Macrobiotus kamilae, Coughlan & Stec, 2019
|
publication ID |
https://doi.org/ 10.5852/ejt.2019.573 |
|
publication LSID |
lsid:zoobank.org:pub:7619772F-2300-442E-8950-69559172360E |
|
persistent identifier |
https://treatment.plazi.org/id/AA314AF2-9A60-47E3-9EB3-B8B249FC1580 |
|
taxon LSID |
lsid:zoobank.org:act:AA314AF2-9A60-47E3-9EB3-B8B249FC1580 |
|
treatment provided by |
Plazi |
|
scientific name |
Macrobiotus kamilae |
| status |
sp. nov. |
Macrobiotus kamilae View in CoL sp. nov.
urn:lsid:zoobank.org:act:AA314AF2-9A60-47E3-9EB3-B8B249FC1580
Figs 8–15 View Fig View Fig View Fig View Fig View Fig View Fig View Fig View Fig
Etymology
We take great pleasure in dedicating this new species to the friend of the second author, Kamila Zając, who is a young malacologist and a PhD student at the Institute of Environmental Sciences, Jagiellonian University, Kraków, Poland.
Material examined
77 animals (including 19 simplex) and 42 eggs. Specimens mounted on microscope slides in Hoyer’s medium (63 animals + 32 eggs), fixed on SEM stubs (10+10) and processed for DNA sequencing (4+0).
Holotype
INDIA – Chuy Province • ♀; Camel’s Back Road, Mussoorie , Dehradin District , Uttarakhand State; 30°27′28″ N, 78°04′41″E; 2001 m a.s.l.; moss on rock; IZiBB IN.030.08. GoogleMaps
Paratypes
INDIA – Chuy Province • 71 paratypes; same collection data as for holotype; IZiBB IN.030.01– 06, IN.030.08–12, IN.030.14–15, IN.030.18–19 GoogleMaps • 47 eggs; same collection data as for holotype; IZiBB IN.030.13, IN.030.16–17, IN.030.20 GoogleMaps .
Description
Animals (measurements and statistics in Table 4 View Table 4 )
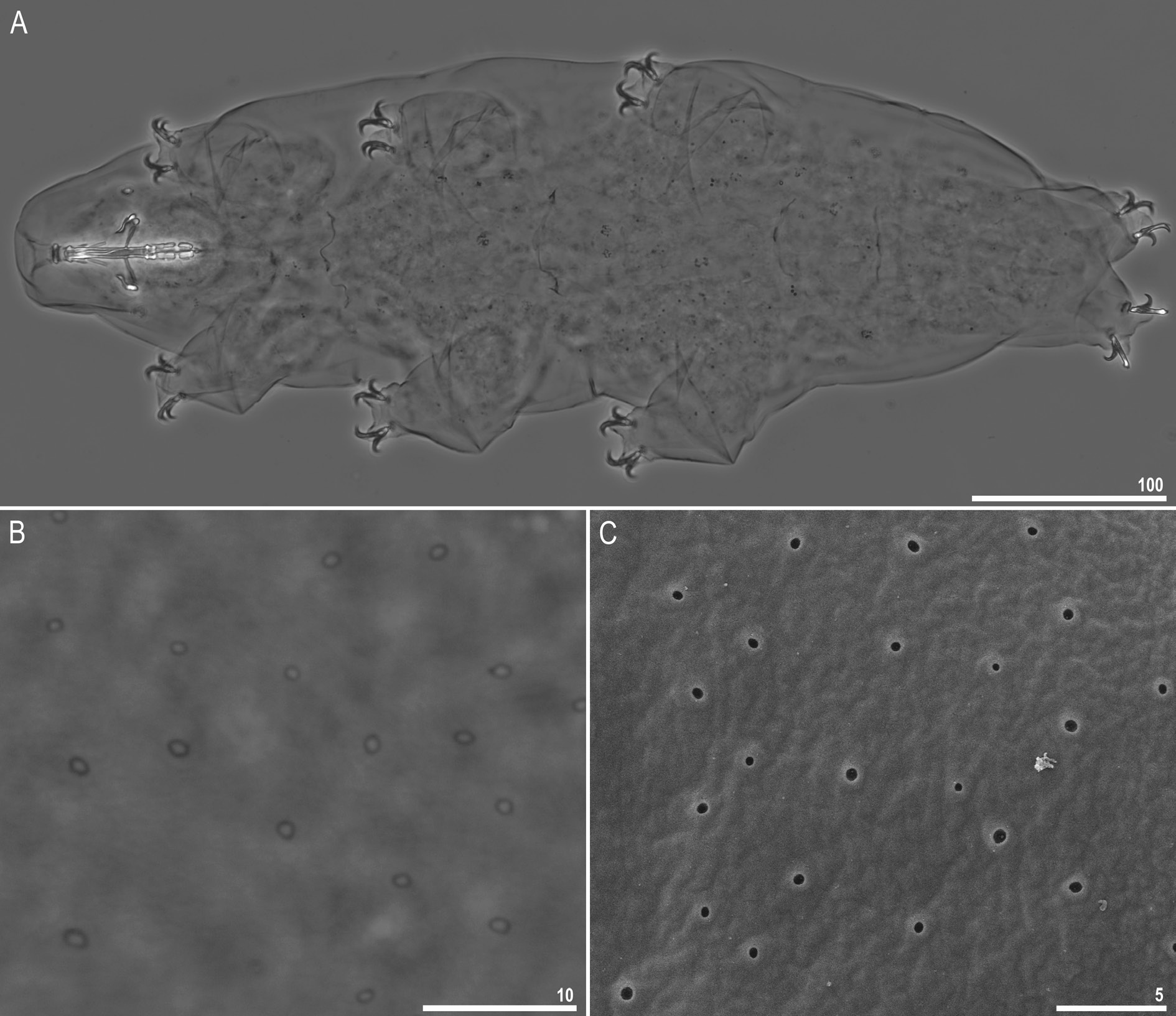
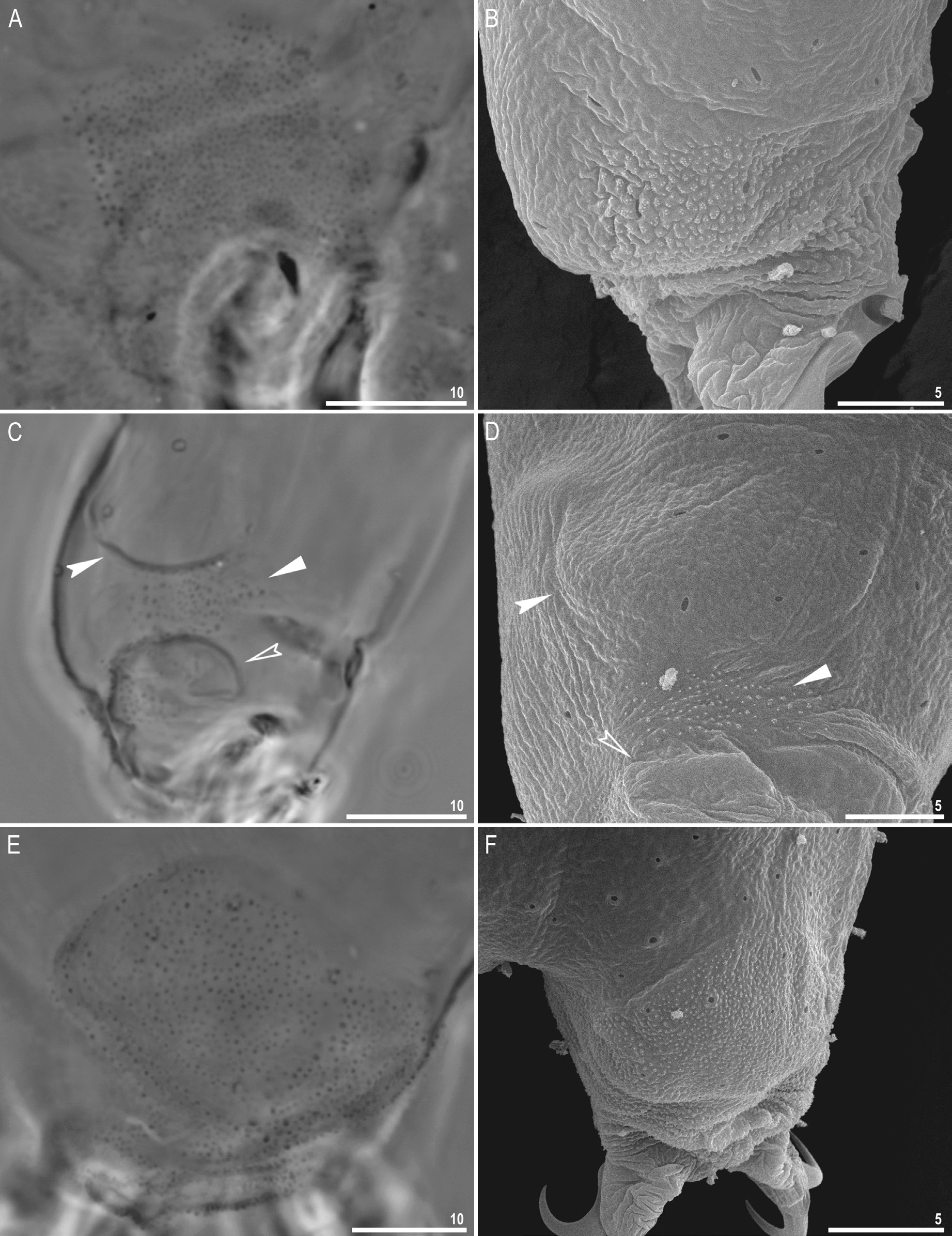
Body transparent in juveniles and yellowish in adults, but transparent after fixation in Hoyer’s medium ( Fig. 8A View Fig ). Eyes present in live animals as well as in specimens mounted in Hoyer’s medium. Small round and oval cuticular pores (0.3–0.8 μm in diameter), visible under both PCM and SEM, scattered randomly on entire body ( Fig. 8 View Fig B–C). Granulation present on all legs ( Fig. 9 View Fig A–F). A patch of clearly visible granulation present on external surface of legs I–III ( Fig. 9 View Fig A–B).A cuticular bulge/fold (pulvinus) present on internal surface of legs I–III, with a faint cuticular fold and a patch of granulation between them ( Fig. 9 View Fig C–D). Both structures visible only if legs fully extended and properly oriented on slide. Cuticular granulation on legs IV always clearly visible and consisting of a single large granulation patch on each leg ( Fig. 9 View Fig E–F). In addition to granulation on legs, three patches of granulation on body located dorso-laterally between legs III and IV, with granule size and density increasing from 1 st to 3 rd patch ( Fig. 10 View Fig A–E).
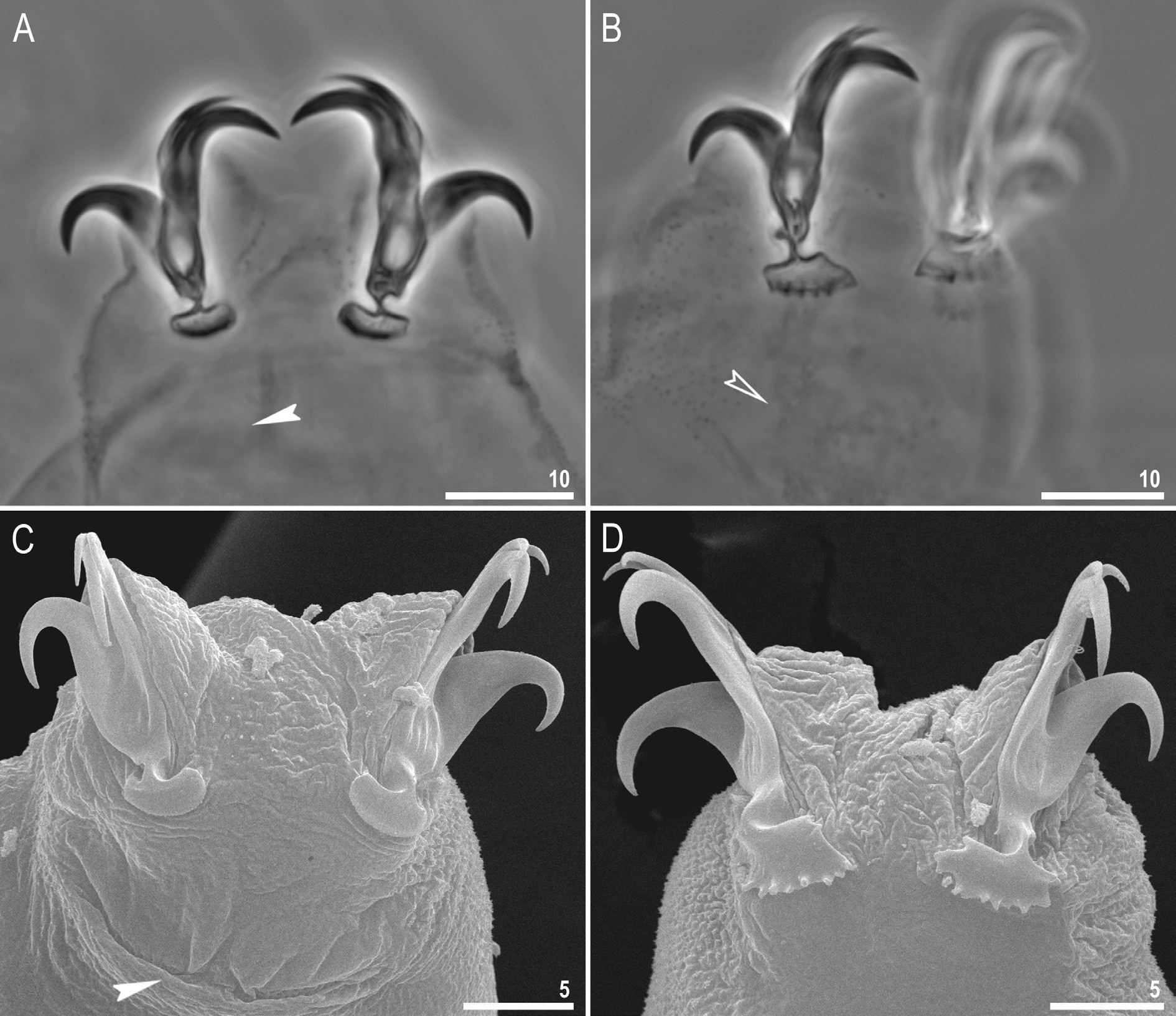
Claws long and slender, of the hufelandi type ( Fig. 11 View Fig A–D). Primary branches with distinct accessory points, a long common tract and with an evident stalk connecting the claw to the lunula ( Fig. 11 View Fig A–D). Lunulae I–III smooth ( Fig. 11A, C View Fig ), whereas lunulae IV clearly dentate ( Fig. 11B, D View Fig ). Cuticular bars under claws are absent. Double muscle attachments are faintly marked under PCM but clearly visible under SEM ( Fig. 11A, C View Fig , respectively). A faintly marked horseshoe structure connecting the anterior and the posterior claw is visible only in PCM ( Fig. 11B, D View Fig ).
Mouth antero-ventral with ten peribuccal lamellae and a circular sensory lobe ( Figs 12A View Fig , 13A View Fig ). Buccopharyngeal apparatus of the Macrobiotus type ( Fig. 12A View Fig ). Under PCM, the oral cavity armature is of the patagonicus type, i.e., with only the 2 nd and 3 rd bands of teeth visible ( Fig. 12 View Fig B–E). However, in SEM all three bands of teeth are visible, with the first band being situated at the base of peribuccal lamellae and composed of a single row of small cone-shaped teeth. The second band of teeth is situated between the ring fold and the third band of teeth and comprises 2–4 rows of small cone-shaped teeth, slightly larger than those in the first band ( Figs 12 View Fig B–E, 13B–C). Under PCM the second band is faintly visible in large as well as small specimens ( Fig. 12 View Fig B–E). The teeth of the third band are located within the posterior portion of the oral cavity, between the second band of teeth and the buccal tube opening ( Figs 12 View Fig B–E, 13B–C). The third band of teeth is discontinuous and divided into the dorsal and ventral portions. Under PCM, the dorsal teeth are fused and seen as one distinct transverse ridge, whereas the ventral teeth appear as two separate lateral transverse ridges and a median tooth which is sometimes divided into two roundish teeth ( Fig. 12 View Fig B–E). In SEM, both dorsal and ventral teeth are also clearly distinct ( Fig. 13 View Fig B– C). Under SEM, the margins of the dorsal portion of the third band are slightly serrated with two clearly visible peaks ( Fig. 13B View Fig ), whereas the ventral teeth are separated with a medio-ventral tooth slightly anterior to the lateral teeth ( Fig. 13C View Fig ). Pharyngeal bulb spherical, with triangular apophyses, two rodshaped macroplacoids and a small triangular microplacoid ( Fig. 12A View Fig , F–G). The macroplacoid length sequence 2 <1. The first macroplacoid exhibits a central constriction, whereas the second macroplacoid is faintly sub-terminally constricted ( Fig. 12 View Fig F–G).
Eggs (measurements and statistics in Table 5 View Table 5 )
Laid freely, yellowish, spherical or slightly ovoid ( Figs 14A View Fig , 15A View Fig ). The surface between processes is of the hufelandi type, i.e., covered with a reticulum ( Figs 14E View Fig , 15 View Fig B–F). Meshes of the reticulum small and rounded, irregular in size (mesh diameter 0.3–0.8 µm), with interbasal meshes slightly larger than peribasal meshes but peribasal meshes do not form rings around process bases ( Figs 14E View Fig , 15 View Fig B–F). The nodes of reticulum are often narrower than the mesh diameters visible in PCM and SEM ( Figs 14E View Fig , 15F View Fig ). Eggs have 26–32 processes on the circumference, 29 on average ( Fig. 14A View Fig ). Processes are of the inverted goblet shape with slightly concave trunks and concave terminal discs ( Figs 14 View Fig C–D, 15B–E). Terminal discs round, with faintly indented margins ( Fig. 15 View Fig B–E). Each terminal disc has a distinct concave central area which may contain some scattered granulation within, which is also always present on the margin (visible only under SEM; Fig. 15E View Fig ).
Reproduction
The new species is dioecious. No spermathecae filled with sperm have been found in gravid females on the freshly prepared slides. However, in males the testis, filled with spermatozoa, is clearly visible under PCM up to 24 hours after mounting in Hoyer’s medium ( Fig. 14F View Fig ). The new species does not exhibit male secondary sexual dimorphism traits such as lateral gibbosities on legs IV.
DNA sequences
We obtained sequences for all four of the above-mentioned DNA markers. All sequenced fragments were represented by single haplotypes except the COI, in which two distinct haplotypes were present:
The 18S rRNA sequence (GenBank: MK737070 View Materials ), 1015 bp long.
The 28S rRNA sequence (GenBank: MK737064 View Materials ), 793 bp long.
The ITS-2 sequence (GenBank: MK737067 View Materials ), 381 bp long.
The COI haplotype 1 sequence (GenBank: MK737920 View Materials ), 658 bp long.
The COI haplotype 2 sequence (GenBank: MK737921 View Materials ), 658 bp long.
Phylogenetic analysis
The phylogenetic analysis, based on available COI sequences of M. hufelandi spp., conducted in our study showed that M. noongaris sp. nov. and M. kamilae sp. nov. indeed belong to this group. The analysis recovered two highly supported clades ( Fig. 16 View Fig ). The first grouping (blue nodes) is of species with typical processes of inverted goblet shape and whitish body (with the only exception being M. cf. recens , which has processes in the shape of thin cones devoid of terminal discs). In contrast to the first clade, the second group (red nodes) is of species with yellowish body and morphological modifications of egg processes (flexible filaments on the terminal discs or processes without terminal discs). Interestingly, the two new species described within this study, which both exhibit typical inverted goblet-shaped processes, have been found to cluster together with the species which have modified egg processes. However, M. kamilae sp. nov. has a yellowish body, which conforms to the second characteristic of this clade, whereas M. noongaris sp. nov. has a whitish body.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |