Smittina imragueni, Matsuyama, Kei, Titschack, Jürgen, Baum, Daniel & Freiwald, André, 2015
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4020.1.3 |
|
publication LSID |
lsid:zoobank.org:pub:70F8893D-2140-452F-A7DB-BA11A1AF8ADF |
|
DOI |
https://doi.org/10.5281/zenodo.6118925 |
|
persistent identifier |
https://treatment.plazi.org/id/03E42D7E-9459-857F-FF5D-F8B17BF1401E |
|
treatment provided by |
Plazi |
|
scientific name |
Smittina imragueni |
| status |
sp. nov. |
Smittina imragueni n. sp.
( Figs 6 View FIGURE 6 , 7 View FIGURE 7 )
Material examined. Holotype: SMF 40005, station 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex. Paratypes: SMF 40006, station 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex; SMF 40007, station 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex; SMF 40008, station 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex; SMF 40009, station 14904-1, 17°32.559ʹ N, 16°39.805ʹ W, 510 m, Tamxat Mound Complex; SMF 40010, station GeoB 14911-1, 17°28.910ʹ N, 16°41.509ʹ W, 450 m, Tamxat Mound Complex. Smittina cervicornis : NHMUK 1911.10.1.1428, Adriatic, 34 m; NHMUK 1887.12.9.585, Cape Verde Islands, 183–274 m; NHMUK 1944.1.8.281, Cape Verde Islands, 183–219 m; SMF 9811, Riou Island, 34 m; SMF 40011, Adriatic, 15 m.
Etymology. The species name is derived from the Imraguen, a tribe of Mauritanian fishermen inhabiting the area around Banc d’Arguin.
Diagnosis. Erect, bilaminar Smittina with an oval suboral avicularium on the lyrula within the peristome, orientated longitudinally, almost normal to the direction of growth. Spines absent.
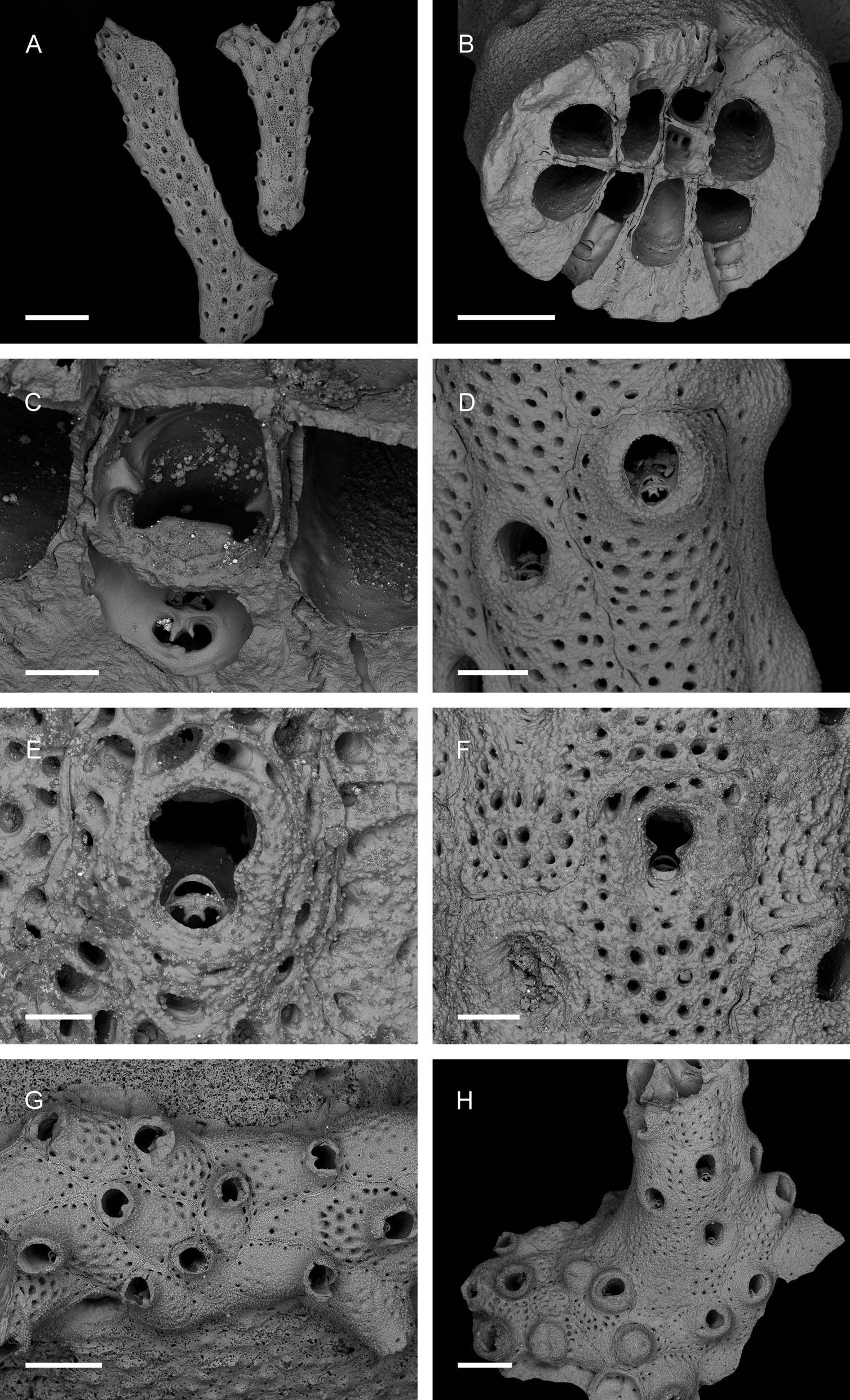
Description. Colony erect, bilaminar, multiserial. Dichotomously branching, internodes flattened ( Fig. 6 View FIGURE 6 A). The laminae of each branch are symmetrical to each other with respect to autozooidal arrangement. Autozooids arranged in diagonal series consisting of four autozooids ( Fig. 7 View FIGURE 7 A). Neighbouring zooids on narrow side of branch are on same level ( Fig. 7 View FIGURE 7 B). Zooids more or less rectangular, distal end rounded, surface convex, boundaries distinct. Sutures in primary calcification straight in cross-sections; serrated in area of secondary calcification ( Fig. 6 View FIGURE 6 B). Septular pores simple. Frontal shield cryptocystidean, evenly porous. Primary orifice elongate-semicircular, deeply immersed, lyrula about half as wide as orifice, truncate. Condyles rounded, directed proximomedially. Oral spines absent ( Fig. 6 View FIGURE 6 C–E). Proximally, but still on lyrula, distinct oval avicularium, nearly normal to direction of growth. Rostrum oval, rounded, proximal border finely serrate, crossbar complete, with bifid ligula ( Fig. 6 View FIGURE 6 C–F). Thick cylindrical peristome develops through secondary calcification, projecting from colony surface, almost concealing primary orifice and in later ontogeny incorporating and almost concealing avicularium. Secondary calcification leads eventually to flattened colony surface, owing to immersion of peristomes and avicularia; peristomial rim forms circular or oval secondary orifice that eventually becomes constricted in middle by ‘pseudocondyles’, separating avicularium from orifice and resulting in keyhole shape ( Fig. 6 View FIGURE 6 F). Zooids early in astogeny form encrusting base ( Fig. 6 View FIGURE 6 G, H); these zooids have lyrula with median ridge, and peristomes sometimes closed by secondary calcification during ontogeny, leading to bulbous appearance ( Fig. 6 View FIGURE 6 H). Ancestrula same shape as autozooids, but smaller and with fewer pores ( Fig. 6 View FIGURE 6 G). Ovicells not observed.
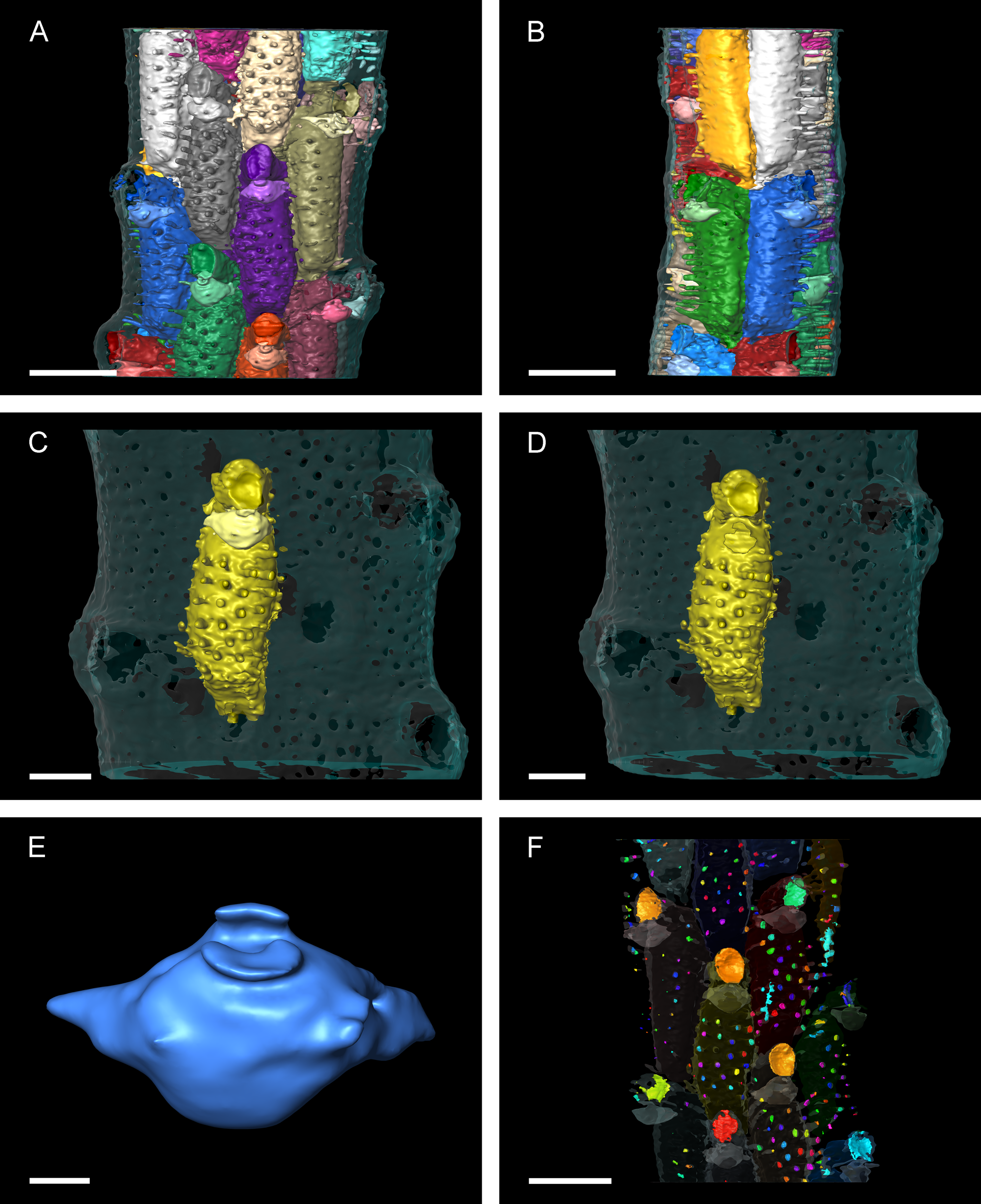
Micro-CT imaging and quantification. Interior zooidal chamber larger transversely than basal-frontally ( Fig. 7 View FIGURE 7 A, B). Avicularium connected mediofrontally to zooidal chamber, immediately proximal to orifice ( Fig. 7 View FIGURE 7 C, D); avicularian chamber shaped like rugby ball, orientated transversely and perpendicular to rostrum, and tapering laterally around zooidal chamber. Two or three frontal pores arise from avicularian chamber, indistinguishable from other frontal pores originating from autozooidal chamber ( Fig. 7 View FIGURE 7 E). Frontal pores cylindrical ( Fig. 7 View FIGURE 7 C, D).
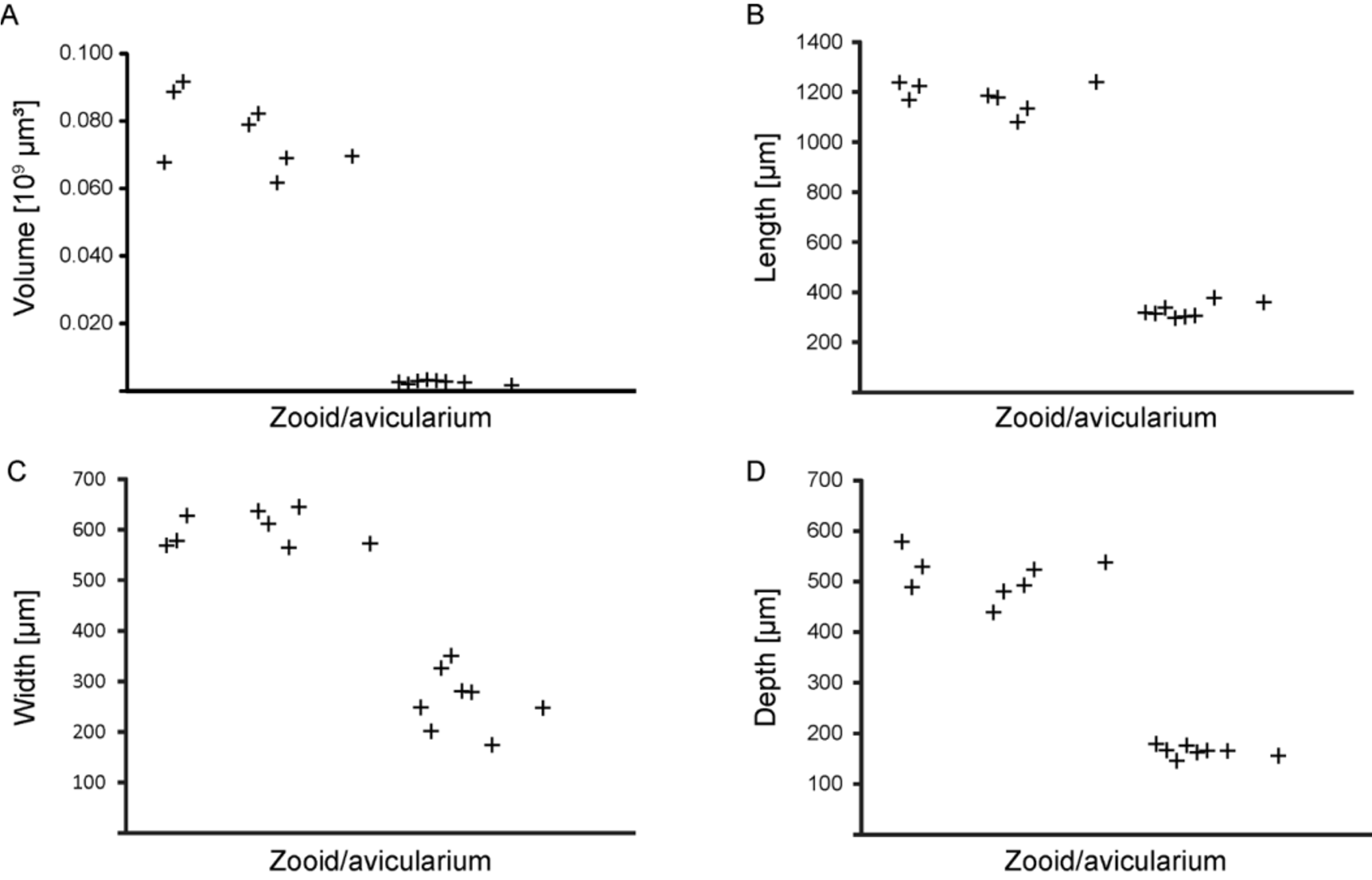
Values for the volume and the maximum extent of all three spatial axes of the zooidal and avicularian chambers clearly show two distinct groups (n = 16) ( Fig. 8 View FIGURE 8 ). The variance for both groups differs depending on the character measured. Zooids show the smallest variation in the width of the zooidal chamber ( Table 6 View TABLE 6 ), whereas avicularia show the smallest variance in depth ( Table 7 View TABLE 7 ).
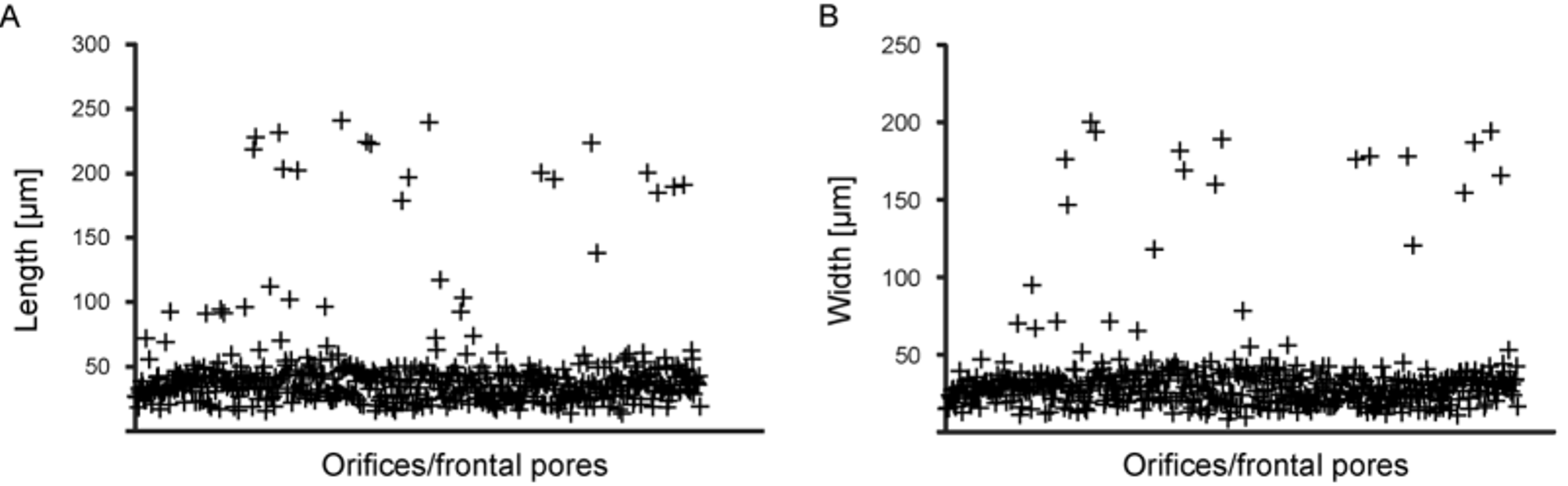
Orificial and frontal-pore measurements form three clusters ( Figs 7 View FIGURE 7 F, 9): a large cluster of 441 data points plots between 20 and 50 µm; a second group of 18 data points between 180 and 240 µm, with a mean of 209 µm; and a third group of 56 data points ranging in size from 50 to 140 µm. This last group is most evident in the length plot ( Fig. 9 View FIGURE 9 A), while in the width plot separation from the second group is less distinct ( Fig. 9 View FIGURE 9 B).
Difficulties in species identification arise from intracolony variation owing to increasing calcification during ontogeny. While peristomes and avicularia are easily discernible in younger parts of the colony, they become scarcely discernible in older parts. The zooidal boundaries also become more irregular and partly occluded. These limitations can be overcome by using micro-CT imaging techniques or by viewing distal branch segments in the early ontogenic stage. As the internal basal and lateral walls were not completely resolved during the scan, owing to comparatively low acceleration voltage resulting in only a fragmentary reconstruction, we cannot describe the distribution and shape of communication pores. It is evident from the reconstructed transverse stack that new zooids originate from the median lamina, simultaneously on each side.
Remarks. Smittina imragueni n. sp. closely resembles S. cervicornis ( Pallas, 1766) . Smittina cervicornis is one of the bryozoans mentioned earliest in the literature, as references to this species can be traced back to Imperato (1599), who described and figured an erect, laminar, branching species from the Mediterranean as “ Porus cervinus ”. However, confusion exists concerning the actual type, as Pallas’s collection appears to be lost ( Kuklinski & Taylor 2008) and no lectotype has been designated. Pallas also did not provide any figures.
Smittina imragueni n. sp. differs from an Adriatic specimen of S. cervicornis View in CoL (NHM 1911.10.1.1428) in the position of the avicularium. In the Adriatic colony, it is positioned in the same plane as the frontal surface, whereas in S. imragueni it extends from the lyrula along the proximal peristomial margin, resulting in a more oblique orientation relative to the direction of colony growth. The distal end of the rostrum is also more acute in the Adriatic specimen. It is noteworthy that this Adriatic specimen, which is part of the Canon Alfred Merle Norman collection, has a type label that is amended by “?part of type material”. A small included note states that it was originally described as “ Porella tubulifera Heller ” and was apparently sent by Camil Heller. Another species from the Adriatic (SMF 40011) has the avicularium oriented 35° to 45° from the direction of zooidal growth, which still appears less oblique than in S. imragueni . The avicularium itself also has an acute rostrum. Material from the Adriatic figured by Hayward & McKinney (2002) as Smittina cervicornis View in CoL seems to be this species. A western Mediterranean specimen from Riou Island (SMF 9811) has the same more-frontal orientation of the avicularium as the Adriatic specimens, but the shape of the rostrum appears similar to that in S. imragueni , though it is even more acute. At present, it is uncertain whether the Riou Island specimen is conspecific with Adriatic S. cervicornis View in CoL . Two other specimens at the NHM, from the Cape Verde Islands, also recorded as S. cervicornis View in CoL , which were originally described as Porella laevis View in CoL var. subcompressa Busk, 1884 (NHM 1887.12.9.585 and NHM 1944.1.8.281), show the same orientation of the avicularium as the Mediterranean species, but differ from S. imragueni and S. cervicornis View in CoL in other aspects of zooid morphology and in having differently shaped and also dimorphic suboral avicularia.
Another similar species is the less well-known Smittina colletti ( Jullien & Calvet, 1903) View in CoL , which differs from S. imragueni in the orientation and the relative size of the suboral avicularium and S. colletti View in CoL also has a characteristic median denticle on top of the lyrula. The zooidal sizes given by Harmelin (1969) for S. colletti View in CoL are significantly smaller than those for S. imragueni .
Strong secondary calcification and intracolony variation complicate identification for these three species, and close attention should be given to the shape and orientation of the suboral avicularium. Further studies might reveal the true identity of Smittina cervicornis View in CoL s.s.
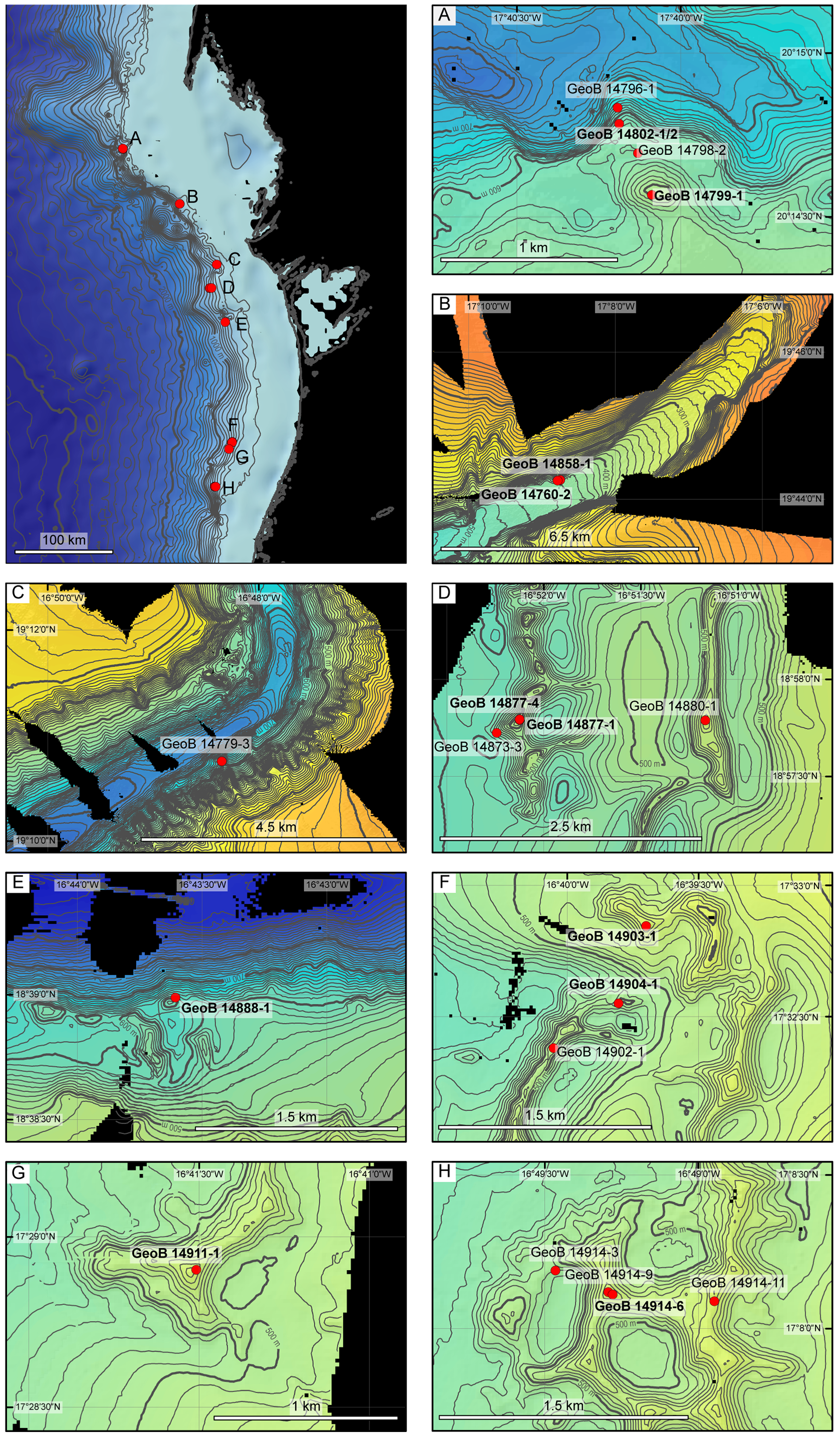
Distribution. Smittina cervicornis occurs mainly in the Mediterranean ( Zabala & Maluquer 1988; Rosso 2003), but it has also been reported along the West African coast, from Morocco ( Canu & Bassler 1925, 1928; d’Hondt, 1978) to Equatorial Guinea ( Cook 1968, 1985) and the Canaries ( Aristegui & Cruz 1986). Its northern extent is the coast of Brittany ( Harmelin et al. 1994). The bathymetric range seems to be from shallow coastal water down to 120 m ( Cook 1968; Harmelin et al. 1994; Hayward & McKinney 2002), but Canu & Bassler (1928) reported a depth of 400 m in the Bay of Biscay. Smittina colletti has also been reported from the Bay of Biscay ( Jullien & Calvet 1903; Harmelin 1969) and the Western Mediterranean ( Gautier 1962; Harmelin 1969), in waters deeper than 100 m. The short account of the different forms of “ Smittina cervicornis ” given above suggests that this is in fact a species complex, containing at least three species. Smittina imragueni n. sp. is so far known only from cold-water coral and adjacent habitats off Mauritania, at depths of 414 to 600 m, where it forms small, branched colonies attached to the dead coral framework in the basal part of small patches of living and dead colonies of the corals Lophelia pertusa ( Linnaeus, 1758) and Madrepora oculata ( Linnaeus, 1758) . In the area investigated, S. imragueni occurred south of 18.5° N and in the Arguin Canyon ( Table 1 View TABLE 1 ; Fig. 1 View FIGURE 1 ).
TABLE 6. Measurements (in µm) of internal zooidal chambers for Smittina imragueni n. sp. (paratype, 40010), made from the virtual dataset.
| Autozooid Volume (10 9 µm3) | Length (µm) | Width (µm) | Depth (µm) |
|---|---|---|---|
| 1 0.070 | 1240 | 538 | 572 |
| 2 0.068 | 1237 | 579 | 569 |
| 3 0.092 | 1223 | 529 | 628 |
| 4 0.079 | 1185 | 439 | 637 |
| 5 0.082 | 1177 | 480 | 611 |
| 6 0.089 | 1168 | 489 | 578 |
| 7 0.069 | 1134 | 524 | 645 |
| 8 0.062 | 1080 | 493 | 565 |
| Mean 0.076 | 1181 | 509 | 601 |
| SD 0.010 | 51 | 40 | 31 |
TABLE 7. Measurements of avicularian chambers for Smittina imragueni n. sp. (paratype, 40010), made from the virtual dataset.
| Avicularium Volume (10 9 µm³) | Width (µm) | Length (µm) | Depth (µm) |
|---|---|---|---|
| 1 0.003 | 377 | 174 | 166 |
| 2 0.002 | 359 | 248 | 156 |
| 3 0.003 | 306 | 279 | 166 |
| 4 0.003 | 301 | 280 | 162 |
| 5 0.003 | 337 | 326 | 145 |
| 6 0.003 | 296 | 350 | 176 |
| 7 0.002 | 314 | 201 | 167 |
| 8 0.003 | 317 | 248 | 179 |
| Mean 0.003 | 326 | 263 | 165 |
| SD 0.0005 | 27 | 55 | 10 |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Smittina imragueni
| Matsuyama, Kei, Titschack, Jürgen, Baum, Daniel & Freiwald, André 2015 |
Smittina colletti (
| Jullien & Calvet 1903 |
var. subcompressa
| Busk 1884 |