Lonchaea sylvatica (Lonchaeidae), Beling, 1873
|
publication ID |
https://doi.org/10.1080/00222933.2015.1010314 |
|
DOI |
https://doi.org/10.5281/zenodo.4330130 |
|
persistent identifier |
https://treatment.plazi.org/id/03F4879D-FFA3-5058-9232-B6B8FC5D5F1D |
|
treatment provided by |
Carolina |
|
scientific name |
Lonchaea sylvatica (Lonchaeidae) |
| status |
|
Mycophagy: Lonchaea sylvatica (Lonchaeidae) View in CoL and Stegana coleoptrata (Drosophilidae) .
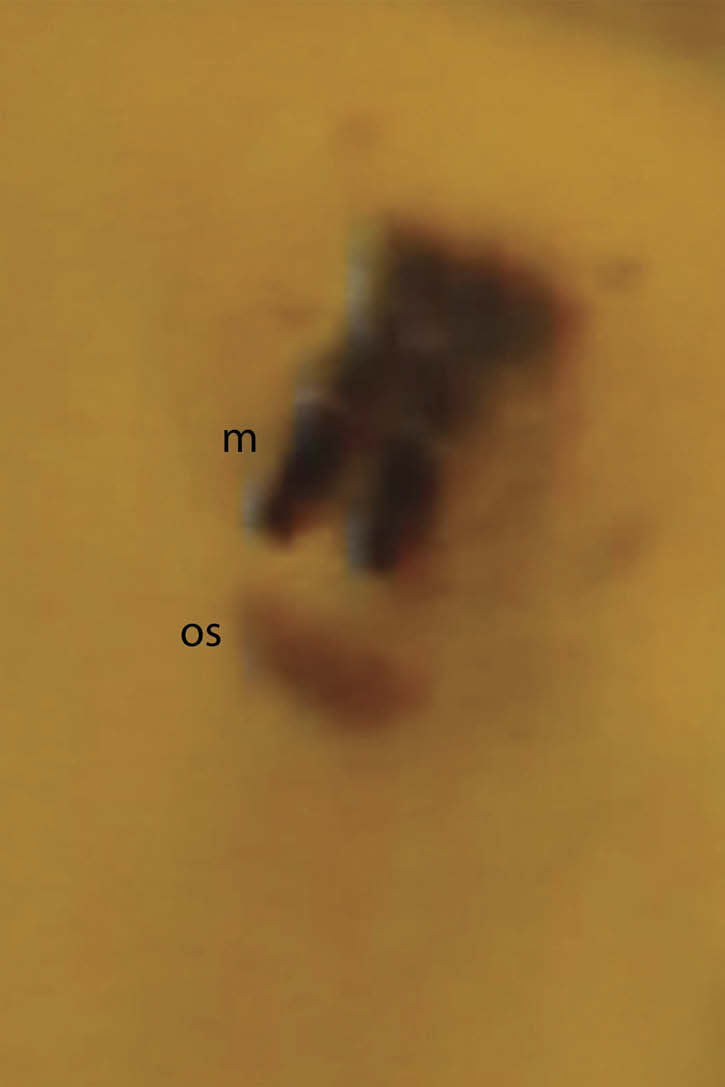
The larvae of L. sylvatica and S. coleoptrata feed on sooty moulds growing under bark lenticels of fallen aspen, Populus tremula L. and especially birch, Betula spp. ( Figure 51 View Figure 51 ). They can also feed on soft solid to firm biofilm under bark, more so L. sylvatica . The heights of these spaces is often less than the heights of larvae and access is restricted++ to +++. The distinctive features of these two species are the shapes of the prothorax, the pseudocephalon and the position, size and shape of the mandibles. Like phytophages, the prothorax is truncate anteriorly, rather than tapered ( Figure 52 View Figure 52 ), the cephalic lobes are fused and the pseudocephalon is domeshaped lacking cirri. The mandibles are blunt-tipped and, in resting position, they incline almost at right angles relative to the intermediate sclerite ( Figure 52 View Figure 52 ). Oral plates are present with a small region of dark sclerotisation (Films 19–20, Figures 53 View Figure 53 and 54 View Figure 54 ). Mandibles are similar to those of saprophages with rectangular bases and non-aligned apodemes for muscle attachment ( Figure 52 View Figure 52 ). Compared with L. sylvatica , the prothorax in S. coleoptrata is proportionately more narrow, segment borders are coated in spicules, the anterior spiracles are more dorsal in position and the mandibles are elongate and fused medially. The mandibles of S. coleoptrata are also long relative to the length of the head skeleton of the other higher cyclorrhaphan larvae studied here ( Table 3). Other features of S. coleoptrata are the short, deep intermediate sclerite aligned smoothly with the basal sclerite (i.e. it abuts the basal sclerite evenly), the greater degree of sclerotisation indicated by black colouration and the absence of cibarial ridges and dental sclerites. The narrow development site means that when moving and feeding the body is compressed, less sweeping takes place, lunging occurs repeatedly over the same part of the substrate and feeding tracks tend to be straight or in slightly curving lines. Both species have relatively short head skeleton pivots and lunge distances and slow lunge rates ( Table 2, Films 19–20). The mandibles barely move apart during elevation and depression, but unlike phytophages, they extend from the pseudocephalon (Films 19–20).
Performance consequences
Lonchoptera lutea : in this larva, the high width to length ratios of rear compartment segments and flattening enhance the ability to hold on and squeeze between adhering substrates with the sclerotised plates protecting the dorsal surface. Fusion between the plates of the metathorax and first abdominal segment probably reinforces the rear compartment during shovelling, i.e. fusion braces against the tendency of the rear compartment to move when the front one shovels forward. The fused plates of the seventh abdominal and anal segments similarly brace the rear end at the start of peristalsis. However, flattening and sclerotisation restrict manoeuverability and lonchopterid larvae do not make sharp changes in direction, move effectively along narrow substrates nor move round sharp angles such as leaf edges. Conversely, the width of abdominal segments facilitates the sinusoidal mode of locomotion which, among the species studied here, is unique.
The relatively narrow front compartment facilitates shovelling, but as a feeding mechanism, shovelling seems less effective compared with brushing, scooping and spot-sucking, the other feeding mechanisms observed for feeding on watery biofilm ( Table 4). For instance, in shovelling, the trough moves forward and the tendency will be for biofilm to move with it. There is also the tendency for biofilm to flow off the end when the trough is lifted up. Furthermore, the trough is fleshy and, compared with sclerotised structures, poor at penetrating into and loosening other than watery biofilm.
Mitigating against these limitations, the floor of the trough is supported mechanically by the sclerotised labial rods which originate on the basal sclerite and, relative to it, angle down by about 35 degrees. This imposes a fixed inclination to the trough which supports shovelling. Contraction of labial muscles may flatten the fleshy apex which further encourages biofilm on to the trough. To prevent it flowing off the trough, it tilts up at the end of shovelling and the labial muscles probably relax allowing the margin to expand with body fluids and so act as a barrier. The trough also has a pair of extrudable, tapering cones which, as it is lifted up, swing round in front and may also help prevent biofilm flowing off.
Apart from sclerotised labial rods supporting the floor of the trough, the sides are supported by parastomal bars and basal mandibular sclerites, and muscle action encourages the flow of biofilm towards the pharynx. In locomotion the mandibles do not grip substrates and are not important. In any case, this would be complicated as the mandibles are not at the front of the body and are orientated at right angles to its long axis.
Polyporivora picta : the cylindrical body shape of this larva is more efficient than a flattened one for tunnelling. The ability of the segments of the rear compartment to contract, and hence shorten and broaden, is also important for gripping the sides of the tunnel. By frictional force, holding on is supported by the short, stiff setae which cover the body. The tapered shape of the middle compartment creates space for lunging and the ability of the head skeleton to pivot and protract and retract provides a fine level of adjustment for placing the labial teeth against the tunnel face. Moving the labial teeth down the tunnel face tends to force ripped and torn hyphae towards the entrance to the pharynx ready to be sucked in. This process is probably facilitated by the mandibles which depress to guide rasped hyphae between the upper labial teeth, but are otherwise of minor significance. The oral plate attached to the ventral margin of the pseudocephalon ( Figure 6A View Figures 6 ) supports rasping by action of muscles attached to it which contract and so pull back the pseudocephalon to expose fully the labial teeth.
Microdon analis : as with L. lutea , the high width to length ratios of the abdominal segments enable this larva to move sideways and rotate on a point which is advantageous in confined ant galleries. Elongate projections supporting the antennomaxillary organs extend their reach during sweeping for prey. Prey capture was not observed, but presumably when the antennomaxillary organs sense prey, the head skeleton protracts, the mandibles grab it in a pincer-like action, the head skeleton retracts and the front compartment contracts. These actions pull the prey under the upper section of the metathorax where the prey is pierced. How this occurs is unclear. By depressing the labial plate and sclerites, space would be created between them and the mandibles, and the prey is possibly pulled into this space where it is pierced by the spines at the back of the labial plate and the long, incurved teeth at the back of the mandibles. Additional force would be available to pierce and tear prey if it was rubbed against the floor of the ant gallery. Such processes are facilitated by the position of the mandibles at the front of the head skeleton. The ability of the pump to work independently of the mandibles, i.e. several pumping actions per lunge, means that food can be imbibed simply by protracting the head skeleton into the prey body. During pumping, the mandibles are relatively inactive and unimportant. However, unlike M. cinctus , no seal is made between the larva and its prey and some prey fluids and tissue spill out and are lost.
Melangyna cincta View in CoL : at the start of peristalsis, this larva prevents slippage using a unique, grasping bar at the apex of the anal segment. High levels of collapsibility and extendability underpin the fine control and exceptional flexibility of this larva. These features suit locomotion on the varied topography of plant substrates. Such abilities are enhanced by the shifting border between the two main body compartments which, in particular, modify the distance over which lunges are made. Once a prey aggregation is located, it is an advantage not to disturb it as more than one aphid is required for development. Having a modifiable lunge distance is energy-efficient because larvae can stay close with short lunges capturing the nearest aphids. The unique overlap between, particularly the prothorax and mesothorax, means that anterior spiracles are removed from a position where they might interfere with feeding and frees the prothorax to retract into a cup-shape to hold prey in combination with sticky saliva and the triangular-shaped sclerites on the lateral margins of the prothorax. Such a mechanism for holding prey creates a seal and prevents loss of prey fluids and tissues. Overlap may also provide space for the head skeleton to pivot and protract and retract when the prey is lifted up, i.e. protractor and retractor muscles originate further backwards than if no overlap occurred. Unlike M. analis View in CoL , the labrum and labial plate are tapered and project together at the front of the head skeleton, which is an efficient shape and position for piercing prey. The mandibles have no active role in feeding. They lack muscles and are embedded in and support the sides of the head skeleton. This creates a nozzle at the apex of the head skeleton which increases velocity of intake by concentrating sucking power ( Figure 12C View Figures 12 ). To suck in food, like M. analis View in CoL and P. picta View in CoL , the labium depresses to expose the nozzle entrance. By altering the direction of protraction, the head skeleton can reach different areas inside the prey. Lifting prey up has two advantages, alarm pheromones emitted by captured aphids are dispersed harmlessly above aphid aggregations and it prevents prey escaping by walking away ( Roberts 1970; Rotheray and Gilbert 2011).
Higher Cyclorrhapha : observation of movement in Petri dishes reveals that at the start of peristalsis, spicules on the anal lobe are pressed against the substrate and probably prevent the anal segment from slipping. As peristalsis moves forward, gripping power is maintained by spicule-bearing integument either side of segment borders spreading out forwards anterior of the borders and backwards behind it; the spicules are often orientated this way too. At these borders there are always more spicules on the posterior than the anterior segment which probably reflects the emphasis for forward more than reverse locomotion, i.e. there is a greater need to resist slipping backwards than forwards. Spicules presumably provide gripping power by frictional forces, the results of which were probably the effective locomotion observed when larvae moved on dry, filter paper as opposed to slipping, body rocking and lack of progress which occurred on a substrate they found problematic to grip, the smooth, dry surface of a plastic Petri dish. The segments of the rear compartment are relatively uniform in size and shape which provides an even spread of gripping power, especially during sweeping and lunging when this compartment is stationary and the middle and front ones are mobile.
The middle body compartment sweeps and rarely grips a substrate, which explains the relative absence of spicules. The division between the middle and the front compartments is just ahead of the anterior spiracles. Beyond the anterior spiracles, the prothorax inclines or bends to reach food or a substrate. The anterior spiracles are rigid structures that do not bend or collapse, and this may explain why the border between these compartments is beyond them. In addition, in this position, the anterior spiracles remain elevated during lunging and better protected from immersion and blockage with loose or wet material. Their position also effects locomotion. This is because, when the mandibles depress to grip a substrate, the next peristaltic wave causes the mesothorax to fold over the stationary prothorax but usually the fold stops just behind the anterior spiracles ( Figure 22 View Figure 22 ), perhaps because the spiracles might be damaged or would cause damage if they were taken into a fold. This limit on folding is breached in C. frigida and C. vomitoria ( Figure 23B View Figures 23 ). In these species, the small size of the prothorax relative to the mesothorax appears to make this possible. It means that, when folding into one another, there is space between them sufficient to accommodate the anterior spiracles. When the mandibles grip the substrate, the face of the prothorax, the anterior fold, is also pressed against the substrate ( Figure 23A and 23B View Figures 23 ). In almost all species, the anterior fold is coated in spicules that probably protect the prothorax and help in holding on.
The border between the middle and front compartments is also seen in the puparium. The anterior spiracles are on the antero-dorsal margin of the puparium in all higher cyclorrhaphan species examined here. The prothorax beyond the spiracles is inclined as it is during lunging but, in the puparium, bending goes further and the prothorax is turned to almost 180 degrees into the puparium. At its end, the pseudocephalon remains attached and within it are the mandibles followed by the rest of the head skeleton. Hence, the apex of the puparium is the point where it inclines just ahead of the anterior spiracles.
Among the species examined here, the form of both the head skeleton and the pseudocephalon is variable but in general, the head skeleton protracts and retracts, and the mandibles depress which extends the mandibular hooks from their sheathes in the pseudocephalon. The mandibular hooks gather food or, in locomotion, grip a substrate. Between the mandibles is the oral cavity into which food is gathered prior to it being sucked into the pharynx, which at this point, is in the form of the nozzlelike atrium.
Saprophagy: compared with mycophagy and phytophagy in which firm or compacted tissue needs removing and zoophages in which prey have to be captured and pierced, food gathering in saprophages appears less challenging. Liquidised food needs gathering for which the suction pump in the head skeleton appears well suited. As do ensheathed mandibles which, by virtue of the fleshy sides of the pseudocephalon, provide a greater surface area than mandibular hooks alone for gathering liquid food and larvae are usually immersed in and surrounded by food ( Table 1). Although saprophages share some features, in the nine species assessed, seven distinctive feeding mechanisms were recorded ( Table 4).
In most saprophages, the mandibular base is subrectangular and indented ( Figure 16 View Figure 16 ). Indentation probably adds mechanical resistance to forces that might distort the mandible when the mandibular hooks are gripping or pulling across hard substrates. Furthermore, the mandibular hook is often flat to scalloped which, by a greater surface area, is an effective shape for gathering liquid or soft food. The mandibles usually move in an inclined plane, i.e. they separate on depression. Inclination opens and closes the oral cavity and, in locomotion, separated mandibles make two points of contact with the substrate, helping to anchor and stabilise the front end of the body.
A hinge joint links the mandibles to the intermediate sclerite. It consists of a groove in the anterior face of the intermediate sclerite which articulates with a ridge on the rear face of the mandible ( Figures 17 View Figure 17 and 18 View Figure 18 ). The joint slopes inward relative to the horizontal plane and it is this slope that enables inclined mandibular movement. The muscles controlling mandibular movement are inserted via tendons to slight apodemes or projections, elevator muscles to the inner, upper corner and depressor muscles to the outer, lower corner of the rectangular base ( Figure 16 View Figure 16 ). Because of the width of the mandible and their position on it, these apodemes are not in the same vertical plane but displaced laterally from one another. This arrangement means they work with the joint during movement i.e. independent of the joint itself; elevator muscles would tend to pull the mandible up and towards the midline, whereas depressor muscles would tend to pull the mandible down and away from the midline. Due to the position of the dental sclerities below and under the mandible base and that depressor tendon envelops them ( Figure 16 View Figure 16 ), mandibular depression pulls the rear margin of the oral cavity back and down which, in conjunction with retraction of the labial lobe, exposes the opening to the atrium and acts as a critical backstop, helping to retain food in the oral cavity.
The pseudocephalon and hence, the oral cavity, is longer than wide in saprophages and at the border with the prothorax, the pseudocephalon folds and unfolds evenly as a unit, unlike the pseudocephalon of phytophages and mycophages, see below. This shape and pattern of folding optimises the degree the oral cavity is projected to gather food. The characteristic systems of anastomising ridges or cirri that coat the sides of the oral cavity and lead to the opening of the atrium presumably both protect the pseudocephalon and guide fluids into the cavity, but details of the latter process are unclear. Despite spicules coating the anterior face of the prothorax, the prothorax and indeed other parts of the integument are often covered in scars, appearing as black marks on the integument ( Figures 23A View Figures 23 and 35 View Figure 35 ).
Silba fumosa , Palloptera trimacula and Chymomyza costata : the larva of these three species are the most generalised of saprophages examined here. For instance, over the range recorded for all saprophages, their middle and front body compartments are not especially manoeuvrable, lunge times are neither fast nor slow, and head skeleton protraction distances are neither long nor short. Their most distinctive features are the middle and front body compartments which taper the least but are relatively long. The lack of taper suggests they are not specialised for extracting food from particularly confined spaces. The relatively long middle and front compartments might be an adaption for optimising reach, but given that these larvae are usually immersed in and surrounded by biofilm, there seems to be no particular advantage of a long over a short reach.
Food gathering in S. fumosa appears even more generalised compared with the other two species in that this species has an open oral cavity, the inside margin of the mandibular hook is rounded not flattened and the prothoracic anterior face lacks spicules. The advantage of a closed oral cavity is that biofilm will be isolated inside simply by placing it down into biofilm. With an open cavity, the two lobes have to be drawn and held together. Compared with a flattened or scalloped mandibular hook, biofilm will tend to flow either side of a rounded state and, without protection for the anterior fold, food gathering may be confined to a subset of places where the chances of injury are slight, such as smooth biofilm on smooth substrates.
Lonchaea hackmani and Neophyllomyza acyglossa : compared with other saprophages, the distinctive features of these two species are their relatively small mandibles and oral cavities and short, tapered middle and front body compartments. These features suggest that the muscles of these body sections are correspondingly short and approximated. Also distinctive is their unusual capability to fold the prothorax ( Figures 29 View Figure 29 and 30 View Figure 30 ). All these features contribute to an ability to make fine, controlled movement. Such control facilitates food gathering from inside crevices and coating networks of fibres where these larvae usually feed.
The most obvious difference between these two species is the elongate, flexible intermediate sclerite of N. acyglossa . The significance of this remarkable feature is presumably the enhancement it gives to manipulating the head skeleton in food gathering.
Meiosimyza platycephala : one of the features of this species is that the middle and front compartments do not contract or fold but remain extended during sweeping. This is probably a consequence of decaying leaves being relatively flat and where the mechanical resistance of watery biofilm is low. Facilitating the feeding mechanism of this species are the rows of setae-like projections covering the bridge across the front of the oral cavity. When the oral cavity is lowered into biofilm and retracted, these setae brush the substrate that not only gathers biofilm but also creates a wave of it which bulges up into the oral cavity. These setae project further than the ventral margin of the thorax and, during protraction, the front section must lift to prevent them brushing biofilm away on the outstroke. The setae are protected by sclerotisation. The lunge distance and range of mandible movement is low, but with the oral cavity filled by bulges of biofilm, a wide range of movement is not required. Compared with other higher cyclorrhaphan saprophages, the mandibles play a reduced role in food gathering. The wide atrium supported by a sclerotised apex to the epipharygneal plate facilitates the flow of biofilm through to the basal sclerite where microbes are separated from excess water by the cibarial ridge filtering mechanism.
For protection and effective grasping, spicules are extensive in M. platycephala , covering the ventral and dorsal surfaces. Furthermore, the larva is able to flatten its body which facilitates squeezing through confined spaces.
Clusia flava : feeding lunges were the slowest of the species recorded here, taking minutes not seconds to complete. Most lunging time is spent with the apex of the prothorax pressed against sapwood and this is when ingestion takes place via repeated pumping. Repeated pumping probably draws biofilm into the head skeleton not only from the surface covered by the apex but also from biofilm soaked into and from the surrounding area, aided by an eversible channel that can form across the flattened apex. This explains the length of time the head is pressed against the substrate during lunging.
The flattened apices of the prothorax and pseudocephalon facilitate spot-sucking. The surrounding sensilla probably enable the pressure applied to the substrate to be assessed. An inverted, tubular oral cavity facilitates capillary action and some biofilm will enter without suction from the pump. Depression of the mandibles opens the entrance to the oral cavity, but the mandibles have little further role in food gathering. Cibarial ridges are absent and their loss implies a continuous passage of food, which facilitates prolonged sucking over one spot. However, a poorly sclerotised head skeleton suggests that pumping actions are relatively weak, i.e. the head skeleton is not braced or buttressed against distortion caused by contracting muscles. Absence of cibarial ridges and food entering the oral cavity by capillary action might mean that a strong pump, and hence a large, well-sclerotised head skeleton, is not so important.
The presence of spicules on the dorsum and ventrum of the thorax is unusual as is the slow rate of locomotion. Spicules probably reflect a need to protect the integument, as suggested by the sometimes, numerous black scars on the integument ( Figure 35 View Figure 35 ). Slow locomotion probably reflects a compromise between the difficulties of squeezing through sapwood and minimising energy expenditure. Even when removed from sapwood, this larva retains a slow rate of progress.
Coelopha frigida and Callicera vomitoria : the distinctive feature of these two species is the wide range of angles and directions achieved during sweeping and lunging. Such high levels of manoeuvrability are facilitated by a high level of folding between increasingly smaller and tapered segments of the middle and front body compartments. Such manoeuvrability takes advantage of food which, in a deep volume, is available in almost any direction. Furthermore, both species have a fleshy bridge across the anterior margin of the oral cavity and food can be gathered by simply extending the oral cavity forward. The crenulated ridges coating the distal end of the oral cavity in C. frigida presumably assist scooping food and/or protect its surface. In C. vomitoria , the operation of the bridge is more complex due to the presence within it of the oral sclerite and the paired oral plates. These plates incline during mandibular depression, which helps cut through and isolate portions of food. The rapid isolation of food that these plates facilitate in part explains the very rapid rate of feeding observed in this species. The other requirement of rapid feeding is a pump strong enough to withstand the forces involved. This may explain the high level of sclerotisation in the basal sclerite of C. vomitoria . It was not possible to confirm if saliva was secreted extra-orally during feeding in C. vomitoria , but at the rate of ingestion recorded, extra-oral digestion seems unlikely. Apart from scalloped mandibles, another feature aiding rapid ingestion in these two species is a wide atrium which by the provision of space, facilitates ingestion of lumpy or soft-solid food.
Mycophagy and phytophagy: compared with saprophages, food gathering in mycophages and phytophages is a mechanically demanding process. The mandibles must engage relatively hard or compacted tissue with sufficient force to fragment it. The mycophages and phytophages examined here share certain features that facilitate this process. For instance, to enhance leverage, the head skeleton pivots or see-saws up and down rather than, as in saprophages, protracting and retracting forwards and backwards.
In saprophages, the pseudocephalon extends back and forth evenly. With up and down movement, this does not occur and dorsal and ventral margins fold in opposing directions during pivots, i.e. when the head skeleton pivots up, the dorsal margin of the pseudocephalon folds or collapses while the ventral margin extends and unfolds and the reverse on moving down. This is facilitated by the domed rather than elongate shape of the pseudocephalon. This imposes, however, the problem of ensuring the oral cavity remains open and does not collapse on the down stroke. This is critical because food is gathered into it during the down stroke. In saprophages, dental sclerites keep the oral cavity open during mandibular depression, but in the mycophages and phytophages examined here, dental sclerites are absent. The oral cavity is kept open by an oral plate that extends from the rear margin of the oral cavity under the head skeleton. This structure can be almost as long as the basal sclerite and moves in co-ordination with it. A pivoting head skeleton also explains the truncate shape of the prothoracic apex in mycophages and phytophages. Compared with the narrow taper of the prothorax in saprophages, a truncate apex in mycophages and phytophages probably increases space and modifies the angles of protractor and retractor muscles, enabling them to pivot the head skeleton.
Finally, in saprophages the mandibular hooks are relatively long, narrow, sharptipped and separate on depression. These features are not as effective for tearing plant and fungal tissue as the mandibular hooks of mycophages and phytophages which are parallel-moving, short, wide and blunt-tipped. This type of mandibular hook concentrates rasping in a smaller area and protects the tips by spreading their surface area. In most phytophages, surface area is additionally increased by accessory teeth.
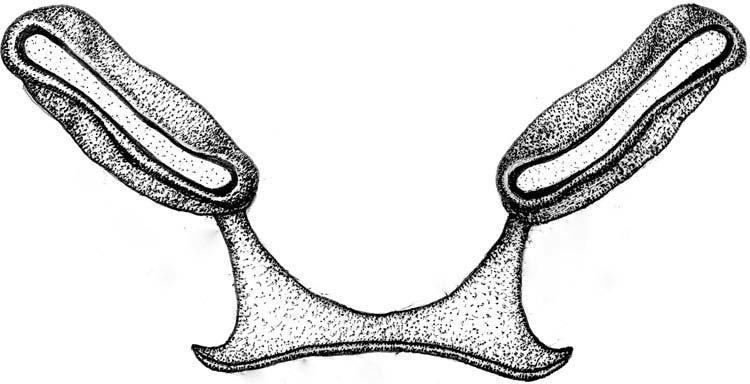
Lonchaea sylvatica and Stegana coleoptrata : these larvae feed most frequently on sooty fungi. But they can also feed on firm biofilm, both food types co-occurring at their development sites under the bark of fallen wood ( Figure 51 View Figure 51 , Table 1). The narrow range of head skeleton and mandibular movement that characterises these larvae facilitates repeated lunging at the same place on the substrate. This appears to be required to loosen and gather hard food. Such a requirement for repeated lunging and spatial restrictions within development site probably explains the linear shape of feeding tracks. The morphological differences between L. sylvatica and S. coleoptrata suggest that the latter species is more completely modified for feeding on hard food. For instance, the prothorax of S. coleoptrata is more wedge-shaped and the anterior margins of segments are coated in spicules. These characteristics probably facilitate feeding in restricted spaces, perhaps by feeding in a twisted or sideways position. Also, as a proportion of head skeleton length the mandibles of S. coleoptrata are twice as long as those of L. sylvatica (50% versus 25%) and they are fused together. Length and fusion adds mechanical strength to the mandibles.
Other distinctive head skeleton features of S. coleoptrata are the greater degree of sclerotisation, absence of cibarial ridges and a shorter, deeper intermediate sclerite which is smoothly aligned with the basal sclerite. These features brace the head skeleton to a higher level than that of L. sylvatica . A deep intermediate sclerite also provides space for fragments of food to mix with saliva. The absence of cibarial ridges means that food is not concentrated in the head skeleton but passes rapidly through it.
Tephritis vespertina , Chaetostomella cylindrica , Acidia cognata , Botanophila seneciella and Pegomya solennis : in lunging, these phytophagous larvae maintain the mandible in one position, rather than it depressing continuously. This enhances rasping power, but elevator and depressor muscles must work in conjunction to hold the mandible in position. This may, in part, explain the vertical alignment between elevator and depressor muscle apodemes, and hence the characteristic shape of the mandible base in phytophages. An additional influence on the position of the depressor apodeme is avoiding interference with a short mandibular hook. The depressor apodeme would probably be in the way if located in the same relative position as in saprophages.
Lunges of this type involve the intermediate and basal sclerites because mandibular muscles originate on the basal sclerite and these sclerites must be strong enough to withstand the forces involved to hold the mandible in one position. Added strength is exemplified by the characteristic, complete sclerotisation of the intermediate sclerite and heavy sclerotisation of the vertical plate. Furthermore, in the tephritids, additional bracing occurs via smooth alignment between the intermediate and basal sclerites, similar to that of S. coleoptrata . However, the cornua in phytophages appear weak. There is abrupt termination of sclerotisation partway along the ventral cornu, and there are windows and areas lacking sclerotisation in the dorsal cornu. In phytophagous larvae, cibarial ridges are absent and, possibly as a consequence, the terminal valve is reduced. There is probably a trade off on the ventral cornu between the need to resist distortion via sclerotisation and the reduced need for sclerotisation resulting from the loss of cibarial ridges and terminal valve. This explains the characteristic pattern of sclerotisation in the ventral cornu. A factor explaining the pattern of sclerotisation in the dorsal cornu is probably space. The films reveal that, in live phytophagous larvae, the dorsal cornu bends along these windows. Bent over dorsal cornua reduce the height of the head skeleton. This may compensate for lack of space caused by up and down movement of the head skeleton and narrow, confined feeding places .
With greater forces required to gather food, phytophagous larvae require a very firm grip of the substrate. This is in part facilitated by the restricted spaces they occupy, such that lateral and dorsal aspects of the body are in contact with substrates. For instance, in leaf mines, larvae are on their sides and hold on with spicule-coated, lateral margins and, in flowerheads, larval bodies are, on all sides, in contact with the substrate. Furthermore, the two leaf mining larvae have unfolded bodies when feeding whereas flowerhead larvae are folded and are, in consequence, short but broad. These shapes are retained even when larvae are removed from their development sites and they suggest adaptive processes to conserve body size within confined spaces and optimise body shape for holding on.
The larva of C. cylindrica is unusual among the flowerhead species in feeding on the receptacle. Receptacle tissue is dense and hard relative to that of the florets and there are certain features of the larva of C. cylindrica absent in the two other flowerhead feeders that may facilitate gathering this harder quality of food. To better brace the head skeleton, it is almost completely sclerotised and the front margin of the basal sclerite tapers and makes a complete junction with the intermediate sclerite. Also, the basal sclerite is braced more effectively by the symmetrical angle between the two cornua ( Figure 41C View Figures 41 ). In the other flowerhead species, the angle of divergence suggests that the ventral cornu is mainly involved in bracing ( Figure 41A and 41D View Figures 41 ).
Comparing the two leaf mining larvae, there are considerable differences between them. The larva of P. solennis is characterised by an extraordinary group of characters, unique among the species studied here, a crenulated pseudocephalon, a conspicuous fleshy bar on the prothoracic apico-ventral margin, and asymmetrical pseudocephalon and mandibles. The prothoracic bar helps prevent rasped food from being moved past the oral cavity during lunging, and because fluids cannot be compressed, the crenulations on the pseudocephalon allow excess fluids to pass between them. Asymmetry creates a level of flattening that suits the narrow leaves of the food plant used by this species. In particular, the relative shape and position of the two mandibles means that teeth on both can be bought into action when lunging. These remarkable features are absent in the other similar-sized leaf miner examined here, A. cognata , but this species mines a leaf about twice as thick.
| A |
Harvard University - Arnold Arboretum |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
InfraOrder |
Cyclorrhapha |
|
Family |
|
|
Genus |