Doropygus humilis Stock, 1967
|
publication ID |
https://doi.org/ 10.11646/megataxa.4.1.1 |
|
DOI |
https://doi.org/10.5281/zenodo.6422227 |
|
persistent identifier |
https://treatment.plazi.org/id/03C487CB-EE21-3B47-FF4D-FF63FEECFCED |
|
treatment provided by |
Plazi |
|
scientific name |
Doropygus humilis Stock, 1967 |
| status |
|
( Fig. 244 View FIGURE 244 )
Syn.: Doropygus curvipes Gotto, 1975: 169 , fig. 3. new synonym. Doropygus apicatus Stock, 1967 , new synonym.
Material examined. 2 ♀♀ (MNHN-IU-2018-1869) and 1 dissected ♀ (figured) from Styela canopus (Savigny, 1816) : Bermuda; 1 ♀ (MNHN-IU-2018-1870) and 1 dissected ♀ from S. canopus , Madagascar (24°59 Ś, 47°05 É), depth 15-17 m, 09 May 2010.
1 ♀ (MNHN-IU-2018-1871) from Distomus hupferi (Michaelsen, 1904) Biaçores ; 3 ♀♀ (MNHN-IU-2018- 1872) and 1 dissected ♀ from D. hupferi, Biaçores.
1 ♀ (dissected) from Polycarpa cartilaginea (Sluiter, 1898) : Guadeloupe.
3 ♀♀ (MNHN-IU-2018-1873) and 1 dissected ♀ from Microcosmus exasperatus Heller, 1878 , Noumea, New Caledonia.
1 ♀ (dissected) from Stolonica inhacae (Millar, 1956) , Ibo, Mozambique.
1 ♀ (dissected) from Polycarpa plantei Monniot C., 2002 , Victoria harbour, Mahé Is., Seychelles, collected by Richmond, 1995.
1 ♀ (MNHN-IU-2018-1919) from Pyura ocellata Monniot F.,2016 , French Guiana (05°38.4 Ń, 52°29.2 Ẃ), Stn CP 4386, depth 46-47 m, 05 Aug 2014.
1 ♀ (MNHN-IU-2017-2174, dissected) from Polycarpa salutis Monniot F., 2016 , (06°31 Ń, 52°36 Ẃ), GUYANE 2014, Stn CP 4381, depth 114-118 m, 04 August 2014.
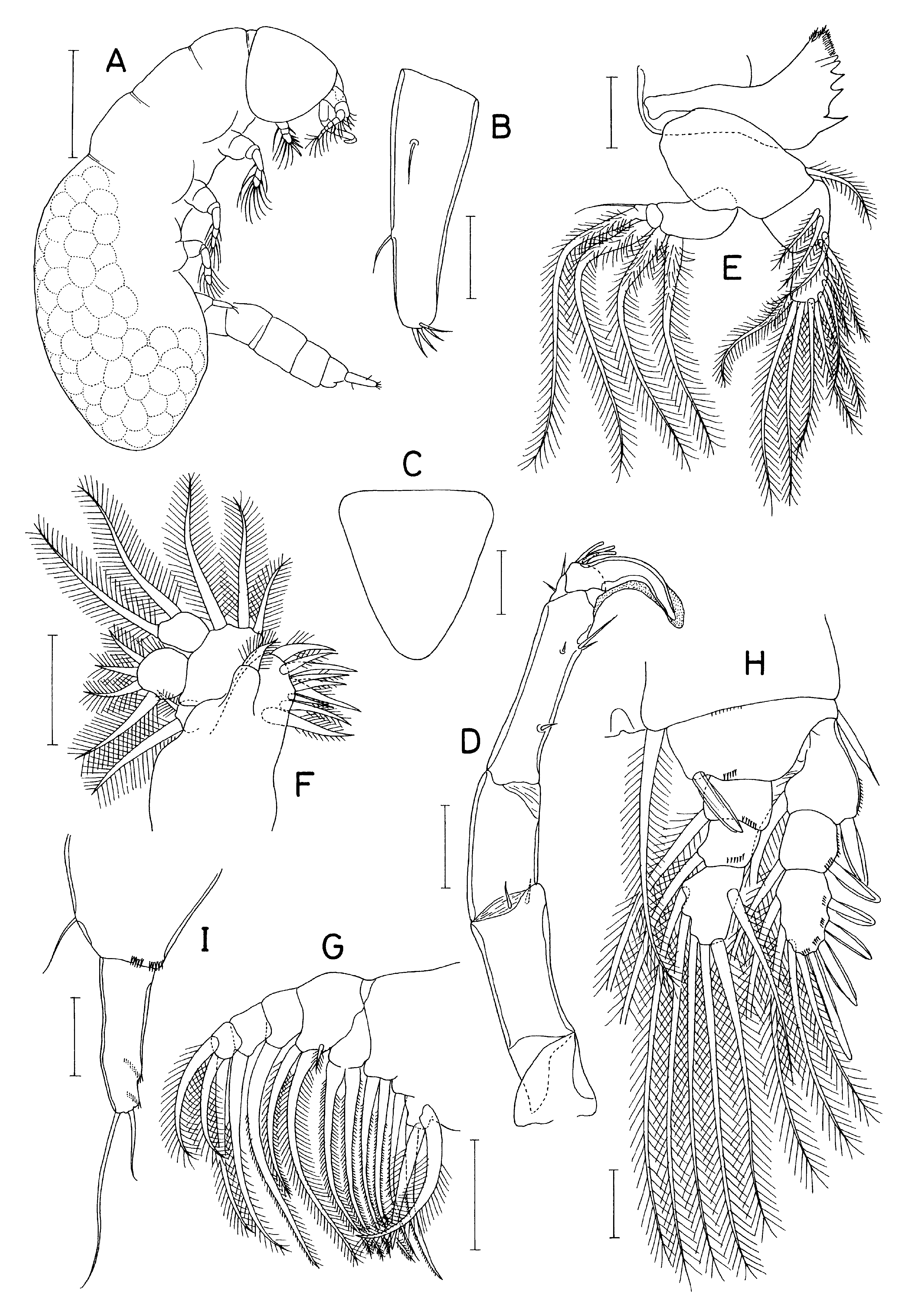
Supplementary description of female. Body ( Fig. 244A View FIGURE 244 ) relatively narrow; bodylength 2.96 mm: prosome 2.24 mm long. Dorsal cephalic shield distinctly defined. Metasomeindistinctly segmented. Fourthpedigerous somite forming elongate oval bood pouch, almost twice as long as wide, longer than anterior part of prosome, withrounded posterior margin. Freeurosome slender, 5-segmented. Anal somite characteristically tapering posteriorly, with deep posteromedian incision. Caudal ramus ( Fig. 244B View FIGURE 244 ) taperingevenly towards apex, 3.1 times longerthanwide (159×52 μm) and 1.85 timeslongerthan anal somite; 2 proximal setae positioned at 26 and 63% of ramus length.
Rostrum ( Fig. 244C View FIGURE 244 ) triangular, slightly longerthan wide, evenly tapering towards blunt apex. Antennule 9-segmented; first and second segments much broader than distal segments; armatureformula 3, 16, 3, 3, 5, 3, 2, 2+aesthetasc, and 7+aesthetasc; setae crowded and generally long; 2 larger setae on first segment about twice as long as width of segment; 2 pinnate setae on first segment, 3 on second, and 1 each on third, fifth, and sixth. Antenna ( Fig. 244D View FIGURE 244 ) narrow, 4-segmented; coxa short; basis twice as long as wide (97×48 μm), with 2 setae distally; firstendopodal segment unarmed, 1.8 times longerthanwide (77×43 μm); compounddistalendopodal segment about 4.3 times longerthan wide (137×32 μm); armed with 9 setae (3 distal subequal in length and at most one-third as long as terminal claw) plus terminal claw 75 μm long, 0.55 times as long as segment, gently curved with blunt tip, claw fringed with hyaline membrane along concave margin and distal part of convex margin.
Labrum as in D. pulex . Mandible ( Fig. 244E View FIGURE 244 ) with coxa and basis same as in D. pulex ; exopod indistinctly segmented, armed with 4 large equal setae and 1 rudimentary, thread-like seta distally, latter occasionally absent: endopod indistinctly articulated from basis, armed with 4 and 8 setae on first and second segments, respectively; 2 largest distal setae on second segment subequalin length. Paragnath lacking spinules at apex. Maxillule ( Fig. 244F View FIGURE 244 ) with 9 setaeon arthrite; setaon coxal endite more than twice as long as wide; epipodite with 2 very unequal setae; basis with 3 unequal setae on medial margin; exopodwith 4 setae distally, medial 3 subequally small, longer outer seta about twice length of medial 3; endopodwith 2 large, subequal setae. Maxilla ( Fig. 244G View FIGURE 244 ) with 8 setaeonsyncoxa (lacking proximal smallseta on fourth endite), 3 setae on basis, and 1, 1, and 3 setae on first to third endopodal segments, respectively. Maxilliped as in D. pulex , with 9 setae on first segment and 2 subequal, large setae on short second segment.
Leg 1 ( Fig. 244H View FIGURE 244 ) segmented and armed as in D. pulex , but inner coxal seta large (extending beyond distal tip of endopod); outer seta on basis flagellate distally and naked or weakly pinnate; inner distal spine on basis 54 μm, extending beyond distal border of first endopodal segment, fringed with membrane along lateral margins. Legs 2–4 as in D. pulex , except that inner coxal seta and inner setae on endopod larger and densely pinnate, and basis of leg 4 lacking outer seta.
Leg 5 ( Fig. 244I View FIGURE 244 ) with protopod bearing thin outer seta and mediodistal row of minute spinules; exopod 2.7 to 4.1 timeslongerthan wide, armedwith 2 thin, unequal setae distally and 2 or 3 rows of minute spinules on dorsomedial surface; longer outer seta as long as exopodal segment, about 2.5 times longerthan inner seta.
Remarks. Stock (1967) described D. humilis as an associate of an unidentified solitary ascidian (a member of either the Styelidae or the Pyuridae ) collected in the Red Sea. Doropygus curvipes Gotto, 1975 , which was described as an associate of Cnemidocarpa radicosa (Herdman, 1832) (as C. etheridgii Herdman, 1899 ) in Sydney Harbour, Australia ( Gotto, 1975), is here synonymised with D. humilis as there are no significant differences between these two species. Seo & Lee (1998) also reported this species under the name D. curvipes found in Styela clava Herdman, 1881 on the eastern coast of Korea. In the present account, eight additional ascidian species are reported as hosts of D. humilis , not only from the Indo-Pacific but also from the Atlantic Ocean. The wide geographical distribution of D. humilis may be linked to the global introductions of certain ascidian species, such as Styela canopus , that are known to serve as hosts of this copepod.
Doropygus apicatus Stock, 1967 was originally described mainly on the basis of the female, but the specimens examined by Stock (1967) were juvenile females; as indicated by his illustrated mandible which contains internally the developing gnathobase of the next moult stage. The antenna of D. apicatus has the same characteristic blunt terminal claw bearing hyaline membrane as found in D. humilis , and we consider that D. apicatus is probably based on copepodid stages of D. humilis . Here we propose to treat D. apicatus as a junior subjective synonym of D. humilis .
Doropygus humilis , as a member of the D. pulex complex, is distinguishable from other species of the complex by its characteristic antenna in which the terminal claw is fringed with hyaline membrane. This feature has not previously been reported in other species of Doropygus .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
SubPhylum |
Tunicata |
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |