Rhopalomyia hirtipes ( Osten Sacken 1862 )
|
publication ID |
https://doi.org/ 10.5281/zenodo.188745 |
|
DOI |
https://doi.org/10.5281/zenodo.6217062 |
|
persistent identifier |
https://treatment.plazi.org/id/074287C9-FFC1-E303-FF01-F9B359D53801 |
|
treatment provided by |
Plazi |
|
scientific name |
Rhopalomyia hirtipes ( Osten Sacken 1862 ) |
| status |
|
Rhopalomyia hirtipes ( Osten Sacken 1862)
Cecidomyia hirtipes Osten Sacken 1862: 195 ; Felt, 1908: 363 ( Rhopalomyia View in CoL ).
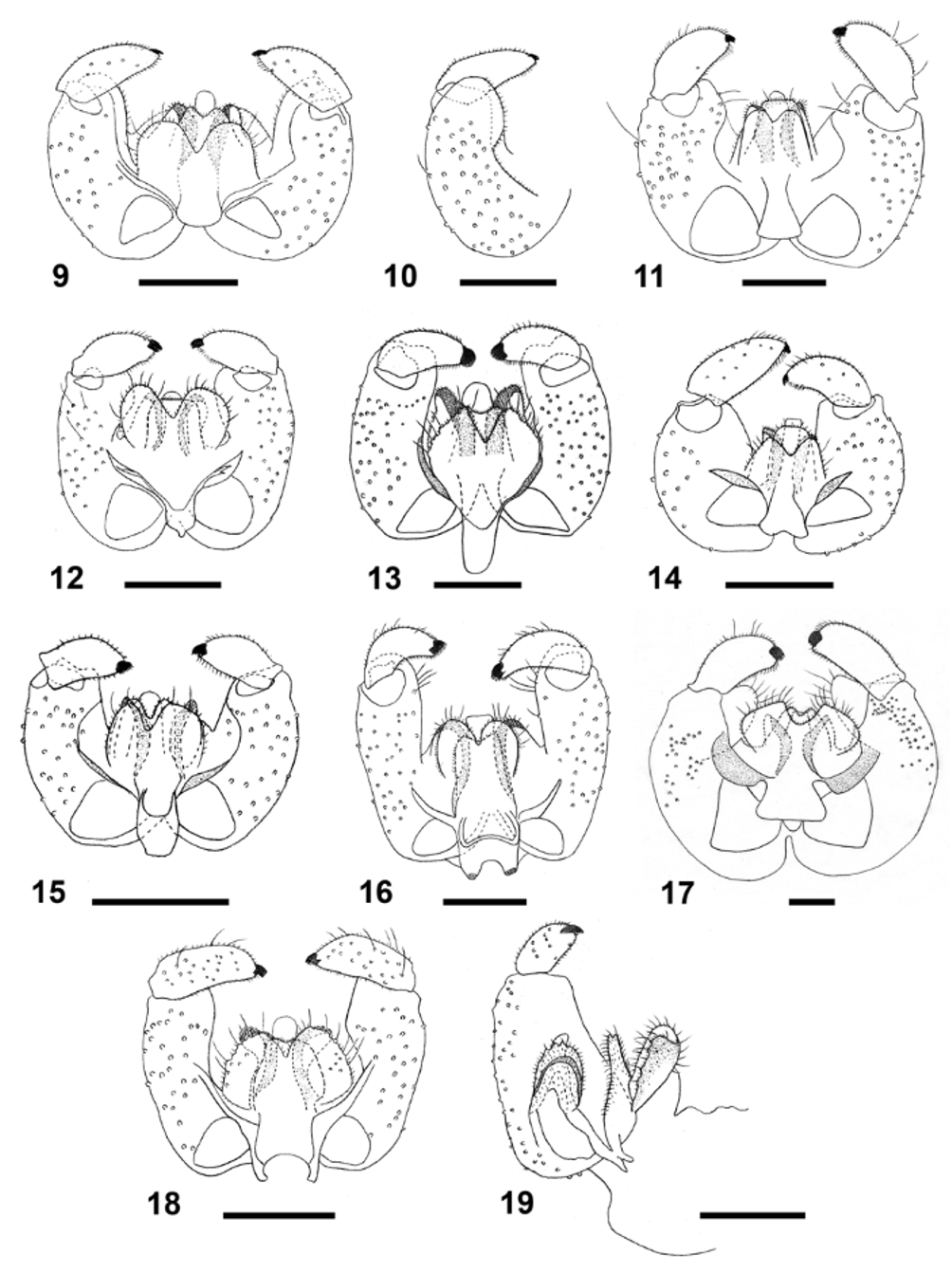
Adult: This is the largest of the Rhopalomyia View in CoL species on goldenrods, which is also striking due to the bright orange-red color of the abdomen and the dark wings and legs. Most of the body is covered by dark, elongate scales. Antenna with 21 flagellomeres in male, 20–22 in female; necks of male flagellomeres III–VII 0.44–0.70 times as long as nodes, necks of female flagellomeres 0.23–0.40 times as long as nodes. Palpus 2-segmented, second segment at least 1.5 times as long as first, with numerous strong, dark setae. Wings dark and densely covered by dark, hair-like setae; wing length 4.0– 4.1 mm in male, 4.0– 4.2 mm in female. Legs densely covered by dark scales. Male terminalia ( Fig. 17 View FIGURES 9 – 19 ): gonocoxite massive, almost bulbous, with prominent apicoventral projection bearing long setae, strongly setose mediobasal lobes, wide, truncate gonocoxal apodeme with strongly sclerotized arms; gonostylus cylindrical and robust, setose and setulose throughout, with small brush-like tooth; aedeagus conical, truncate; hypoproct conical, with shallow notch, setose and setulose; cerci wide and robust, bearing numerous long setae, separated by deep, rounded notch. Female abdomen (Fig. 34): tergites weakly sclerotized; tergite 7 rectangular but more sclerotized in mid- than in lateral parts, with large distal group and smaller mesolateral groups of strong setae; tergite 8 slender Y-shaped, sometimes barely sclerotized along mid part, thus appears to be divided into two longitudinal rods, each with anterior trichoid sensillum and no other perceptible setae; ovipositor 6.5–7.0 times as long as tergite 7.
Pupa ( Figs. 48–49 View FIGURES 48 – 55 ): Bright orange; sclerotized parts black. Large and robust. Antennal bases short and wide, rounded dorsally, with tiny tapering tip anteriorly. Posterior margin of antennal bases wide V-shaped in frontal view. Frons without projections or ridges, considerably wider than long, posterior margins V-shaped. Abdominal segments with tiny barbs all over.
Type material: Cecidomyia hirtipes Osten Sacken. Neotype designated here: male, USA, Eldridge Wilderness, NY, 14/IX/1987, M.V. McEvoy, reared from Solidago juncea . The neotype is designated in order to clarify the taxonomic status of R. hirtipes (Osten Sacken) , whose type series is lost. The original description of the insect and its gall match those of the specimens we reared. The neotype is so labeled and is deposited together with associated permanent microscopic slides of two females and six larvae of the same series in the USNM. This species was based on specimens collected by Osten Sacken in the environs of Washington DC, from S. juncea . These specimens are neither in the MCZC nor in the USNM and we consider them lost.
Other material examined (all from S. juncea ): 1 male, 1 female, USA, NY, Glen Lake, 4/IX/1906, E.P. Felt; 1 male, 1 female, USA, Hoxie’s Gorge, NY, 8/IX/1969, A. Spence; 6 larvae, USA, Hector Ridge, NY, 22/VII/1987, M.V. McEvoy; 1 male, 2 females, USA, Eldridge Wilderness, NY, 14/IX/1987, M.V. McEvoy; 2 pupae, USA, Liberty Valley Rd., PA, 29/IX/2005, T. Dowling; 1 male, 4 females, 2 pupae, 1 exuviae, USA, Liberty Valley Rd., PA, 17/IX/2006, N. Dorchin and M. Wise. Felt (1915) mentioned Elizabethtown and Albany, NY, Springfield and Magnolia, MA, and Evanston, IL as other localities in which galls of this species were collected.
Host: Solidago juncea
Gall and biology: Galls of this species appear at the base of the stem in mid June to mid July ( Fig. 58 View FIGURES 56 – 61 ). They mostly occur just above the ground but are occasionally found up to 60 cm above it ( Fig. 60 View FIGURES 56 – 61 ), and are surrounded by a rosette of long leaves. It is therefore clear that the gall develops from vegetative buds rather than from rhizomes. The young gall is wide at base and has a tapered tip ( Fig. 58 View FIGURES 56 – 61 ), which disappears as the gall matures. During July and August, the gall becomes ovoid, 8–25 mm long and 6–36 mm wide and often changes its color from green to brownish, resembling a small potato ( Figs. 59, 60 View FIGURES 56 – 61 ). Galls are spongy and usually multi-chambered, containing 1–30 individual larvae in separate chambers, with an average of 8 larvae per gall ( Spence 1969). As long as the larvae feed, they face downwards inside their chambers, but before the onset of pupation, in early August, they turn to face upwards. Shortly before adult emergence, the gall splits open at its apex into several lobes, similar to the shell of a hickory nut ( Fig. 61 View FIGURES 56 – 61 ). Adults emerge from early to late September, after which the gall shrivels and dries on the stem. Emergence of all adults from the same gall may continue over more than a week. Some galls fail to split, resulting in death of the pupae inside the gall.
Galls of this species can be very difficult to locate and were rare even in fields where S. juncea was the dominant plant. Although Spence (1969) recorded more than 1200 galls over a 2-year period in central NY, in 1987 they were found in only one field site in that area, and during 2005–2007 we found them in the same single locality out of many fields that were surveyed in central PA. The life history and behavior of R. hirtipes were studied in detail by Spence (1969); mating occurs on the plant a few hours after emergence and females begin to oviposit about 1.5 hours later, depositing eggs mainly under old leaf sheaths on lower parts of the stem, but also on rhizomes and among axils of leaves that surround the gall from which they emerged. Larvae hatch from the eggs about 3 weeks after oviposition and overwinter in the soil. In early spring, the larvae become active and embed themselves in small buds near leaf bases or at the bases of new shoots.
Remarks: This is the largest Rhopalomyia species on goldenrods. It is distinct for its inflated male gonocoxites with their apicoventral projection, wide gonocoxal apodeme, and very widely splayed arms of the 8th abdominal tergite in the female (Fig. 34), which is almost T-shaped rather than Y-shaped as in other Rhopalomyia species on goldenrods. Rhopalomyia hirtipes shares a large number of over 20 antennal flagellomeres with R. solidaginis and R. thompsoni , but differs from them in having relatively short flagellomere necks in the male (very long in R. solidaginis and R. thompsoni ) and relatively long flagellomere necks in the female (absent in R. solidaginis and R. thompsoni ).
| USNM |
Smithsonian Institution, National Museum of Natural History |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Rhopalomyia hirtipes ( Osten Sacken 1862 )
| Dorchin, Netta, Mcevoy, Miles V., Dowling, Todd A., Abrahamson, Warren G. & Moore, Joseph G. 2009 |
Cecidomyia hirtipes
| Felt 1908: 363 |
| Osten 1862: 195 |