Martialis heureka Rabeling & Verhaagh, 2008
|
publication ID |
https://doi.org/10.5852/ejt.2015.120 |
|
publication LSID |
lsid:zoobank.org:pub:54714320-5726-44CB-8FF5-60E0B984873D |
|
DOI |
https://doi.org/10.5281/zenodo.3795051 |
|
persistent identifier |
https://treatment.plazi.org/id/038E878C-FF8C-B148-FD9C-FCF6FBCF1986 |
|
treatment provided by |
Carolina |
|
scientific name |
Martialis heureka Rabeling & Verhaagh, 2008 |
| status |
|
Martialis heureka Rabeling & Verhaagh, 2008 View in CoL
Martialis heureka Rabeling & Verhaagh, in Rabeling et al. 2008 : 14914, figs. 1–2 (worker). BRAZIL, Amazonas: Manaus, Headquarters of Empresa Brasileira de Pesquisa Agropecuária (EMBRAPA) Amazônia Ocidental, kilometer 28 highway AM 010, 2°53’S, 59°59’W, elev. 40–50 m, 9 May 2003, ex leaf litter at dusk, primary tropical lowland rainforest. (C. Rabeling) [MZSP].
Male description
MEASUREMENTS (n=3). HL 0.35–0.42, HW1 0.35–0.40, HW2 0.44–0.49, MAL 0.04–0.06, MDL 0.10– 0.11, SL 0.20–0.23, PDL 0.10–0.13, A3L 0.17–0.21, AAL 0.17–0.21, EL 0.17–0.19, EW 0.14–0.17, OOD 0.14–0.15, LOD 0.04–0.05, MOD 0.04–0.05, ML 0.64–0.78, MLL 0.16–0.18, MLW 0.17–0.20, MTL 0.29–0.37, MTW 0.36–0.45, PFL 0.39–0.48, MFL 0.43–0.53, PTH 0.16–0.19, PTL 0.20–0.23.
INDICES. CI 0.94–1.02, CS 0.35–0.41, SEI 83.6–86.2, SI 55.6–58.4, EI 83.0–85.6, EYE 88.1–88.7, MI 26.7–27.8, OBI 80.1–82.8, OMI 3.46–4.74, MNI 2.00–2.26, MTI 79.9–83.2, FI 88.3–91.3, PTI 76.9– 83.2. Small, but body variable in overall size ( Fig. 11C View Fig ).
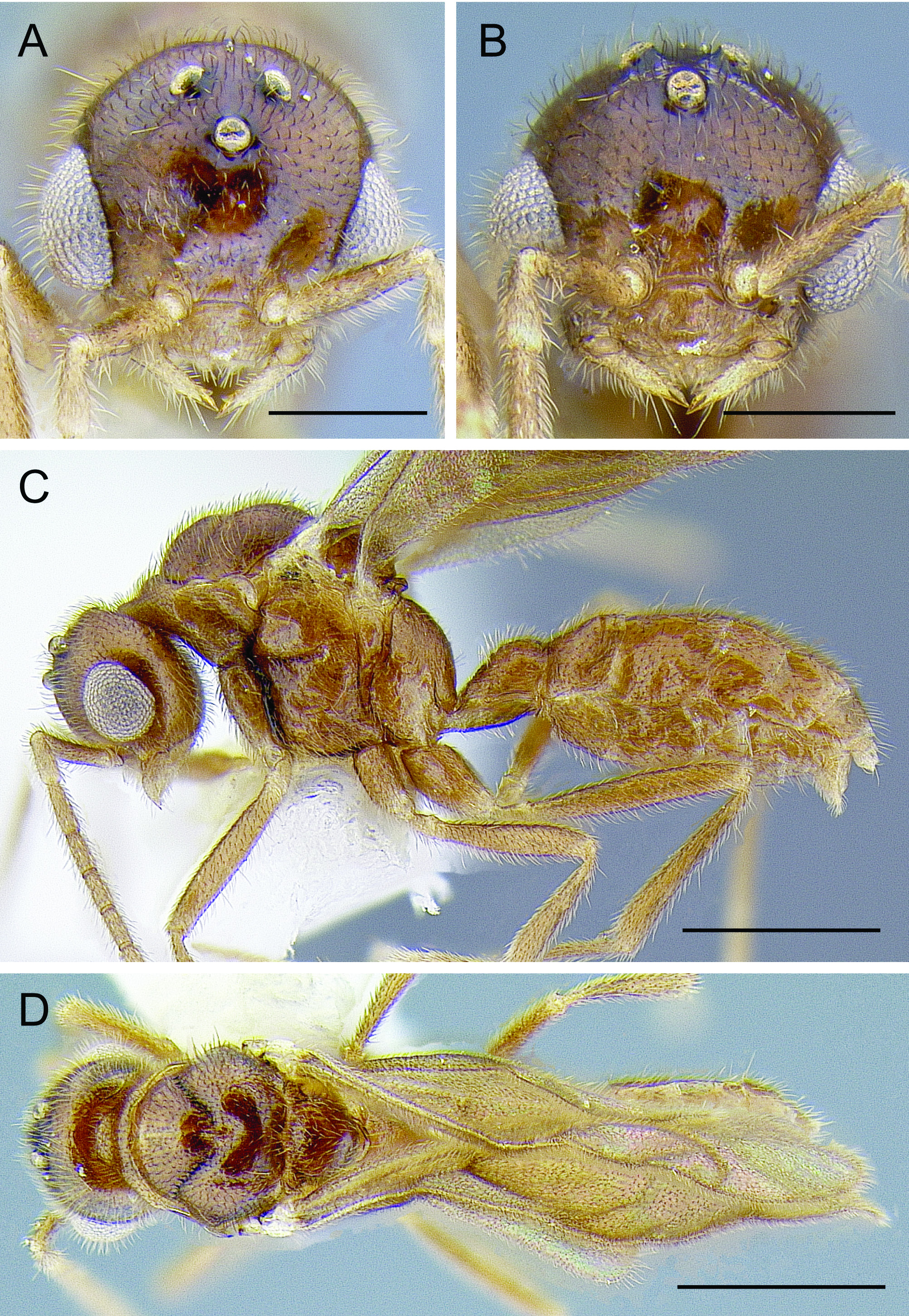
HEAD ( Fig. 11 View Fig A–B). In full-face view head about as broad as long excluding eyes, broader than long including eyes. Palpal formula 2,1; palps short, not reaching hypostomal margin. Stipes simple, lacking carinae on medial surface. Labrum very small, medially emarginate, setose; lateral margins distant from mandibular bases by somewhat less than maximum lateromedial labrum length; labrum lacking basolateral trigger setae observable in workers. Mandibles linear, narrow; lateral and medial margins weakly tapering to apex; masticatory mandibular margin reduced, bidentate; apical tooth asymmetrical, larger than symmetrical basal tooth; mandalus enlarged, diameter equal to maximum mandible width. Clypeus reduced; anterior margin broadly emarginate; medial clypeal portion maximum anteroposterior length about 1.5 maximum antennal socket diameters; posterior clypeal margin produced between antennal toruli. Supraclypeal area arc-shaped, anteroposteriorly longer than maximum antennal socket diameter. Antennal toruli situated anteriorly, with anteriormost portion of torular arch anterad anterior tentorial pit. Frons and ocellar area bulging. Occipital carina present, weakly developed, obscured in full-face view by vertex, not enclosing occiput. Compound eyes bulging strongly; medial margin weakly convex; posterior margin weakly emarginate; compound eye narrower dorsally than ventrally. Ocelli small, situated distant from compound eye. Hypostomal margin reduced, lacking lamina. Antenna 13-merous; scape longer than maximum compound eye diameter and slightly more than 2 x pedicel length; pedicel cylindrical, long, about 4/5 x antennomere 3 length; funiculus filiform, elongate, reaching metasoma when laid against mesosoma.
MESOSOMA ( Fig. 11 View Fig C–D). Pronotal neck continuous with remainder of sclerite in dorsal view;main portion of pronotum swollen, muscular; anteromedian pronotal face convex in profile view, short, dorsoventral height of pronotum from pronotal neck about 1/3 x mesoscutum height in profile view; lateral pronotal face concave. Mesoscutum broader than long in dorsal view (length 0.80–0.83 x width); anterior and posterolateral areas swollen. Notauli distinct, crossribbed, meeting at body midline, not extending to transscutal line although narrow longitudinal line present from notauli to transscutal line. Parapsidal lines impressed, slightly divergent. Parascutal carinae nearly linear; weakly sinuate. Scutoscutellar sulcus unimpressed. Axillae small and widely situated. Mesoscutellum high and convex in profile view; not modified. Metascutellum small, lateromedial width slightly less than one half anteroposterior length; in profile view metascutellum strongly produced. Metanotal trough deep, small, circular. Mesopectus with oblique longitudinal sulcus, anterior terminus of sulcus nearly contacting posterolateral pronotal corner. Spiracular sclerite inconspicuous. Lower metapleural area strongly offset from upper metapleural area by deep, broad, margined sulcus. Metapleural gland orifice occluded; presence of internal metapleural gland not visible through metapleural sclerite. Propodeum parabolic in profile view, dorsal face about as long as and continuous with posterior face; propodeal spiracle circular, small; propodeal lobe weakly developed, carinate, clearly visible in anterolateral oblique view.
METASOMA ( Fig. 11C View Fig ). Petiole nodiform, pedunculate; anteriormost portion of petiolar tergum offset by parabolic carina; petiolar tergum and sternum fused, longitudinal lateral carinae not suggestive of suture; petiolar node shallow, in profile view anterodorsal face nearly linear, dorsum weakly convex, posterior face very weak; petiolar sternum linear for most of length, posteriorly narrowed, ventral petiolar surface with paired diverging carinulae; subpetiolar process absent. Abdominal segment III slightly reduced and differentiated from segment IV; helcium axial, sternal presclerite visible in profile view, not obscured by tergal presclerite; abdominal posttergite and poststernite III not fused; abdominal sternum III prora present as anterolateral bosses subtending helcium, anteromedian area of sternum concave. Abdominal terga IV–VIII and abdominal sterna IV–IX normally developed, not reduced or obscured in situ. Abdominal tergum VIII posterior margin unmodified. Abdominal sternum IX apically ligulate, narrow, posterior margin very narrowly convex, nearly triangular.
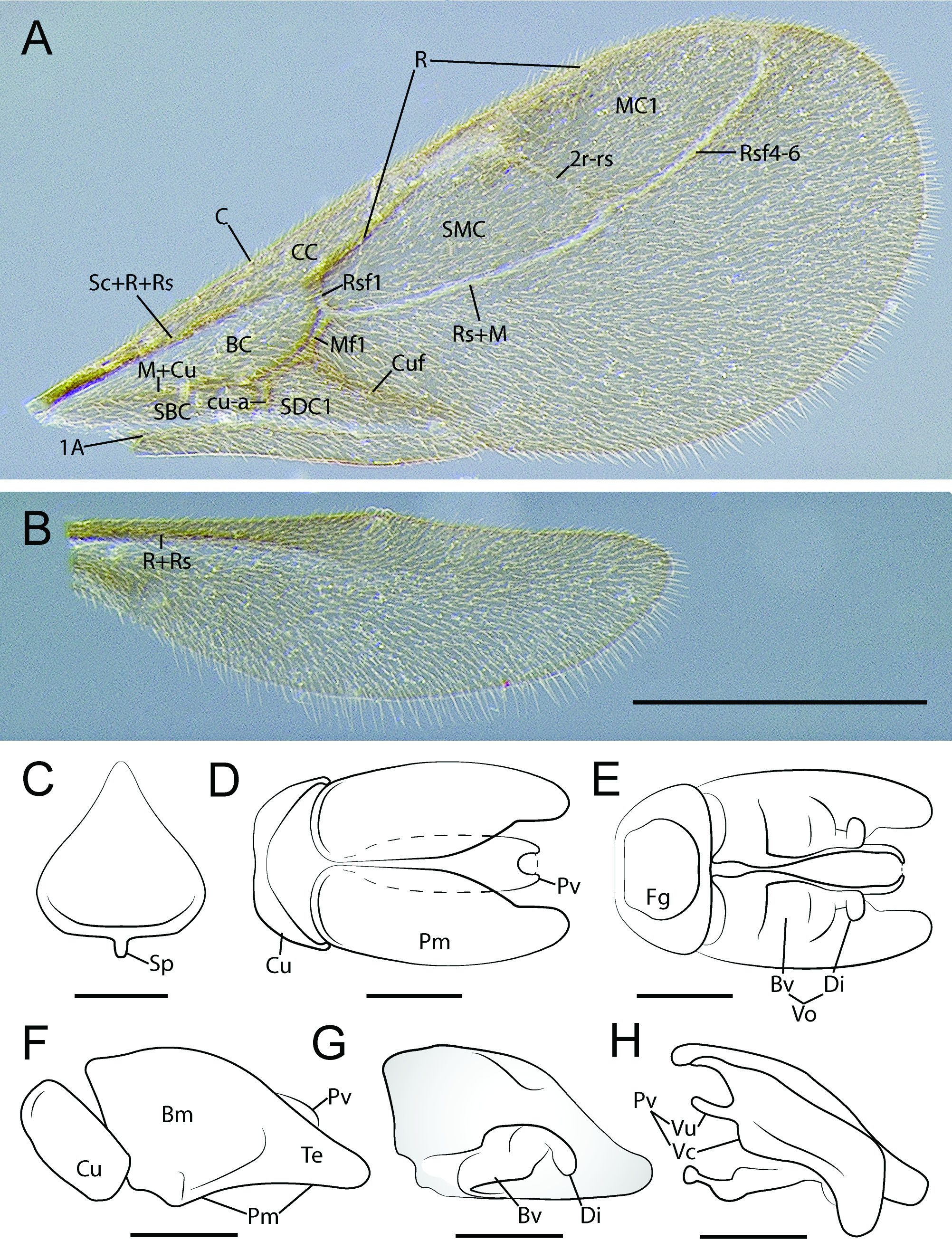
FOREWING ( Fig. 12A View Fig ). Tegulum reduced, subrectangular, longer than broad. Wings weakly infuscated, completely covered in fine setose layer. Pterostigma poorly-developed, only anterior enclosing abscissa tubular. Wing venation Ogata type IVa: Submarginal cell 1+2 and marginal cell 1 closed, 1m-cu absent, thus discal cell 1 open. Costal vein tubular to pterostigma. Rsf1 slightly more than 1/2 x length of and meeting Mf1 obliquely. Rs+M continuous with undifferentiated Mf2-3 until meeting very short 2rsm. Rsf2+3 absent. 2r-rs very long, longer than combined lengths of Rsf1 and Mf1; 2r-rs directed posteroapically, not orthogonal with anterior wing margin. Rsf4–6 tubular to Rf, enclosing marginal cell. Mf4–6 absent. Crossvein cu-a incompletely tubular, situated basad Mf1. Cuf divergent with respect to Rs+M+Mf2+3. 1A extending only slightly beyond cu-a, not enclosing subdiscal cell 1.
HINDWING ( Fig. 12B View Fig ). Hindwing venation reduced, only R+Rs and 1A tubular; R not reaching anterior wing margin; 1A short, weakly indicated. Three hamuli present. Claval region poorly developed.
GENITALIA ( Fig. 12 View Fig C–H). Pygostyles absent.Abdominal sternum IX spiculum short; anterior margin linear, curving posterolaterally near lateral margins; lateral margins short, slightly divergent; posterolateral margins weakly concave, tapering strongly to acute, narrowly rounded apex. Cupula dorsal and lateral faces about as broad as telomeral base; lateral face narrowing ventrally to narrow bar-like ventral face. Basimere and telomere more-or-less continuous, basimere weakly shouldered dorsomedially anterad telomeral base; dorsomedian margins of basimeres parallel for about half length of paramere; telomere acutely triangular in profile view; basimere and telomere with ventrolateral layer of posteroventrallydirected setae. Basivolsella lateromedially broad; base transversely connected with basimere; cuspis
absent; digitus clavate, apex swollen and directed ventrally; digital stem short. Valvura short, linear, directed anteriorly and situated at about 2/3 valviceps height; valviceps linear, dorsoventrally short; valviceps dorsomedially fused for most of length; in profile view dorsal valviceps margin weakly convex, ventral margin concave, edentate, valviceps apex weakly convex, subrectangular, produced ventrally; dorsolateral valviceps face convex, margined by lateral apodeme which extends almost to apex before curving ventrad and contacting ventral margin; ventrolateral valviceps face concave; phallotreme situated at aedeagal apex, sclerotic aperture formed by valviceps circular.
COLORATION. Body almost uniformly brown to brownish yellow; extremities slightly lighter colored.
SCULPTURATION. Body weakly sculptured overall; head with fine piligerous punctae; mesonotal piligerous punctae coarser, posterolateral mesoscutal area above parascutal carina roughened; dorsomedian scutoscutellar area finely and densely anteroposteriorly striate; mesoscutellum weakly roughened; metascutellum with fine transverse carina subtending posteriorly produced portion of disc; mesopectus and metapleuron smooth, shining, slightly rough; propodeum finely striate, striae extending from anterior margin down along lateral faces, dorsal propodeal face weakly rugose, posterior face mostly smooth; petiole mostly smooth and shining, with lateral longitudinal carinulae; abdominal segment III mostly smooth and shining; abdominal segments posterior to segment III weakly sclerotized.
SETATION. Head, median pronotal portion including pronotal neck, mesonotum, and procoxae covered by dense layer of somewhat short, uniform, weakly curved, erect to suberect setae, longer setae present on these areas very sparsely; clypeus lacking clypeal brush of worker, although setal layer denser than on remainder of head capsule; setae sparse and subdecumbent to nearly appressed on pronotal lateral face, mesopectus, metapleuron, and propodeum; setae somewhat denser on metasoma, but not as dense as on head and mesonotal dorsum; petiolar setae elongate, linear, setae on remaining segments shorter and curved; setae on legs, including meso- and metacoxae, about as dense as on metasoma, mostly subdecumbent with a few longer suberect setae present.
Distribution
Neotropical: Known only from the Amazon basin near Manaus ( Amazonas, Brazil).
Discussion
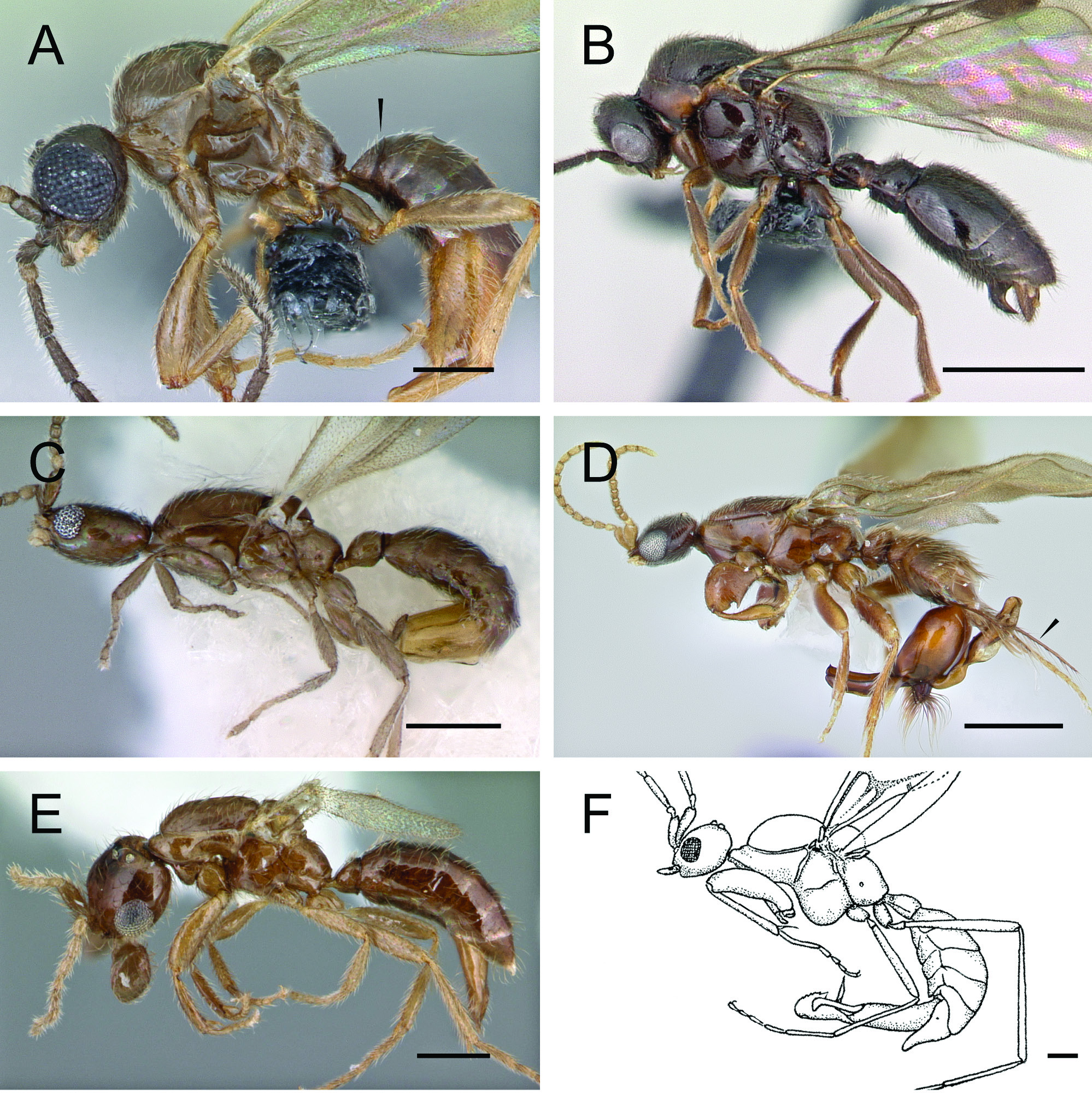
Described from a single stray worker from the Amazon just north of Manaus, Brazil, Martialis heureka Rabeling & Verhaagh, 2008 is one of the most significant taxa in the Formicidae described in recent years. Displaying a bizarre mixture of pleisiomorphic and autapomorphic traits, the species was attributed to its own subfamily, the Martialinae . This decision was supported by further morphological study ( Brandão et al. 2010) and multi-locus molecular phylogenetic reconstruction ( Rabeling et al. 2008; although see Kück et al. 2011). Rabeling et al. (2008) recovered Martialis as the sister to all remaining extant ants including the Old World subfamily Leptanillinae , while a reanalysis by Kück et al. (2011) found the converse. Given this debate and the mysterious biology of the species, Martialis is of high interest. Here the male of Martialis is described for the first time based on material from the Biological Dynamics of Forest Fragments Project (BDFFP [English], PDBFF [Portuguese]) study region, about 50 km north of the type locality.
The male of Martialis differs from the worker by standard intercaste dimorphism, e.g., eyes welldeveloped, ocelli and notauli present, alate, flight sclerites developed, mesosoma musculated for flight, but the male also differs notably in several specific characters: mandibles far shorter, reduced relative to worker; labral trigger setae absent; clypeal brush absent; antennal toruli situated more anteriorly; forelegs weak; metatibial gland absent; petiolar node weakly developed; helcium broader; abdominal tergum and sternum III of equal length; cinctus between pre- and postsclerites IV not developed. On the other hand, the male of Martialis displays numerous similarities with the worker, such as linear mandibles, reduced palpal count (although worker count unconfirmed, but certainly less than 3,3), clypeus poorly developed, anterior tentorial pits posteriorly-situated, antennal toruli well-developed (but not quite as cup-like as in worker), scapes long, pedicel elongate, frontal carinae absent, propodeal lobes weakly developed (contra the initial worker diagnosis), tibial spur formula 1,1, basipetiolar carina present, petiolar tergum and sternum fused, abdominal segment III differentiated from IV, and sculpture and setation remarkably similar, although the somatic sclerites of the male are generally less strongly sclerotized. The two castes are similar in several other specifics, but this brief list captures most of the significant features.
Specimens were examined from collecting events in January, February, April, and October of 1985. The BDFFP study region displays weak seasonality of rainfall and day length, with the months of June through December roughly representing the “dry season”, July through September being the driest (Bierregaard Jr. et al. 2001). It is possible that Martialis flights occur year-round, although the sampling of BDFFP material examined for this study is too small to confidently assert the flight phenology. While the range of Martialis has only been extended by about 50 km by the discovery of the male, the quantity of males recovered exceeds that of workers by an order of magnitude ( 25 males vs. 3 workers). Thus, Martialis may be recovered via alates more readily than workers. The use of Malaise traps and flight intercept traps should be encouraged for studies of ant diversity, particularly for species with cryptic habits. Although our knowledge of flight phenology is poor, tropical rainforests may be particularly amenable to these studies due to the relatively more year-round flights of Neotropical ( Kaspari et al. 2001a, 2001b) than of Nearctic ants ( Dunn et al. 2007).
As the Manaus region is considered the ecological “crossroads” of the Amazon where a high proportion of species ranges overlap (Bierregaard Jr. et al. 2001), it will be valuable to sample for Martialis in other Amazonian regions. Moreover, it is of interest whether the fragmented populations of Martialis in the BDFFP plots have survived the intervening 30 years. This may not be the case, as ant communities have been observed to hemorrhage in BDFFP study plots ( Vasconcelos et al. 2001), although hypogaeic ants may be less sensitive to habitat changes than epigaeic ants. The placement of the Malaise samples relative to the edges of the study plots is unknown. As well, the gyne of Martialis remains unknown. It is possible that this caste will be ergatoid, but the presence of alate gynes cannot be ruled out. In general, the male of a given ant species is more frequently collected via Malaise traps than females; thus it is possible that although only males were encountered, alate gynes could still be present. Regardless, the natural history of Martialis will be fascinating to uncover.
Material examined
Holotype worker examined at MZSP. Specimen was cleared by non-destructive extraction of DNA, which allowed for examination of internal characters. All males were examined from the following collecting events at the Fazenda Esteio study area of the Biological Dynamics of Forest Fragments Project in Amazonas, Brazil, with an elevation of about 90 ± 10 m, collected by Bert Klein (WWF) via Malaise trap in 1985: Plot 1112 “Cidade Powell”, 2.38692° S, 59.87494° W, ± 100 m, 26 Feb. GoogleMaps ( 5 specimens) and 1 Oct. ( 1 specimen), 1 hectare Amazonian rainforest fragment; plot 1208 “Cidade Powell”, 2.37204° S, 59.87252° W, ± 250 m, 22 Oct. GoogleMaps ( 1 specimen), 10 hectare Amazonian rainforest fragment; plot 1301 “Florestal”, 2.38897° S, 59.85012° W, ± 500 m, 23 Jan. GoogleMaps ( 4 specimens), 24 Apr. GoogleMaps ( 7 specimens), and 2 Oct. GoogleMaps ( 7 specimens), 100 hectare Amazonian rainforest fragment. ( Note: data extrapolated from Bierregaard Jr. et al. 2001, and PDBFF & INPA-SI 2014 ; latitude and longitude recorded from Google Earth with error estimates to account for uncertainty of exact plot location; half the male material examined remains at INPA.) GoogleMaps
Shared apomorphies of the basal ants
Of particular interest for the “basal ant” problem ( Brady et al. 2006; Rabeling et al. 2008; Kück et al. 2011) is the relationship of the Leptanillinae , Martialinae , and the Amblyoponinae . While the relationships within the formicoid clade ( Dorylinae , myrmeciomorphs, dolichoderomorphs, Formicinae , ectaheteromorphs, Myrmicinae ) have crystallized in the past decade ( Moreau et al. 2006; Brady et al. 2006, 2014; Ward et al. 2015), the relationships of the “poneroids” ( Agroecomyrmecinae , Amblyoponinae , Paraponerinae , Ponerinae , and Proceratiinae ) are still unresolved ( Ward 2014). The poneroids may constitute a clade or a grade, depending on placement of the root on the ant tree of life ( Brady et al. 2006).
The dilemma of rooting the ant tree of life was highlighted by the discovery of M. heureka , which has been recovered as sister to the remainder of the extant Formicidae ( Rabeling et al. 2008) , a result contested by Kück et al. (2011), who recovered Leptanillinae as sister to the extant Formicidae . These results not only disagree in topology, but may be subject to the biases of long-branch attraction and CG-bias ( Ward 2014). As summarized by Ward (2014), one of the major questions of ant systematics is whether Martialis and/or the Leptanillinae are sister to the extant Formicidae or whether they are highly derived poneroids. Unpublished molecular phylogenetic analyses by P.S. Ward (discussed in Ward 2014), in which the outgroups are excluded, recover Martialis and the Leptanillinae as part of a bipartition comprising part of the poneroids; moreover, these analyses recover Opamyrma as sister to the Leptanillinae . Considerable uncertainty thus exists, even with molecular data. The present work seeks, in part, to render this problem more tractable by providing novel morphological characters that are shared by Martialis , Apomyrma , Opamyrma , the Leptanillinae , and the Amblyoponinae .
The male of Martialis is more generalized morphologically than most Leptanillinae , although some Protanilla display a mosaic of generalized and specialized characteristics. Martialis males differ from all known male Leptanillinae by the following characters: mandibles linear, meeting at head midline, bidentate apically; antennal toruli situated posterad anterior portion of antennal torulus; lower metapleuron dorsoventrally longer than anteroposteriorly broad; metanotal trough pit-like (vs. oblong); propodeal lobe present; first submarginal cell enclosed by tubular abscissa; marginal cell 1 closed; petiole pedunculate; genitalia more generalized. The worker of Martialis is superficially similar to Protanilla and Anomalomyrma . The natural history of all three of these taxa is virtually unknown.
Male Martialis , in comparison with those of the Amblyoponinae , excluding Apomyrma , differ by lacking several amblyoponine apomorphies, such as the anteroventral petiolar tergum collar, modified peg-like setae on clypeus and labrum. Martialis further differs from the Amblyoponinae without Apomyrma by the following characters: mandibles linear (rather than curved and subfalcate); clypeus reduced; antennal toruli strongly developed, conspicuous (vs. inconspicuous); metascutellar trough pit-like; petiolar tergum and sternum fused; helcium axial; and helcial sternite projecting ventrad helcial tergite in profile view.
The male of the amblyoponine Apomyrma , however, displays several characters that are on the one hand very leptanilline in nature and on the other are similar to Martialis . Apomyrma has reduced, nub-like mandibles, lacks propodeal lobes, and has an infraaxial petiole, as in the Leptanillinae . Additionally, the pro- and mesonotum is elongated, similar to most of the Leptanillini . The petiole of Apomyrma is unlike other Amblyoponinae , as it is infraaxial and lacks the anterior tergal collar, but the petiole differs from Martialis in being tergosternally unfused. Unlike Martialis , Apomyrma has a distinct metapleural gland orifice and short, robust legs. Martialis is easily distinguished from Apomyrma . The male of Opamyrma is unknown, but would inevitably be valuable to describe and compare.
Are the workers of Apomyrma , Opamyrma , and the Leptanillinae convergently similar due to subterranean habits? Nothing is known yet of the habits of Martialis . The workers are superficially similar to the
Sphecomyrminae in mesosoma and metasoma form relative to the Leptanillinae and Amblyoponinae , although several sphecomyrmine genera have peg-like setae as in the Amblyoponinae . Martialis males are superficially similar to males tentatively identified as Sphecomyrma ( Grimaldi et al. 1997) , differing mainly in having antennae situated anteriorly, reduced venation, tergosternal petiolar fusion, a tibial spur formula of 1,1, and having abdominal segment III reduced. The extinct genus Baikurus differs similarly, except the mandibles are curved and palps are longer; the petiole is not visible in the specimen illustrated by Grimaldi et al. (1997).
Below is presented a list of apparent morphological apomorphies shared among the Amblyoponinae (excluding Apomyrma and Opamyrma ), Martialinae , Apomyrma , Opamyrma , and Leptanillinae , with groups presented by increasing qualitative similarity. Pleisiomorphic conditions are presented in brackets next to the respective apomorphic conditions.
Apomorphies shared among all five taxa:
1. Compound eyes reduced (worker) (note 1). [Compound eyes not reduced in most Formicidae .]
2. Petiole anteriorly tergosternally fused (worker, gyne) (note 2). [Petiole anteriorly tergosternally unfused in Formicidae .]
Notes:
1. Proposed by Ward (1994) as a putative synapomorphy of the Amblyoponinae , Leptanillinae , and Apomyrma , but prone to homoplasy.
2. Ward (1994) proposed this as a putative synapomorphy of the Amblyoponinae , Leptanillinae , and Apomyrma .
Apomorphies shared by Martialis , Opamyrma , Apomyrma , and the Leptanillinae :
1. Frontal carinae lost (worker, gyne) (note 1). [Frontal carinae present, lobe-like in Amblyoponinae and other poneroids, excepting Proceratiinae .]
2. Antennal toruli directed dorsally or anterodorsally rather than laterally (female castes) (note 1). [Antennal toruli directed more-or-less laterally in Amblyoponinae and other poneroids, excepting Proceratiinae .]
3. Compound eyes completely absent (worker). [Compound eyes present in most Formicidae .]
4. Occiput enclosed by occipital carina (worker, gyne) (note 2). [Occipital carina not enclosing occiput in other Formicidae .]
5. Occiput enlarged, such that it is visible in full-face view (worker, gyne). [Occiput smaller, not visible in full-face view in other Formicidae .]
6. Parascutal carina situated very low on mesoscutum, almost completely obscured by wing base and thus inconspicuous (male). [Parascutal carina raised on mesoscutum, near height of mesoscutal dorsum, only partially obscured by wing base, conspicuous; in Formicidae .]
7. Spiracular sclerite absent, metapleural spiracle unconcealed (male) (note 3). [Spiracular sclerite present, concealing metapleural spiracle; in Formicidae .]
8. Pygostyles absent (male) (note 4). [Pygostyles present for Formicidae ; see note below.]
9. Valviceps linear to arched, with concave ventral and convex dorsal margin in ectal view (male). [Valviceps more-or-less elliptical, with both dorsal and ventral margins convex in ectal view in other poneroids.]
10. Valviceps lateral apodeme evenly linear, extending almost to valviceps apex before curving ventrad, delimiting convex dorsolateral and concave ventrolateral face (male). [Lateral apodeme sinuate, wavy, extending to valviceps apex but not curving ventrad; dorsolateral and ventrolateral faces more or less flat in other poneroids.]
11. Valviceps ventral margin edentate (male) (note 5). [Valviceps ventral margin dentate in other poneroids.]
12. Valviceps dorsomedially fused (male). [Valviceps unfused and articulating dorsomedially in most other Formicidae .]
13. Characters of the sting apparatus (worker, gyne) (note 6).
Notes:
1. Also observed in Dorylinae .
2. The genus Apomyrma is an exception as no occipital carina occurs.
3. The spiracular sclerite has been lost in various lineages of Formicidae , including the entire Myrmicinae and Tatuidris .
4. Pygostyles have been lost in numerous lineages of Formicidae and thus constitute a relatively weak character. Within the poneroid group, the Agroecomyrmecinae , Ponerinae , and Paraponerinae retain pygostyles; in the Proceratiinae , only Probolomyrmex has lost pygostyles. The Amblyoponinae are somewhat more complicated, with loss of pygostyles occurring at least twice: one or more times in the XMAS clade ( Myopopone , Mystrium + Xymmer ), and once outside of the XMAS clade. Pygostyles are retained in the OCP clade and in Stigmatomma and Adetomyrma of the XMAS clade, thus spanning the root of the Amblyoponinae . In formicoids, loss of the pygostyles has been adduced as an apomorphy of the Dorylinae ( Bolton 1990c) . The bizarre, elongate, filamentous structures of some unassociated leptanilline males are, amazingly, appendages of the basimere ( Fig. 10D View Fig ).
5. Absence of ventral valviceps teeth has evolved several times, including the amblyoponine genus Adetomyrma ( Yoshimura & Fisher 2012b) .
6. Kugler (1992) indicated as synapomorphic a set of sting apparatus characters applying to Apomyrma , Protanilla , and Leptanilla ; these characters also seem to occur in Martialis . These characters are, in short, lack of medial lobe on quadrate plate, fulcral arm short and lacking lateral extensions. Notably, the anal plate of Apomyrma and Protanilla is absent and no anal plate was found for Martialis ( Brandão et al. 2010) ; this may, however, be an artefact of the poor preservation of the Martialis specimen examined by Brandão et al., and waits to be confirmed for Protanilla ( Kugler 1992) . The present author admittedly has insufficient expertise with the sting apparatus to critically evaluate these characters, however. Future researchers are encouraged to examine the sting apparati of the poneroids, as knowledge of this group is poor ( Kugler 1992) and as understanding the sting apparatus might assist in providing a more detailed picture of the evolution of the early branching ant lineages.
Apomorphies shared by Opamyrma , Apomyrma , and the Leptanillinae :
1. Lateral bases of mandibles set deep in pits, suggestive of a trap-jaw mechanism (female castes). [Pleurostoma posterad mandibular insertion without a deep pit for reception of mandible; mandibles without trap-jaw mechanism in other poneroids, excepting Mystrium .]
2. Clypeus raised dorsally, distinctly margined posteriorly (worker, gyne). [Clypeus flush with frons, lacking posterior margination in other Formicidae .]
3. Entire clypeus anteriorly produced (not just median portion), with rectangular anterolateral margins (worker, gyne) (note 1). [Clypeus not produced anteriorly; anterolateral corner obtuse in other Formicidae .]
4. Labrum with peg-like setae (worker, gyne) (note 2). [Labrum without dentiform setae in other Formicidae , excepting Amblyopone .]
5. Thickened setae present on ventromedial mandibular face (worker, gyne). [Thin setae present on ventromedial mandibular face in other poneroids, with some exceptions.]
6. Male mandibles strongly reduced, nub-like (male) (note 3). [Mandibles long, falcate to linear in Amblyoponinae and Martialis .]
7. Basivolsella lateromedially narrow in ventral view (male) (note 4). [Basivolsella lateromedially broad in ventral view in other poneroids, excepting various proceratiine species.]
8. Digital stem elongate and strongly arched (male) (note 4). [Digital stem short, linear to weakly curved in other Formicidae .]
9. Larva elongate, slender, and club-shaped (“leptanilloid” in terminology of Wheeler & Wheeler 1976) (note 5). [Larva pogonomyrmecoid, myrmecioid, or platythyreoid in other poneroids; see Wheeler & Wheeler 1976 for definition of larval forms.]
Notes:
1. The clypeus of Leptanilla is strongly produced as a subrectangular median process in addition to the anterior clypeal migration.
2. Polymorphic in Leptanillinae : present at least in Anomalomyrmini . Also present in Amblyopone , Onychomyrmex .
3. Mandibles secondarily elongated in Noonilla and enlarged in Scyphodon .
4. The genitalia of Leptanillinae are subject to extreme modification, but this state is visible in species with more-generalized genitalia. The male of Opamyrma is unknown.
5. May apply to Martialis ; larval caste unknown for Martialis and Opamyrma .
Apomorphies shared by Opamyrma and Apomyrma :
1. Promesonotal flexion extreme (worker, gyne?) (note 1). [Promesonotal articulation flexible, but not enhanced.]
2. Propleurae bulging in profile view (worker, gyne?) (note 2). [Propleurae flat, only weakly produced anteriorly beyond pronotum, if at all.]
3. Scapes clavate (worker, gyne?).
4. Petiolar tergum extending anteroventrally and fusing medially, forming collar (worker, gyne?) (note 3).
Notes:
1. Promesonotal flexion is enhanced in other Formicidae , including some Leptanilloides (Dorylinae) , and the Leptanillini .
2. Also present in Leptanillini , Xymmer (Amblyoponinae) , and Leptanilloides .
3. The tergal-tergal fusion “collar” around the petiolar base is not present in the male of Apomyrma , whereas such a collar is present in both the male and worker of Xymmer .
Brief global diagnoses of subfamilies, based on males
Note
The treatment of the subfamilies below follows the current systematic classification of the Formicidae ( Bolton 2003; Brady et al. 2006, 2014; Ward et al. 2015). The subfamilies are organized by the systematic results of Brady et al. (2006) and Ward et al. (2015), with the “poneroids” in alphabetical order first, followed by the formicoid clade comprised of the Dorylinae , myrmeciomorph clade ( Myrmeciinae , Pseudomyrmecinae ), dolichoderomorph clade ( Aneuretinae , Dolichoderinae ), Formicinae , ectaheteromorph clade ( Ectatomminae , Heteroponerinae ), and Myrmicinae .
| MZSP |
Brazil, Sao Paulo, Sao Paulo, Museu de Zoologia da Universidade de Sao Paulo |
| INPA |
Brazil, Amazonas, Manaus, Instituto Nacional de Pesquisas da Amazoonia, Colecao Sistematica da Entomologia |
| MZSP |
Sao Paulo, Museu de Zoologia da Universidade de Sao Paulo |
| INPA |
Instituto Nacional de Pesquisas da Amazonia |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
SubOrder |
Apocrita |
|
InfraOrder |
Aculeata |
|
SuperFamily |
Formicoidea |
|
Family |
|
|
SubFamily |
Martialinae |
|
Genus |