Spintherobolus Eigenmann, 1911
|
publication ID |
https://doi.org/10.5281/zenodo.10881992 |
|
DOI |
https://doi.org/10.5281/zenodo.10881805 |
|
persistent identifier |
https://treatment.plazi.org/id/03DA87B2-2A39-FFF8-FF5C-F97448C1FB7A |
|
treatment provided by |
Juliana |
|
scientific name |
Spintherobolus Eigenmann |
| status |
|
Spintherobolus Eigenmann View in CoL View at ENA
Spintherobolus Eigenmann, 1911: 167 View in CoL
( type species: S. papilliferus Eigenmann, 1911: 167 View in CoL by monotypy and original designation)
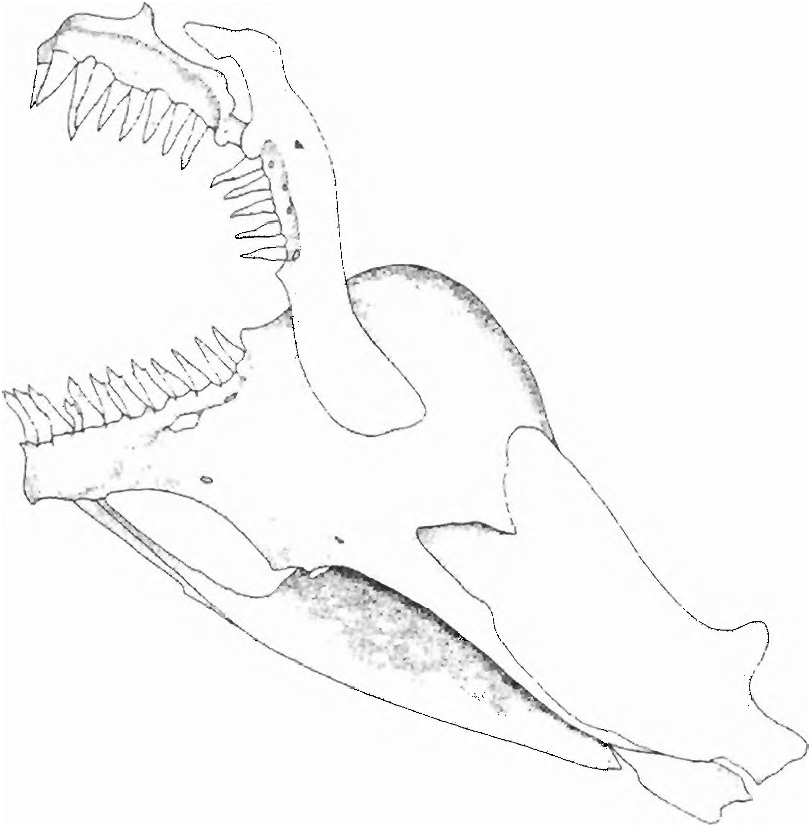
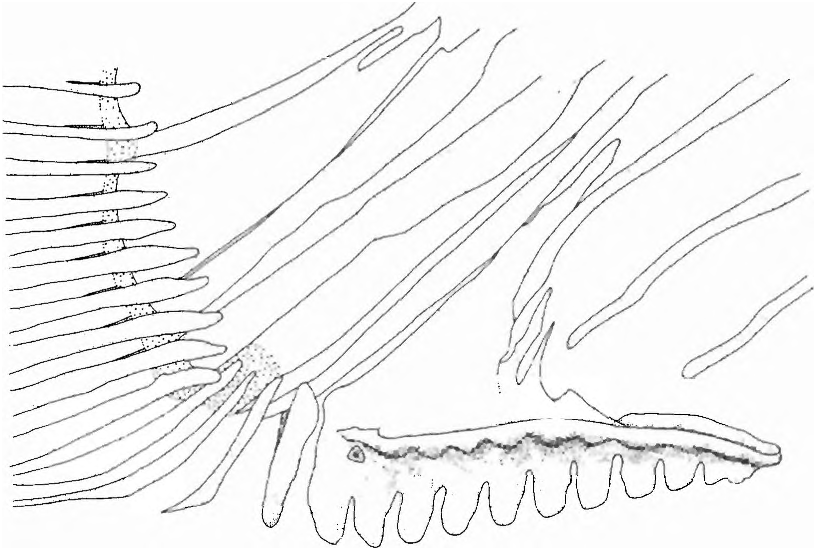
Diagnosis. Jaw teeth elongate and conical or tricuspid ( Fig. 12 View Fig ); anterior ventral procurrent caudal-fin rays with proximal ends anterior to haemal spine of antepenultimate vertebrae fused to one another proximally; symphyseal dentary ar ticulations are smooth, lacking intercalated folded bony surfaces common to most characids; neuromasts on head obvious and present in shallow channels and distributed on head in a char acteristic manner in which one horizontal series in the infraorbital region is crossed by several more or less vertical series ( Figs 4 View Fig , 10 View Fig & 11 View Fig ); dentary with a large anterior fenestra ( Fig. 12 View Fig ) associated with large epidermal, papilla-like structure bearing several neuromasts; anterior pseudotympanum occurs anterior to first pleural rib (see character 21 below for detailed explanation).
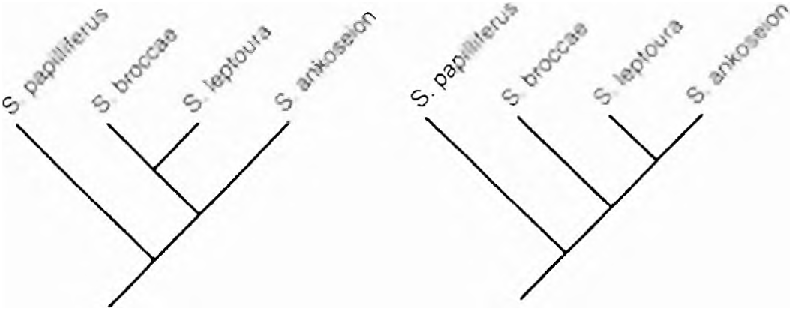
Monophyly of Spintherobolus . The following 14 (15-27, 3 R) synapomorphies indicate monophyly for Spintherobolus . Spintherobolus consists of two sister groups, one (a) containing S. papilliferus and the other (b) the remaining three species. This information is provided at this point to facilitate discussion of the fourteen synapomorphies described below.
(3 R) The jaw teeth are elongate and conical or tricuspid. When tricuspid there are small lateral cusps near the distal apices of what are essentially teeth having an elongate pedicle and a slightly recurved apical cone ( Fig. 12 View Fig ). The anterior dentary teeth of Spintherobolus have three small cusps. Teeth bearing these small cusps are more numer ous than the strictly conical teeth present in the large species, S. papilliferus . The premaxillary teeth are usually conical, but in S. papilliferus they bear some very small lateral cusps in their somewhat laterally expanded distal portions. The maxillary teeth in all species are conical.
Tooth shape, tooth counts and the number of cusps have been extensively used for suggesting cheirodontine and other characid relationships since Eigenmann (1915) used these features in Cheirodontines and other small characids. As discussed by Weitzman & Fink (1983: 341-353), features such as numbers of rows of teeth, counts of the numbers of teeth in a particular jaw bone, the number of cusps in particular teeth, and the shape and form of the teeth and their cusps var ies widely in various lineages of the American Characidae and provide data in support of phylogenetic hypotheses. However, as with many other characters, convergent evolution of such tooth features often cause problems in phylogenetic reconstruction, especially when teeth are not compared and described in enough detail to detect differences and similarities and are used for this purpose without the abundant use of other data.
Conical and tricuspid teeth are found in a number of American characids. The clades within the Cheirodontinae closest to Spintherobolus on the basis of other synapomorphies have five or more cusps, indicating that the low cusp number in Spintherobolus is derived. Some species belonging to other cheirodontine clades also have tricuspid teeth, but their teeth are never elongate and essentially conical in shape at their apices. For example, Odontostilbe microdon ( Eigenmann, 1915: 80) , Cheirodon australe Eigenmann (1927: 44) , and C. kiliani Campos (1982: 154) , although having tricuspid teeth, have these two secondary cusps large enough so that the teeth are broad and the cusps are more or less equal. This cusp number is here considered independently derived, not because of the different tooth shapes, but because of the numerous synapomorphies indicating these Cheirodon species belong to cheirodontine clades different from that of Spintherobolus .
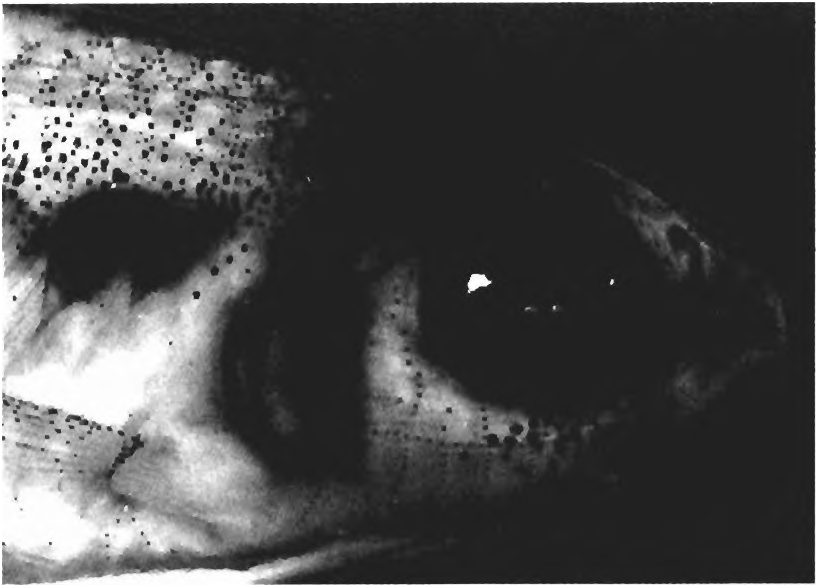
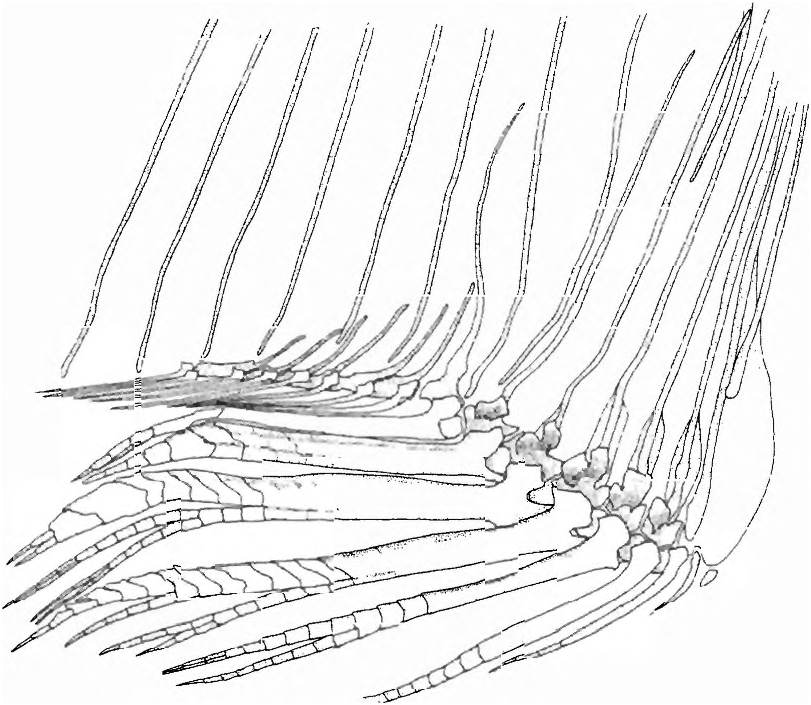
(15) A complex, patterned series of exposed neuromasts are distributed on the head and body ( Figs. 4 View Fig , 10 View Fig & 11 View Fig ). All Spintherobolus species lack most latero-sensory canals on the head and body. Instead there are series of exposed neuromasts distributed often in shallow channels, in a pattern unique to Spintherobolus over the dorsal, ventral, and lateral surfaces of the head. The neuromasts also occur near the posterior border of the body scales and scales at the base of the caudal fin. The neuromast lines of the head for S. broccae were mapped by Sarraf (1997: figs. 2-4), but their terminology equivalents to these lines in other fishes, for example in Arratia & Huaquin (1995: fig. 13) were not attempted. The neuromast lines of the species of Spintherobolus are quite complex and an accurate comparative study of their homology and the nomenclatural equivalency of these lines with those of other characins, catfishes and other teleosts would require a comparative study of the nerve branches serving their neuromasts, a study beyond the scope of the present work. Such a study would perhaps be a useful source of synapomorphies suggesting a phylogeny of the species of Spintherobolus , but we hesitate to use the neuromast lines of Spintherobolus for this purpose without a detailed study of their anatomy and homology.
The neuromasts of Spintherobolus were first identified as “tactile papillae” by Eigenmann (1911: 167). We examined the surface of these organs at 200 x but have no histological evidence to confirm that these are neuromasts. However, these organs are similar in gross anatomy to the neuromasts described for Astyanax mexicanus and histologically examined by Schemmel (1967). They are found distributed in a manner somewhat similar to those figured by Schemmel (1967: 266-267, figs. 4-5) along body scales of this species. In Spintherobolus , these organs are small, rounded, slightly elevated discs that are translucent or have a brown,grange, or red, color. The color intensity varies from collection to collection, ranging from no perceptible color to a relatively dark brown or red. The color possibly depends upon length of time in and the conditions of the preservative. It is also possible that the color is an environmental contaminant because specimens of some collections that have brown or red sediment caught in the mucus around their scales and fins are the ones that most often have colored neuromasts. The disc’ s color, when present, is due to several small, circular areas of color at the surface of the neuromast. These spots of color appear to correspond to sensory cells, each probably bearing a sensory filament. However, sensory filaments or cupulae are not detectable under a dissecting microscope.
Many of the neuromasts are located in shallow epidermal channels of Spintherobolus , especially on the head. The distribution pattern of these organs is approximately the same in all four species except that there are more vertical channels on the cheek (infraorbital region) in S. papilliferus than in the other species ( Figs. 10-11 View Fig View Fig ). Laterally on the cheek a longitudinal curved neuromast channel extends from the posterior border of maxilla to the posterior margin of preopercle. From this channel, at intervals, extend more or less vertical channels, each dorsally reaching the ventral margin of the orbit, and ventrally reaching to near the ventral border of the preopercle. The opercle has an anterior, vertical linear channel near the posterior preopercular border. Posterior to this the opercle bears a series of shallow channels containing neuromasts. Dorsally on the head these organs are mainly distributed in somewhat irregular transverse grooves between the orbits and in two small longitudinal channels between the nares ( Fig. 11 View Fig ). There is a well-developed ventrally facing skin flap extending posteriorly from the area between the dentary and maxilla to the posteroventral end of the preoper cle. This flap partly covers an elongate groove running along its length. The groove with its neuromasts has several medially directed branches. The dentary bears ventral grooves containing neuromasts, these being numerous in the gular area between the two halves of the dentary. Two to six vertically aligned neuromasts are located near the posterior border of many of the body scales. Although many of the scales bear neuromasts, they are not present on all scales. We were unable to detect any repetitive pattern of distribution of neuromasts o^ the body scales. See Arratia & Huaquin (1995) for citation of much of the literature on fish neuromasts.
What appear to be similar organs, also with complex patterns of distribution on the head, accompanied by an absence of laterosensory canals, were described by Poll & Lambert (1964a: 337,1964b: 407) for certain species of the African citharinid characiform Neolebias Steindachner, 1894 . These organs were identified as “lignes d’organes sensoriels ponctiformes”. Note: Although Poll & Lambert used Congocharax Matthes, 1964 for the generic name of those species of Neolebias readily showing the neuromasts, we follow Vari (1979: 330) in recognizing Congocharax as a junior synonym of Neolebias even though Poll & Gosse (1982: 5) provided ample evidence of the different features that can be used to distinguish these nominal genera. We do this because recognition of Congocharax would make Neolebias paraphyletic according to the phylogenetic evidence provided by Vari and not contradicted by additional data extracted from Poll & Gosse and cladistically analyzed along with the data from Vari.
As pointed out by Sarraf (1997: 30), similar organs are found in the species of Phenacogaster and Roeboides Gunther. These were called “cervical pit-lines” by Géry (1972: 10-11). Although cervical is a word referring to a neck, these struc tures are located in shallow channels mostly on the head and putatively assigned as a synapomorphy uniting these two genera by Malabarba & Lucena (1995: 342). We examined these struc tures in species of these two genera and found they have the same apparent gross anatomy and sometimes the same color characteristics as those of Spintherobolus , but with different distributional patterns on the head and body. We agree with Sarraf (1997) that these patterns are independently derived in Spintherobolus on the one hand and Phenacogaster and Roeboides on the other. The surface neuromasts of the laterosensory system and their characteristic distributional patterns found in some species of Neolebias , represent similar but apparently also independently derived features from those present in Spintherobolus or Phenacogaster and Roeboides . As Vari (1979: 329) has shown, the species of Neolebias bearing apparent neuromasts have a derived position in a clade of African characiforms. The exposed neuromasts described for Astyanax mexicanus by Schemmel (1967) seems to be also independently derived from those present in Spintherobolus (compare the neuromast distributional pattern of A. mexicanus in Schemmel (1967: 267, fig. 5) and of Spintherobolus species; Figs. 4,10). None of these outgroup characiform taxa bearing obvious neuromasts and discussed above have all the synapomorphies listed for the Cheirodontinae .
Böhlke (1954: 25) reported “rows of papillae” on the head of Crenuchus spilurus and suggested this genus should be examined when consider ing the relationships of either Spintherobolus or Grundulus . We have examined the heads of several specimens of C. spilurus and fail to find struc tures that we can confidently identify as being similar to the organs we tentatively identify as neuromasts. There are, however, scattered small papillose structures having a central depression or opening. These might be taste buds, but histological examination is needed to confirm such an identification. The Crenuchinae is phylogenetically related to the Characidiinae and both placed in the Crenuchidae by Buckup (1993a: 229; in press) and we found no evidence to contradict Buckup ’ s hypothesis or suggest that Crenuchus is a cheirodontine.
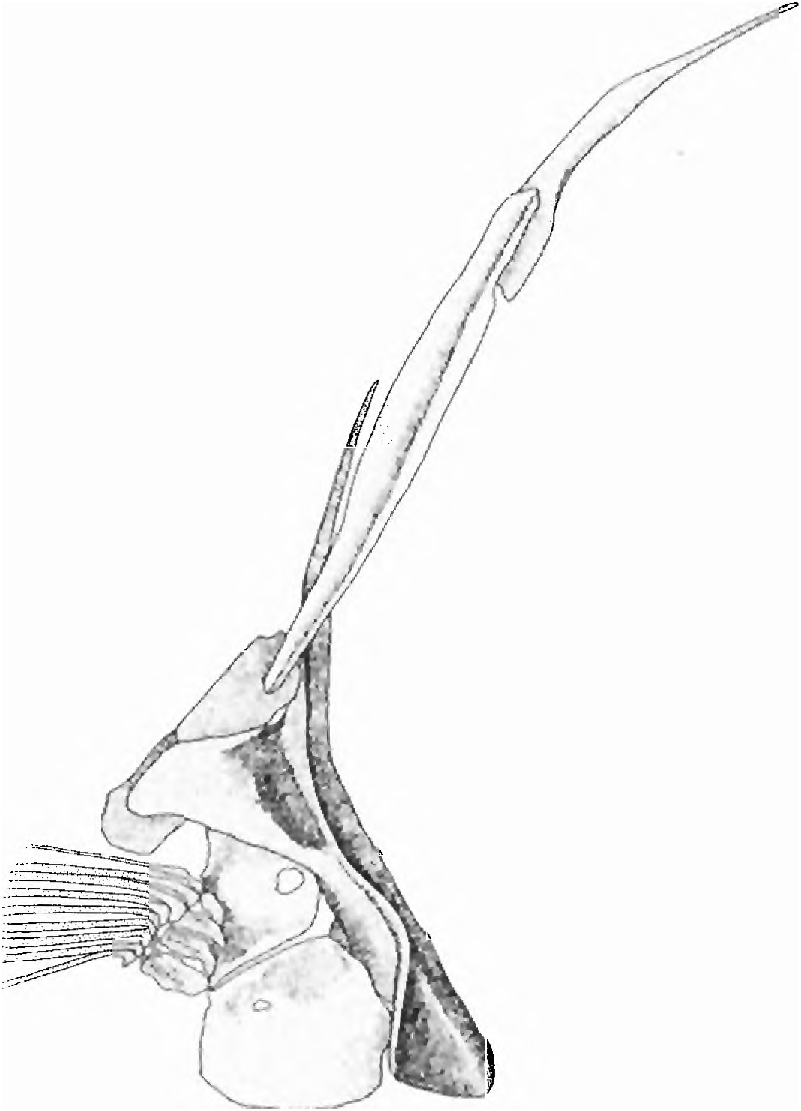
(16) The dentary has a large anterior fenestra ( Fig. 12 View Fig ). Associated with this fenestra is a large epidermal, papilla-like structure surrounded by a deep groove that has its deep internal portion lodged in the dentary fenestra. The external sur face of this papilla bears several exposed neuromasts. The ventral face of the dentary bone, posterior to the fenestra is concave (more so in S. papilliferus than the other species; see also the dentary characters noted below under the synapomorphies that hypothesize subgroup b in Fig ure 3). We suggest the fenestra and the associated nervous and supporting tissues are a derived sensory organ and consider all to be one complex character.
Travassos (1953: 506) first noted the fenestra, but not the associated modified soft tissue. He considered the fenestra to demonstrate a close relationship between S. broccae and S. papilliferus. All four species examined herein have this feature, which is absent in other cheirodontines or characids examined by us.
(17) The adipose fin is absent. The adipose fin loss in Spintherobolus is unique within the monophyletic subfamily Cheirodontinae as rec ognized by us; see also Malabarba (in press). We suggest the absence of an adipose fin in Spintherobolus and Grundulus is homoplastic because of the relationships hypothesized here for Spintherobolus . Eigenmann (1915: 4, 14) briefly discussed the lack of an adipose fin in Grundulus and Spintherobolus and, in part, used this feature to unite these genera in a phyletic group. Géry (1977: 607) followed Eigenmann in this regard.
Adipose fin loss has occurred several times within hypothesized independent clades of characiforms, for example the Characinae ( Priocharax Weitzman & Vari ), Xenurobryconini ( Iotabrycon Roberts , Xenurobrycon Myers & P. Miranda-Ribeiro , and Tyttocharax Fowler ), the Gasteropelecidae ( Carnegiella Eigenmann ), and the Lebiasinidae (some species of Nannostomus Gunther ); see also discussion by Weitzman & Ortega (1995: 147). In these genera the absence of an adipose fin is correlated with small to miniature size. The absence of an adipose fin in the American characiforms is not always associated with miniature size. For example the large species of Lebiasina , family Lebiasinidae , and species of the Erythrinidae lack an adipose fin. The American characid genus Hasemania Ellis was for the most part defined as being like Hyphessobrycon Durbin but without an adipose fin. As currently conceived Hasemania ( Géry, 1977:518) lacks miniature species. We agree with Géry (1977: 518) that this genus is probably polyphyletic.
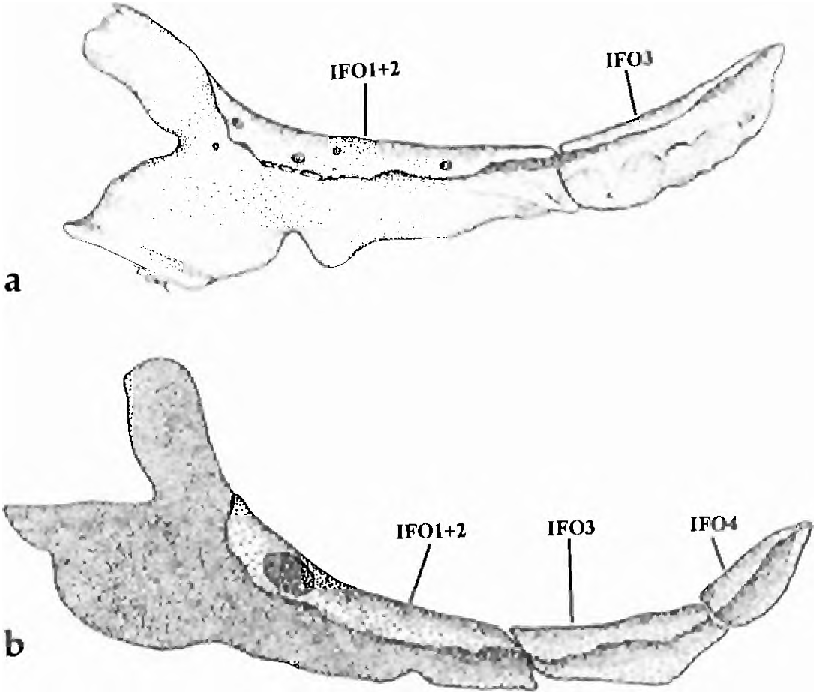
(18, state 1) The infraorbital bones ( Fig. 13 View Fig a-b) are somewhat variable, but some are always reduced in size and number and others possibly fused. They are usually altered as follows. One large bony element is located in the usual charac id position of infraorbital 2. In some specimens ( Fig. 13 View Fig a-b) this bone has an anterodorsal portion that is in the position of infraorbital 1of other characids and a posterior part that may represent infraorbital 2. Thus this bony element may consist of infraorbital 1 and 2 fused to each other. In some specimens this is the only infraorbital bone present. Another infraorbital, much reduced in size and apparently absent in S. broccae (18, state 2; see diagnosis under S. broccae ), is placed in the position of infraorbital 3 ( Fig. 13a View Fig ). Infraorbitals 4 through 6 are almost always absent, at least as ossifications, but in one specimen of S. ankoseion a fourth infraorbital bone appears to be present ( Fig. 13b View Fig ). A well developed antorbital is present in the ventro-lateral wall of the nasal capsule.
Although reductions in the size and apparent loss of infraorbitals are observed in some Cheirodontines, for example Cheirodon australe Eigenmann , no other Cheirodontines have the infraorbitals with the anatomy described here for Spintherobolus . A reduction of the posterior infraorbital bones also occurs in Grundulus bogotensis (Humboldt) but in that species infraorbital 2 and a large infraorbital 3 are well ossified.
Infraorbital bone reduction and apparent loss are common in small and miniature characiforms of divergent clades; see Weitzman & Fink (1983) and Weitzman & Fink (1985) for documentation. However, S. papilliferus and G. bogotensis are both comparatively large and were in part suggested as related by shared infraorbital loss by Eigenmann (1915: 5-6, 14) and Géry (1977: 607). Although the pattern of infraorbital loss in these species differs, this would not preclude consider ing such loss a synapomorphy for the two species. However, Grundulus lacks the synapomorphies uniting the species of Spintherobolus to the Cheirodontinae .
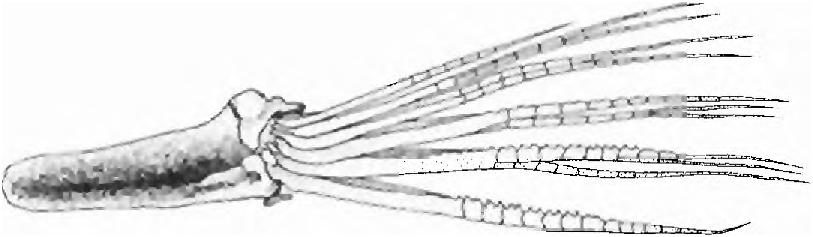
(19) There are one unbranched and five to six branched pelvic-fin rays. The outgroup number of pelvic-fin raysin the Characidae is i,7, and, with rare exceptions, this count is found in all outgroup cheirodontine species. The count in Spintherobolus is i,5 or i, 6 in S. papilliferus and is always i, 5 in the other three species. This is a derived reductive feature for the species of Spintherobolus ( Fig. 14 View Fig ).
A low pelvic-fin ray count occurs in certain miniature or small sized characiforms, for example Carnegiella (see Weitzman, 1960: 217) and Priocharax Weitzman & Vari (1987: 614) , and one might therefore expect a low pelvic-fin ray count in a small species such as S. broccae. However, S. papilliferus although not especially small (to at least 60.8 mm SL) has a low pelvic-fin ray count. Grundulus bogotensis , also not especially small (to at least 67.0 mm SL) also has a somewhat reduced number of pelvic-fin rays, i,6.
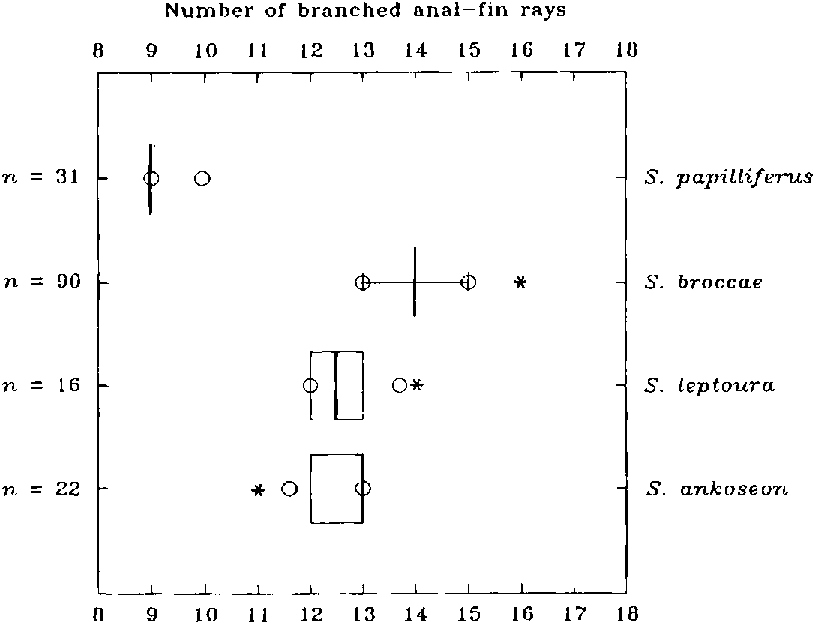
(20) The anal fin has iii-iv unbranched and 9- 16 branched rays ( Figs. 9 View Fig , 15 View Fig ). The number of anal-fin rays in the Characidae is quite variable. The primitive state, however, can be hypothesized as having at least more than 15 branched rays as found in the apparently most primitive American characid species such as the species of Brycon Müller & Troschell. Among the cheirodontine species the number of branched anal-fin rays varies from 16 to 24. Spintherobolus thus has a derived, reduced number and we suggest this as a synapomorphy uniting the species of the genus. A reduced number of anal-fin rays also occurs in the Chilean species of Cheirodon (C. australe Eigenmann , C. galusdae Eigenmann (12-15 branched rays), C. pisciculus Girard , C. kiliani (9-13 )) but this is independently derived according to the hypothesis of cheirodontine relationships proposed herein. Grundulus bogotensis also has a reduced number of anal-fin rays, iv, 11 -12, but again evolved independently of Spintherobolus , based on its lack of synapomorphies of clades A toD.
Branched anal-fin rays in Spintherobolus varies from 13- 16 (x = 14.1; state 1) in S. broccae , 11 - 14 in S. ankoseion and S. leptoura (x = 12.6 for both species; state 2), and 9- 10 (x = 9.1; state 3) in S. papilliferus. We considered these three ranges and their means as different states in our analysis because all were significantly different and can thus be hypothesized as having a different genetic basis. All three states are derived according to outgroup comparison. Grundulus bogotensis also has a small number of anal-fin rays (11-12) and has been suggested as closely related to the species of Spintherobolus by Géry (1977: 607), but again, its anal fin is different from those of the species of Spintherobolus in that the anal fin in G. bogotensis lacks the derived features of the anal-fin rays in the males of the species of Spintherobolus described here. Also, the anal-fin pterygiophores of Grundulus appear derived in that they are all short, having no elongate anterior pterygiophores as found in the species of Spintherobolus and in most characids. Thus the reduced number of anal-fin rays in Grundulus bogotensis seems to be derived, but based on morphology not homologous to the condition found in Spintherobolus species. The most parsimonious hypothesis in the global analysis indicates state 3 (9-10) anal fin rays, is an autapomorphy for S. papilliferus. State 1 (13-16 branched rays) is a synapomorphy for Spintherobolus with an additional reduction of 13-14 branched rays (state 2), as synapomorphic for S. ankoseion and S. leptoura is equally parsimonious with the hypothesis that state 2 is a synapomorphy for Spintherobolus together with an increase (state 1) as an autapomorphy for S. broccae.
(21) An anterior pseudotympanum lies anterior to the first pleural rib. This pseudotympanum consists of a muscle hiatus lateral to the anterior portion of the swimbladder. The juveniles of S. papilliferus and all specimens of the other three species display both pseudotympanums ( Figs. 4-6 View Fig View Fig View Fig , 16). However, in adult S. papilliferus the anterior pseudotympanum is partially filled by muscular tissue and is relatively obscure but still present ( Fig. 17 View Fig ).
A posterior pseudotympanum is situated between the first and second pleural ribs in all cheirodontines including the species of Spintherobolus but all other cheirodontines lack the anterior pseudotympanum. The presence of a muscle hiatus anterior to first pleural rib has been also observed in Atopomesus pachyodus Myers , Paracheirodon axelrodi (Schultz) , and an unidentified species of Axelrodia Géry. However , these species do not have all of the synapomorphies shared by species of the Cheirodontinae . Thus these three species are excluded from that subfamily and relationships with Spintherobolus .
(22) In adult males the anterior ventral procurrent caudal-fin rays, those that have their proximal ends inserted anterior to the haemal spine of the antepenultimate vertebrae, are fused to one another proximally ( Fig. 8 View Fig ). Fusion begins with sexual maturation only in males. Among characiforms this character is unique to species of Spintherobolus , although one species of undescribed cheirodontine not closely related to Spintherobolus also has some fusion among its ventral procurrent rays.
(23) The anterior ventral procurrent caudal-fin rays of males have reduced proximal portions, not rising above the area of fusion between the rays, while the posterior dorsal portions of these rays are fused into a flat compressed plate that inserts between the hemal spine of the preural vertebrae ( Fig. 8 View Fig ). The ventral procurrent caudal-fin rays posteriorly associated with the hemal spine of pu3, pu2, and pul (parhypural) are separate. The proximal portions of the anterior, ventral procurrent caudal-fin rays in males of Spintherobolus species are reduced or lost during sexual maturation. In young specimens and females of Spintherobolus and other Characidae these portions are ossified and well-developed.
(24) The symphyseal dentary joint surfaces are smooth oval articular surfaces, lacking the intercalated and folded bony surfaces found in other characiforms. The articulation is supported by tough ligamentous tissue. A similar condition was found in the cheirodontine Pseudocheirodon arnoldi (Boulenger) , a species that otherwise displays none of the synapomorphies of Spintherobolus and clade B Cheirodontines.
(25) Lateral line reduced to 2-6 perforated scales. Lateral line reduction or loss has been used for defining cheirodontine and other characid genera since Eigenmann (e.g. 1915, 1917). Weitzman & Fink (1983: 391-394) discussed laterosensory reduction in small characids and noted that its loss is often correlated with small size and that the condition has evolved repeatedly in small characids. They also suggested that such reductive characters are phylogenetically significant at some level, but in practice can sometimes prove problematic for use as synapomorphies.
In Spintherobolus we found lateral-line reduction to be a synapomorphy for the species of the genus according to the most parsimonious distribution of the 35 characters used in our analysis of cheirodontine and other characids. Lateral-line counts range from 2-6 in Spintherobolus species (mean 3.7 in S. broccae , 4.0 in S. leptoura and S. ankoseion , 4.6 in S. papilliferus ). In the nearest clade to Spintherobolus , the O. piaba group, the means in lateral-line range from 6.5-9.2. In the next closest clade, the species of Cheirodon , the means range from 6.7-8.8. No other Cheirodontines have the reduced number of pored scales in Spintherobolus . Grundulus also has a small number of perforated lateral-line scales (5-6), but this appears independently derived according to the most parsimonious hypothesis of our data.
(26) The coracoid bone of the pectoral girdle of Spintherobolus is reduced in length, and more or less discoid in shape ( Fig 18 View Fig ). In all other cheirodontines and characids examined by us the coracoid is either derived in other ways, for example in the gasteropelecids ( Weitzman, 1954: 225), or elongate and similar to that described by Weitzman (1962: 41) for Brycon meeki Eigenmann & Hildebrand.
(27, state 1) The eyes of species of Spintherobolus are small and derived compared to other Cheirodontines. Spintherobolus broccae has a mean eye diameter of 28.7 %, S. leptoura a mean of 27.2 %, S. ankoseion a mean of 27.6 %, and S. papilliferus a mean of 21.3 % compared to head length. Cheirodontine outgroups related to Spintherobolus have relatively large eyes, 33.9-40.3 % HL in clade C ( Cheirodon ) and 35.4-41.0 % of head length in clade E ( O. piaba and related species) and many American characids have an eye size ranging from about 25 to 35 % HL ( Weitzman et al., 1994: 55). Small eye size is apparently a reductive synapomorphy for Spintherobolus .
(28) Relatively short pectoral-fin lengths are characteristic of males of S. broccae (15.2-18.7, x = 16.9 % SL), S. leptoura (15.6- 17.9, x = 16.9 % SL), and S. papilliferus (15.7-16.6, x = 16.1 % SL). The males of S. ankoseion have relatively long pectoral fins (18.2-20.8, x = 19.0 % SL) and the outgroup clades C and E also have relatively long pectoral fins, 17.0-20.9, x = 18.5 % SL and 19.6- 21.7, x = 20.2 % SL respectively.
The distribution of this feature is ambiguous in three equally most parsimonious cladograms based on other characters (Figs. 3,19a-b). First, in all three cladograms this feature may be considered a synapomorphy for hypothesizing the monophyly of Spintherobolus , but with a reversal to a longer pectoral fin in S. ankoseion, requiring two steps in each. However, in the cladogram having S. broccae and S. leptoura united as sister species ( Fig. 19a View Fig ) another, two step, equally par simonious hypothesis is supported by the data. This hypothesis indicates that a short pectoral fin was independently acquired in S. papilliferus and in the clade uniting S. broccae and S. leptoura ( Fig. 19a View Fig ). In this hypothesis the character of short pectoral fin does not support Spintherobolus as a clade.
Etymology. Spintherobolus comes from the Greek spinther meaning spark and obelos, to spit. Eigenmann (1915:19) stated “emitting small sparks, referring to the appearance of yellow neuromasts on the head”.
Key to species of Spintherobolus
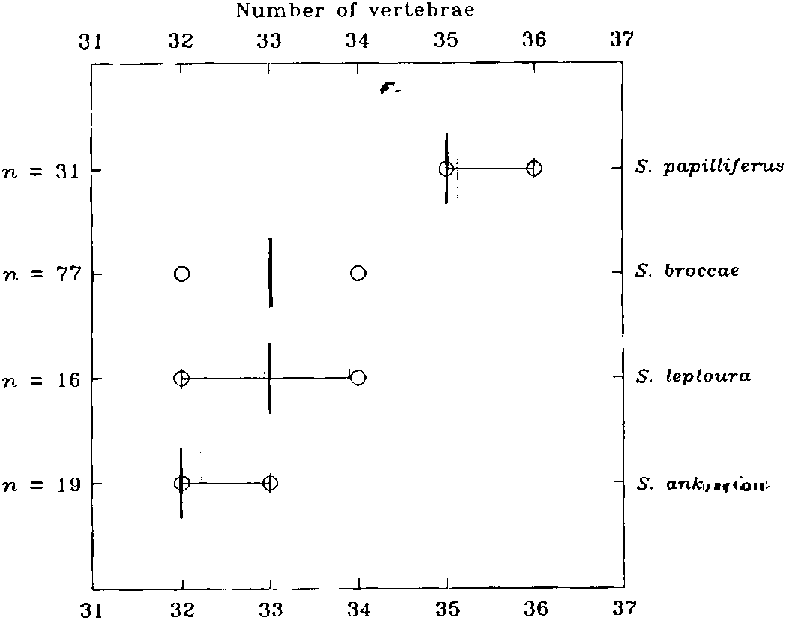
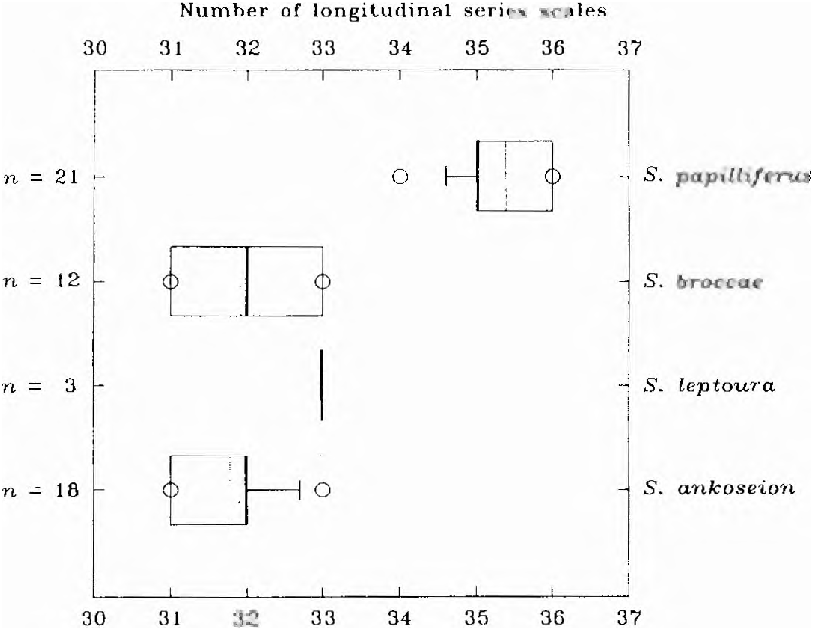
1 -Branchedanal-finrays 11-16 ( Fig. 20 View Fig ); vertebrae32-34( Fig. 21 View Fig );scales in a longitudinalseries31-33( Fig. 22 View Fig );horizontal eye diameterlonger than snout length; coastal Atlanticdrainages,east of Serra Geral. ..
2 - Branchedanal-finrays 9, rarely 10; vertebrae35-36;scales in a longitudinal series 34-36; horizontal eye diameter shorter than snout length; headwaters of rio Tietê, upper rio Parana drainage......... S. papilliferus
2. Branched anal-fin rays 11 -14, usually 12- 13, ›`‹ = 12.6 for both species; ventral procurrent caudal-fin rays 12-16, ›`‹ = 14.0 for S. ankoseıoıı and 14.1 for S. leptoura (Fig. 23); third infraorbital bone present. 3 Branched anal-fin rays 13-16, usually 14- 15, i = 14.1; ventral procurrent caudal-fin rays 14-17, i = 15.4; third infraorbital absent; Rio de Janeiro region and area near Santos, São Paulo). S. broccne
3. Body depth of specimens of both sexes between about 21.0 and 28.0 mm SL 32.4- 385:Á SL, i = 35.8 % (Fig. 24); male body depth regardless of body length 29.3-37.9 "78 SL, i = 34.2 %; male caudal-peduncle depth 12.8- 15.6 % SL, i = 14.8 % (Fig. 25); freshwaters around Baia de Paranaguá, Paraná south to Barra do Sai, Santa Catarina.
S. aııkoseíoıı
Body depth of specimens of both sexes between about 21.0 and 28.0 mm SL 27.5- 33.7 3/11 SL, i = 31.0 3/1) (Fig. 24); male body depth regardless of body length 26.6-29.7 %, i = 28.3; male caudal peduncle depth 11.0- 13.1 % SL, i = 12.3 9i, (Fig. 25); rio Quilombo, rio Ribeira de Iguape drainage, São Paulo. S. Ieptoura
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Order |
|
|
Family |
|
|
SubFamily |
Cheirodontinae |
|
Tribe |
Cheirodontini |
Spintherobolus Eigenmann
| Weitzman, Stanley H. & Malabarba, Luiz R. 1999 |
Spintherobolus
| Eigenmann, C. H. 1911: 167 |
S. papilliferus
| Eigenmann, C. H. 1911: 167 |