Caldwell 1989). Two types of schools are performed, moving or stationary: in the typical moving schools, tadpoles in close physical contact with each other form large, rolling spheroid masses moving slowly in the water column. Tadpoles may spread and graze on the bottom, later regrouping. In the stationary schools, tadpoles are aggregated as a single layer at the surface, and alternate periods motionless with periods of swimming towards the center. In both types, individuals continually swim toward the center of the school, which would presumably reduce the individual chances of being preyed. Morevover, this behavior also confers to the entire school a moving, large dark image that could frighten potential predators ( Caldwell 1989). The same antipredatory function was attributed to the ameboid, loosely aggregated schools of the black tadpoles of Rhinella marina ( Wassersug 1973) .
A different type of school is performed by the bright orange tadpoles of Phyllomedusa vaillantii . These aggregations resemble fish schools in the regular orientation, spacing and coordination of the individuals, which may be found grazing on the bottom, feeding on plankton in midwater head-up at a 45 o angle, or feeding at the surface head-up at a 90 o angle ( Branch 1983). Tadpoles are more gregarious by day than at night; this behavior is mediated by light, as indicated by the tightening of schools if a lamp illuminates the pond during the night. Other species of Phyllomedusa are known to form schools, such as Phyllomedusa tarsius ( Duellman 1978) . Schooling behavior in Central Amazonian tadpoles could be protection against predators. However, at least in Leptodactylus macrosternum schooling results in an increased feeding efficiency. The up and down movements of the school in the water column disrupts the debris on the bottom and puts in suspension particulated detritus and small bottom-dwelling organisms, which constitute the tadpoles’ diet ( Dixon & Staton 1976).
Apart from the presumably aposematic coloration of tadpoles of Boana geographica , Rhinella marina and Phyllodemusa vaillantii , other protective color patterns may be present in Central Amazonian anuran larvae. Camouflage, which in the animal kingdom is a widespread means or reducing the probability of being detected by visuallyoriented predators, appears to be present in many if not most tadpole species occurring in Central Amazon. Camouflage by background color matching appears to be very common, considering the predominance of dull colors such as beige, brown and gray associated with irregular dark spots and blotches (see, e.g., Allobates sumtuosus in Fig. 5D View FIGURE 5 ). The importance of camouflage is further suggested by the observation that in several species, color and color patterns may differ depending on the color of the substrate or water turbidity (see a Leptodactylus knudseni from a clayish puddle, Fig. 54D View FIGURE 54 ), or over ontogeny. Eggs of the aromobatid frog Allobates femoralis are laid in the leaf-litter; after completion of the embryonic development, tadpoles are carried on the dorsum of one of the parents to a temporary water body ( Lima et al. 2012). During the transport phase, tadpoles are black as is the color of the parent’s dorsum; however, coloration changes to beige or brownish-gray in the water, therefore maintaining camouflage, but to a different background (Schiesari, pers. obs.). Camouflage by disruption, i.e., in which color patterns break the shape and/or the outline of the individual, could also be occurring. Several species of Dendropsophus , including D. cf. brevifrons ( Fig. 24A, B View FIGURE 24 ), D. haraldschultzi ( Fig. 25A, B View FIGURE 25 ), and D. parviceps ( Fig. 29A, B View FIGURE 29 ), as well as Lysapsus bolivianus ( Fig. 32D View FIGURE 32 ), present a broad dark band in the posterior half or third of the tail; all these species are nektonic and, therefore, supposedly visible swimmers.
Diet. Information on Central Amazonian tadpole diets is scant. Table 4 suggests that oophagy is widespread (see Figure 76 View FIGURE 76 for a tadpole with a dilated venter due to egg consumption). Necrophagy towards other tadpoles (observed in Rhinella marina , Leptodactylus knudseni , Trachycephalus coriaceus ; L. Schiesari pers. obs.) and other animals (a Leptodactylus knudseni tadpole was observed voraciously eating a dead earthworm; L. Schiesari pers. obs.) is probably also common. Cannibalism towards live tadpoles was observed in just a few cases but is probably common towards early developing embryos judging from the importance of oophagy. Consumption of detritus, periphyton and phytoplankton, which are important basal food sources for generalized anuran larvae (e.g., McDiarmid & Altig 1999) are surely more widespread. Boana geographica is frequently seen grazing on periphyton (L. Schiesari pers. obs.). Allobates femoralis is known to consume detritus ( Weygoldt 1980) as is Trachycephalus resinifictrix (Schiesari et al. 2003a) . All phyllomedusid species occurring in the Central Amazonia are frequently observed suspension feeding at the surface or in midwater, but are also observed grazing. Gut content analysis demonstrated that Phyllomedusa vaillanti ingests a large amount of phytoplankton and particulate matter ( Branch 1983).
Parasites. Although parasitism is an important and widespread type of interspecific interaction, to our knowledge the only record of parasites for any Central Amazonian tadpole is that of an undescribed species of Argulus (Crustacea, Branchiura, Argulidae ). Argulus is an ectoparasite that was observed on a single occasion to infest about 20% of all individuals of Boana geographica in a terra-firme impounded stream (L. Schiesari, E. Fischer and M. Gordo pers. obs.; species identification by J. C. Malta, INPA). Argulid branchiurans are parasites of a number of aquatic vertebrates, including fish, adult amphibians, turtles and caimans, and it is likely that this particular species is generalized with respect to host (J. C. Malta, pers. comm.). The behavior of the tadpoles of B. geographica , which form dense, tightly aggregated schools, may facilitate the rapid spread of a parasite population once a single host individual is infected. Endoparasites are less conspicuous and have not been reported for Central Amazonian tadpoles.
Competition. While natural history observations suffice for detecting the occurrence of predation or parasitism (even if not their importance), competition can only be unequivocally demonstrated experimentally. No experimental studies have been conducted about competitive interactions in Central Amazonian tadpole communities. Nevertheless, intra- and interspecific competition is very likely to be important not only because it is widespread in tadpole assemblages elsewhere but also because high densities of eggs and/or tadpoles of tadpoles of the same or different species are frequently observed in nature. For example, Schiesari et al. (2003a) found up to 16 Trachycephalus resinifictrix individuals per liter in water-filled treeholes, which are constrained habitats characterized by very low food availability. Likewise, small, shallow temporary ponds sometimes present high tadpole densities, including several coexisting species with very similar ecomorphological features (see below:´Ecomorphological guilds´).
Ecomorphological guilds
A guild is a set of species that utilize resources in similar ways and thus potentially compete (e.g., Holt 2009). Altig & Johnston (1989) proposed a system, later revised by McDiarmid & Altig (1999), for the classification of ecomorphological guilds of anuran larvae. As presented in´Reproductive modes and premetamorphic development´, the highest-order division in their classification is the source of nutrients for the embryos and larvae, dichotomized as either endotrophs and exotrophs. Among endotrophs, six guilds were recognized based on the loss of tadpole features, the site of embryo-larval development, and the association between parents and their offspring. Among exotrophic species, 15 guilds were recognized: three common to lentic and lotic habitats, five found exclusively in lentic habitats, and seven found exclusively in lotic habitats. Definition of guilds was based on morphology, known and presumed diet, and known and presumed natural history.
Although their classification system captures the diversity of embryo-larval anuran ecomorphotypes, its application is difficult. This is because ecological information on tadpole food and feeding are scant, but also because branching in their system can be variously defined by habitat (´arboreal´), microhabitat (´benthic´), trophic level (´carnivorous´), size of particle ingested (´macrophagous´), feeding mode (´suspension feeder´) and mechanism of maintaining position in water (´adherent´). Evidently, the autecology of any species is defined by a combination of every one of these attributes (for example, a species could be lentic, benthic, carnivorous and macrophagous at the same time).
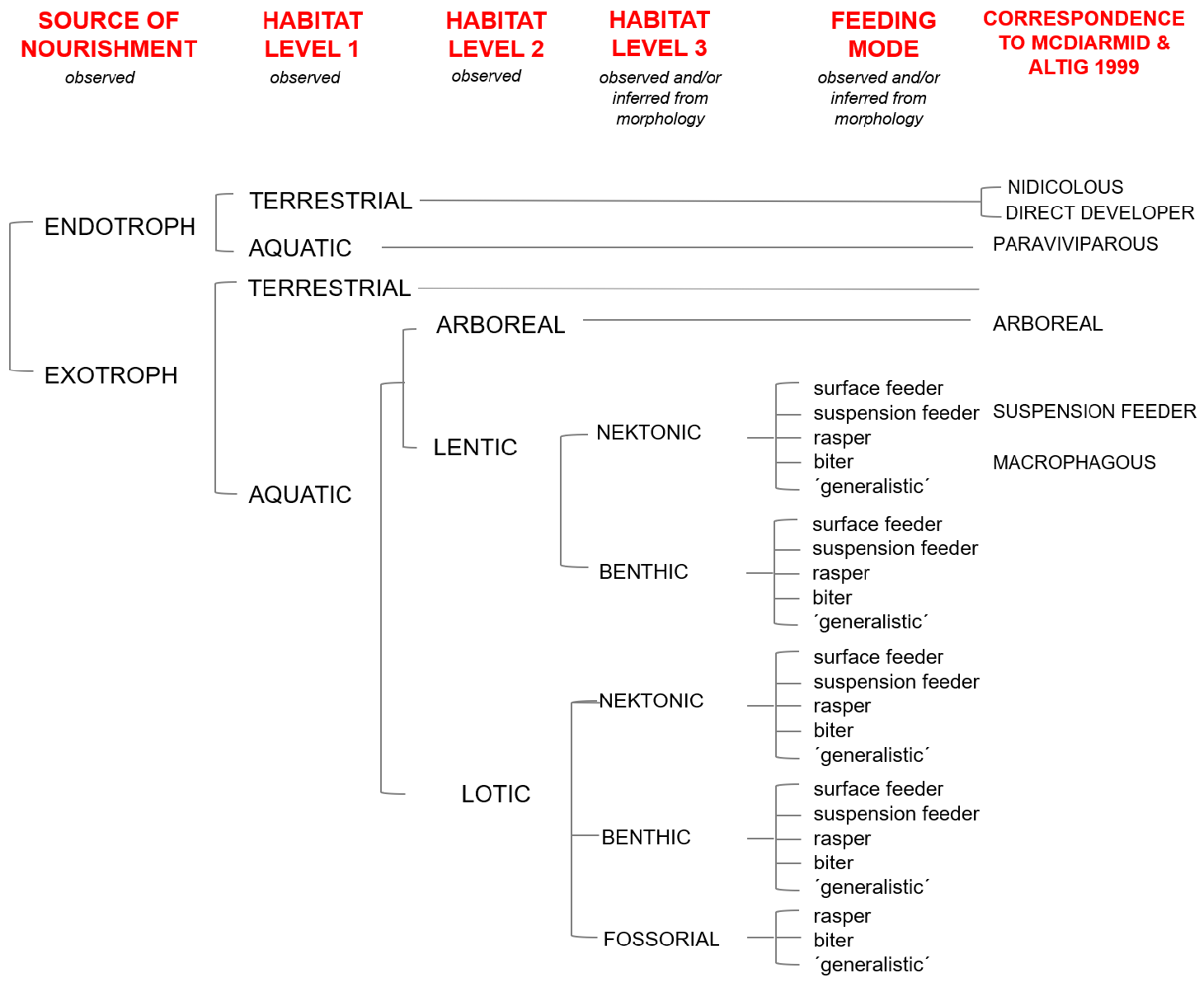
We therefore propose a classification system of tadpole ecomorphological guilds that is inspired, but attempts to circumvent, some of the drawbacks of the classification system of Altig & Johnston (1989) and McDiarmid & Altig (1999). The logical structure of our classification system is presented in Fig. 77 View FIGURE 77 as a decision tree with five hierarchical levels. The first hierarchical level is source of nourishment (endotroph or exotroph). The second, third and fourth hierarchical levels refer to larval habitat including whether terrestrial or aquatic; among aquatic habitats, whether arboreal, lentic surface water body or lotic surface water body; and among surface water bodies, whether the tadpole occupies fossorial, benthic or nektonic microhabitats. The fifth hierarchical level refers to predominant feeding mode as surface feeding, suspension feeding, rasping, biting or generalized (see below). To avoid further subjectivities, guilds are unnamed—they are simply descriptions of the abovementioned attributes (´exotroph, aquatic, lotic, nektonic, generalist feeder´) as is usually the case of traditional bird guilds (e.g., the´foliage- and bark-gleaning guild of insectivorous´). When additional information is available, we add by the end of the description (e.g.,´with a strong reliance on periphyton grazing´). Using this system all endotrophic guilds of McDiarmid and Altig (1999) remained unchanged; however, only a subset of their exotrophic guilds could be retained (´arboreal´,´suspension feeders´) without adjustments.
We first synthesized relevant morphological traits, diet, feeding mode, and position in the water column of all species described in this monograph ( Table 5). Morphological traits included body shape, eye position (i.e., position on the head) and eye direction (i.e., direction of the gaze), fin height, oral disc position, and presence and number of keratinized mouthparts. Because body shape is indicative of position in the water column, body shape was coded as´depressed´ (body height/body width ratio <0.9),´intermediate´ (BH/BW = 1) or´compressed´ (BH/BW> 1.1). Slightly depressed (BH/BW = 0.9) and slightly compressed (BH/BW = 1.1) bodies were marked in grey in Table 5; greater or smaller ratios were marked in black. Eye position and direction were coded as´eyes dorsally or dorsolaterally positioned and directed´,´eyes laterally positioned and directed´ and´intermediate´ (for example, in cases where eyes were dorsolaterally positioned but laterally directed). Fin height was coded as´dorsal and ventral fins shallow´,´dorsal and ventral fins moderately high or high´ and´intermediate´ (for example, when the dorsal fin was shallow but the ventral fin was moderately high). Oral disc position (or mouth position, when an oral disc was not present) was coded as´ventral´,´anteroventral´ or´terminal´. Keratinized mouthparts comprised one column for´jaw sheaths absent´, one column for´jaw sheaths present´ and one column for the basic labial tooth row formula (i.e., LTRF without reference to tooth row gaps). Additional morphological traits that are relevant for assessing resource use (such as, for example, the presence of a large abdominal disc in Atelopus ) were annotated in the column´Other´ (´other morphological observations´).
Feeding habits—diet and feeding mode—were also presented and coded combining our own field observations with published literature based on field observations, gut content analyses or experiments. Because data on tadpole diets are scant, studies conducted outside of Amazonia were also included. Dietary items were classified as´phytoplankton´,´zooplankton´,´periphyton´,´detritus´,´higher plants´,´invertebrates´,´amphibian eggs´,´tadpoles (live)´and´carrion (necrophagy)´. Items reported in the literature as´rare´ or with frequency of occurrence <10% in gut content analyses were ignored. Data on consumption of amphibian eggs were color coded according to the voracity with which eggs were consumed. This was possible because most data on oophagy were quantified in experiments by Magnusson & Hero (1991). Oophagy was reported as absent (<10% of the eggs offered were consumed), slight (10% <x <25%, light grey), moderate (25% <x <50%, grey), and substantial (>50%; black). Some species not tested by Magnusson & Hero (1991) were so voracious egg predators that could be easily classified as´substantial´ egg predators even if the methodology of assessing oophagy was different (e.g., Osteocephalus oophagus , Trachycephalus resinifictrix ). Feeding mode was classified as´surface film feeding´,´suspension feeding´,´rasping´,´biting´, and´swallowing´.´Swallowing´ was used for referring to ingestion of large intact particles (such as amphibian eggs).
Finally, position in the water column was classified as´fossorial´,´benthic´ and´nektonic´. We do not recognize strictly´neustonic´ tadpoles in Central Amazonia even though some species are known to forage in the water surface.
All data presented here on morphological traits, diet, feeding mode, and position in the water column are either observational or experimentally derived. By contrast, ecomorphological guilds are hypotheses based on (i) these information (ii) known or expected correspondence between form, function and natural history (such as in Wassersug 1980 and McDiarmid & Altig 1999) (iii) feeding habits in species morphologically similar and/or phylogenetically related and (iv) ecological plausibility (e.g., it is not a reasonable expectation that suspension feeding is a viable feeding mode for tadpoles in fast flowing water).
In the definition of guilds, source of nourishment (endotroph vs. exotroph) and habitat (level 1: terrestrial vs. aquatic; level 2: among aquatic habitats, arboreal vs. lentic vs. lotic) are all based in the information recorded in Tables 1 and 2. Species living exclusively or predominantly in ponds and puddles are considered lentic, and those living exclusively or predominantly in streams are considered lotic. Species living in floodplain lakes and rivers are considered lentic and lotic because usually associated to shallow shores or floating meadows which are lentic biotopes. In the definition of guilds, microhabitat (fossorial vs. benthic vs. nektonic) and feeding mode (surface feeder vs. suspension feeder vs. rasper vs. biter vs´generalistic´) are based on a combination of behavioral observations, gut content analyses and ecomorphological inferences. For example, unless natural history observations indicate otherwise, species with a combination of depressed bodies, dorsal or dorsolateral eyes, shallow fins and ventral oral disks are considered benthic. Species with compressed bodies, lateral eyes, moderately high or high tail fins and terminal oral disks were considered nektonic. Likewise, unless natural history observations indicate otherwise, species lacking keratinized jaw sheaths are presumed to obtain their food by means of suspension feeding. Tadpoles of species possessing jaw sheaths but no labial tooth rows are presumed to obtain their food by biting, and those possessing labial tooth rows are presumed to be capable of scraping. Tadpoles possessing numerous labial tooth rows are presumed to rely extensively on scraping course material. Natural history observations most often match these expectations. For example, microhylids lack keratinized mouthparts and feed primarily by suspension feeding. Dendropsophus possesses a jaw sheath but no or few labial tooth rows and were observed actively biting other tadpoles. Most tadpoles have labial tooth rows and feed by scraping periphyton and detritus. A´generalist feeder´ is a species whose tadpole either was observed to display several feeding modes or to possess a generalized morphology with no clear indication of predominance of one feeding mode relative to others.
As one example, phyllomedusids have compressed bodies (slightly depressed in P. bicolor ), lateral eyes, fins of intermediate height (as ventral fins are moderately high or high but dorsal fins are invariably shallow), and an anteroventral oral disc with LTRF 2/3. As one might expect based on their morphology, natural history observations demonstrate that they are all nektonic. In Phyllomedusa tarsius , the tail terminates in a very distinct flagellum (not as distinct in other species) capable of independent movement in such a way that tadpoles retain their position in the water column with no noticeable body movement. P. vaillanti was observed surface film feeding, suspension feeding, and rasping surfaces. As expected, its gut contents included phytoplankton, periphyton and detritus ( Branch 1983). Upon dissection P. vaillanti revealed large and deep branchial baskets with dense filter mesh (LS pers. obs) and, in addition, refused to eat amphibian eggs when offered them ( Magnusson & Hero 1991). The morphological and behavioral similarity of all four species permitted us to propose that Central Amazonian phyllomedusids are suspension raspers, i.e., tadpoles that feed by a combination of filtering from the water column and rasping submerged surfaces (as, in fact, proposed by McDiarmid & Altig 1999). Other hypothesized guilds, and the morphological and natural history observations on which their classification is based, are presented in Table 5.
Morphology Diet Feeding mode Position
Body Eyes Fin height Oral disc Mouthparts high
dorsolateral high or absent present plants eggs) live necrophagy
)
Hypothesized Guilds and Species depressed intermediate compressed or dorsal intermediate lateral shallow intermediate moderately ventral anteroventral terminal sheaths jaw sheats jaw LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian tadpoles (carrion (surface feeding feeding suspension rasping biting swallowing fossorial benthic nektonic
ENDOTROPHS
Terrestrial
Endotroph, terrestrial, nidicolous
Anomaloglossus stepheni - - - - - - - - - - - - - - - - - -
Adenomera andreae 0/0 - - - - - - - - - - - - - - - - -
Adenomera hylaedactyla 0/0 - - - - - - - - - - - - - - - - -
Synapturanus ajuricaba - - - - - - - - - - - - - - - - - -
Synapturanus mirandaribeiroi - - - - - - - - - - - - - - - - - -
Endotroph, terrestrial, direct developer
Pristimantis fenestratus - - - - - - - - - - - - - - - - - - - - - -
...Continued on the next page high
dorsolateral or high absent present plants eggs live)
) necrophagy
Hypothesized Guilds and Species depressed intermediate compressed or dorsal intermediate lateral shallow intermediate moderately ventral anteroventral terminal sheaths jaw sheats jaw LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian tadpoles ((carrion surface feeding feeding suspension rasping biting swallowing fossorial benthic nektonic Aquatic
Endotroph, aquatic, paraviviparous
Pipa arrabali - - - - - - - - - - - - - - - - - -
Pipa pipa - - - - - - - - - - - - - - - - - EXOTROPHS
Terrestrial
Exotroph, terrestrial, possibly oophagous
Leptodactylus pentadactylus 1/2 1 - - - Leptodactylus stenodema ?
Aquatic
Exotroph, aquatic, arboreal
Osteocephalus oophagus 2/3 2
2/3-
Trachycephalus resinifictrix 4 2
Ponds and puddles
Nektonic
Exotroph, aquatic, lentic, nektonic, suspension feeder
...Continued on the next page high
dorsolateral or high absent present plants eggs live) necrophagy
)
Hypothesized Guilds and Species depressed intermediate compressed dorsal or intermediate lateral shallow intermediate moderately ventral anteroventral terminal jaw sheaths jaw sheats LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian tadpoles ((carrion feeding surface suspension feeding rasping biting swallowing fossorial benthic nektonic Dendropsophus marmoratus 0/0
Benthic
Exotroph, aquatic, lentic, benthic, generalist feeder
Allobates sumtuosus 2/3 NO
...Continued on the next page
Morphology Diet Feeding mode Position
Body Eyes Fin height Oral disc Mouthparts high
dorsolateral high or absent present plants eggs live)
) necrophagy
Hypothesized Guilds and Species depressed intermediate compressed dorsal or intermediate lateral shallow intermediate moderately ventral anteroventral terminal jaw sheaths jaw sheats LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian (tadpoles carrion (surface feeding suspension feeding rasping biting swallowing fossorial benthic nektonic
Boana calcarata 2/3
Boana fasciata 2/4
Leptodactylus longirostris 2/3
Exotroph, aquatic, lentic, benthic, biting and scraping carnivore
Ceratophrys cornuta 9/9 5
Exotroph, aquatic, lentic, benthic, generalist feeder with a predominance of macrophagy
Exotroph, aquatic, lentic, benthic, generalist feeder with a predominance of macrophagy of animal matter
...Continued on the next page Morphology Diet Feeding mode Position
Body Eyes Fin height Oral disc Mouthparts high
dorsolateral high or absent present plants eggs) live
) necrophagy
Hypothesized Guilds and Species depressed intermediate compressed or dorsal intermediate lateral shallow intermediate moderately ventral anteroventral terminal jaw sheaths sheats jaw LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian tadpoles (carrion (surface feeding suspension feeding rasping biting swallowing fossorial benthic nektonic
Leptodactylus knudseni 2/3 1
Exotroph, aquatic, lentic, benthic and nektonic, generalistic feeder
Leptodactylus macrosternum 2/3
Lakes and rivers
Nektonic
Exotroph, aquatic, lentic and lotic, nektonic, suspension feeder
Dendropsophus walfordi 0/0 6
Exotroph, aquatic, lentic and lotic, nektonic, suspension rasper
Scinax sp.1 2/3
Scinax sp.3 2/3
Exotroph, aquatic, lentic and lotic, nektonic, biter
Dendropsophus haraldschultzi 0/1
Dendropsophus leucophyllatus 0/0
...Continued on the next page high
dorsolateral high or absent present plants eggs) live necrophagy
)
Hypothesized Guilds and Species depressed intermediate compressed dorsal or intermediate lateral shallow intermediate moderately ventral anteroventral terminal jaw sheaths jaw sheats LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian (tadpoles (carrion surface feeding suspension feeding rasping biting swallowing fossorial benthic nektonic Exotroph, aquatic, lentic and lotic, nektonic, generalist feeder
Rhinella marina 2/3
Pseudis paradoxa 2/3
Benthic
Exotroph, aquatic, lentic and lotic, benthic, generalist feeder
Boana punctata 2/3
Boana raniceps 2/3
Boana wavrini 2/3
Leptodactylus leptodactyloides
Streams
Nektonic
Exotroph, aquatic, lotic, nektonic, generalist feeder with strong reliance on periphyton grazing and oophagy
...Continued on the next page high
dorsolateral high or absent present plants eggs live) necrophagy
)
Hypothesized Guilds and Species depressed intermediate compressed dorsal or intermediate lateral shallow intermediate moderately ventral anteroventral terminal jaw sheaths jaw sheats LTRF Other phytoplankton zooplankton periphyton detritus higher invertebrates amphibian (tadpoles carrion (feeding surface feeding suspension rasping biting swallowing fossorial benthic nektonic
3/5-
Benthic
Exotroph, aquatic, lotic, benthic, generalist feeder with a predominance of macrophagy
Boana boans 2/4
2/3-
Exotroph, aquatic, lotic, benthic/gastromyzophorous, rasper
Atelopus manauensis 2/3 7
Fossorial
Exotroph, aquatic, lotic, fossorial, biter
Footnotes
´Other remarkable morphological traits´: 1 elongated tadpole with heavy jaw sheaths; 2 reduced number of tooth rows, external and internal papillae, and folding pattern of filter rows relative to other spp in genus (Schiesari et al. 1996); 3 tail tip capable of independent movement; 4 large and deep branchial baskets with dense filter mesh; 5 massive tadpole with heavy, pointed jaw sheath; 6 mouth modified as an oral tube; 7 large abdominal sucker; 8 heavy, serrated jaw sheaths.
´Diet´ and´Feeding mode´. Coding is based on own observations and literature (see Table 4 for literature on oophagy; Kenny 1969 for diet of S. ruber , D. minutus , L. petersi , and R. marina ; Branch 1983 for diet of P. vaillanti ; Weygoldt 1980 for diet of A. femoralis ; Weygoldt 1986 for diet of D. parviceps ; Duellman & Lizana 1994 for diet of C. cornuta ; Schiesari et al. 1993b for diet of T. resinifictrix ; and Schiesari et al. 1996 for correlates between morphology and food particle size in Osteocephalus and Trachycephalus ).
A useful application of the classification system is to describe the distribution and diversity of ecomorphological guilds within and among habitats ( Table 6). Focusing on the better-known terra-firme amphibian fauna, isolated forest ponds contain 8 ecomorphological guilds, each with one to eight species, for a total of 24 species. Likewise, streamside forest ponds contain 10 ecomorphological guilds, each with 1 to 14 species, for a total of 33 species. Importantly, although 24 and 33 species might theoretically coexist in isolated forest ponds and in streamside ponds, mean number of species per water body is only 4.7 and 1.6, respectively ( Gascon 1991). Overlap in guild composition between these different lentic water bodies is very high, but isolated forest ponds have three times more species recognized as nektonic suspension feeders and nektonic suspension raspers, while streamside ponds have two to three times more species recognized as nektonic biters and benthic, generalist feeders. The distinctive tadpole of Ceratophrys cornuta , classified as a benthic, biting and scraping carnivore, is exclusive to isolated forest ponds whereas species (and therefore guilds) typical of streams are sometimes found in streamside ponds. Nine species belonging to five ecomorphological guilds are found in streams, including the emblematic fossorial biters ( Vitreorana and presumably Hyalinobatrachium iaspidiense , whose tadpoles is unknown) and the benthic/gastromyzophorous rasper Atelopus manauensis possessing an abdominal sucker that permits life in fast flowing streams. Again, even though 9 species might theoretically coexist in streams, mean number of species per water body was 0.4 ( Gascon 1991). Phytotelms contain one ecomorphological guild,´arboreal´, with three species. These are all strict habitat specialists, as were never found in surface water bodies in many months of intensive sampling. Finally, current knowledge permits recognizing only 5 ecomorphological guilds in lakes and rivers, each containing 1 to 7 species for a total of 22 species. These are nektonic, generalist feeders; nektonic, suspension feeders; nektonic, suspension raspers; nektonic, biters; and benthic, generalized feeders.
Final remarks
Naturalists have been long interested in the high diversity of species in tropical rainforests. At the broadest level, the composition and structure of any ecological community is a result of four classes of processes: speciation, dispersal, drift and selection ( Vellend 2010). Speciation, or more generally evolutionary and biogeographic processes, have a fundamental role in determining the pool of species we observe today in Central Amazonia. This is testified both by the recognition of the Guyanan region as an area of endemism for amphibians ( Ron 2000), and by the proposition of topographic events and landscape restructuration as influencing the diversification of the Central Amazonian fauna ( Badgley 2010; Hoorn et al. 2010). From this species pool, however, local communities are assembled by selection (deterministic fitness differences among individuals of different species), drift (random changes in species relative abundances) and ongoing dispersal (movement of organisms across space) ( Vellend 2010).
A major cornerstone of contemporary ecology was the recognition of stochastic processes in driving patterns of species abundances ( Hubbell 2001). Indeed, the last two decades witnessed a heated debate about the relative roles of niche (i.e., selection) and neutral (i.e. ecological drift) processes in driving community structure. Even though chance is inevitable in nature and must contribute to Central Amazonian amphibian population dynamics, especially for rare species, the natural history this monograph conveys provides a repertoire of arguments for strong interspecific niche differentiation helping to maintain species diversity.
Such niche differentiation is evident in the wide interspecific variation in amphibian reproductive strategies, reproductive phenology, or habitat use at the macro, meso and microscales. Indeed, classical habitat segregation, one of the most frequently documented forms of niche partitioning and species packing ( Schoener 1974; Pianka 1975; Toft 1985), is demonstrated by the nearly complete habitat segregation of lotic and lentic larvae, of pond-dwelling and phytotelmata-dwelling larvae among lentic larvae, and even among species of phytotelmata-dwelling larvae. Or, still, by the presence of up to seven reproductive modes (1, 11, 20, 24, 28, 29, 30) and eight ecomorphological guilds in a single isolated forest pond a few tens of square meters in area. Of course, habitat segregation is a pattern that could result from a variety of different processes within selection, including physiological constraints and interspecific interactions such as competition and predation.
Dispersal and dispersal limitation among Central Amazonian amphibians also have the potential to influence community composition and species distributions. At large spatial scales, successful species introductions provide one of the strongest cases for the importance of dispersal limitation in constraining the set of species capable of joining a community. Leptodactylus labyrinthicus , a species from the open phytophysiognomies of Central and Southern Brazil, Argentina and Paraguay successfully invaded Central Amazonia once dispersal limitation was overcome by human-mediated introduction for frog farming ( Carvalho et al. 2013). Likewise, the expansion of generalistic open area species, such as Leptodactylus fuscus and L. longirostris , accompanying the opening of new roads in Central Amazonia is yet another evidence for habitat change favoring species for which closed-canopy forests appear to be a barrier (see Schiesari et al. 2020 for an analogous scenario of expansion of generalistic open area amphibians in newly deforested land). At landscape-level spatial scales, the composition of larval amphibian assemblages in terra-firme forest water bodies are explained both by their environmental attributes and their spatial relations. This constitutes additional evidence that dispersal limitation plays a role in the organization of Central Amazonian amphibian biodiversity ( Almeida et al. 2015). Indeed, species distributions sometimes appear to deviate from what we know about their environmental demands. For example, there is no obvious environmental reason for why Trachycephalus coriaceus is not found in Reserva Florestal Adolpho Ducke north of Manaus, as apparently similar habitats are available to those where the species occurs.
Of course, these and other phenomena that lie at the heart of biodiversity research are strongly dependent on basic descriptive research about who are the species that compose biological communities and where, when, and how they live. Due to the challenges imposed by diversity in itself, but also to the more recent history of investigation and the strong geographic biases in the distribution of researchers (see, e.g., Schiesari et al. 2007), megadiverse regions are those where basic biological knowledge is most lacking. When we in addition consider the rate of deforestation in the Amazon—totaling 419 thousand square kilometers since our study started (1990-2019; INPE 2020), then it is clear that massive funding of biological research in the region is needed.
In this study we describe the larval stages of three-quarters of anuran amphibian species of Central Amazonia, and amass a considerable volume of natural history observations taken over 20 years of fieldwork. We hope that these efforts inspire, and provide the tools, for further studies on the ecological and evolutionary forces shaping Amazonian anuran assemblages, and their conservation.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.