Spyridium, Fenzl
|
publication ID |
https://doi.org/10.1071/SB21034 |
|
DOI |
https://doi.org/10.5281/zenodo.10949890 |
|
persistent identifier |
https://treatment.plazi.org/id/03F60813-FFB1-FFBD-CF21-FBCFAE30F873 |
|
treatment provided by |
Felipe |
|
scientific name |
Spyridium |
| status |
|
Broad biogeographic patterns in Spyridium View in CoL
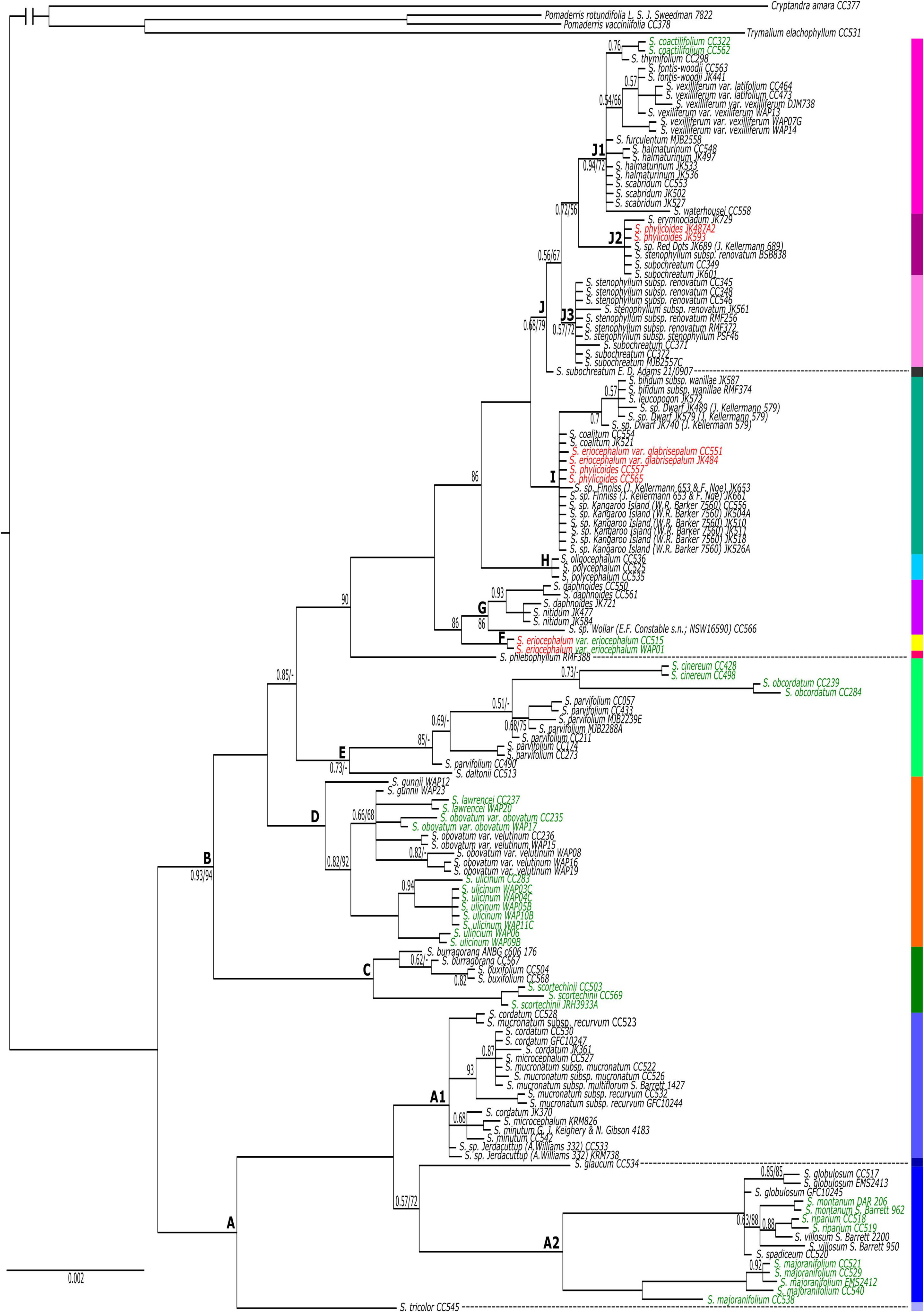
This study has provided the first comprehensive (all species) phylogenetic assessment of biogeographic patterns in Spyridium View in CoL . Our inferences (below) are based on the molecular phylogenies and insight from other studies of biogeography in southern Australian. We focus on broad (continent scale) patterns in general terms. The work presented here could be extended by additional analyses, such as, for example, using dated trees or probabilistic biogeographic modelling (e.g. Ree and Smith 2008; Matzke 2013), although these are not explored here.
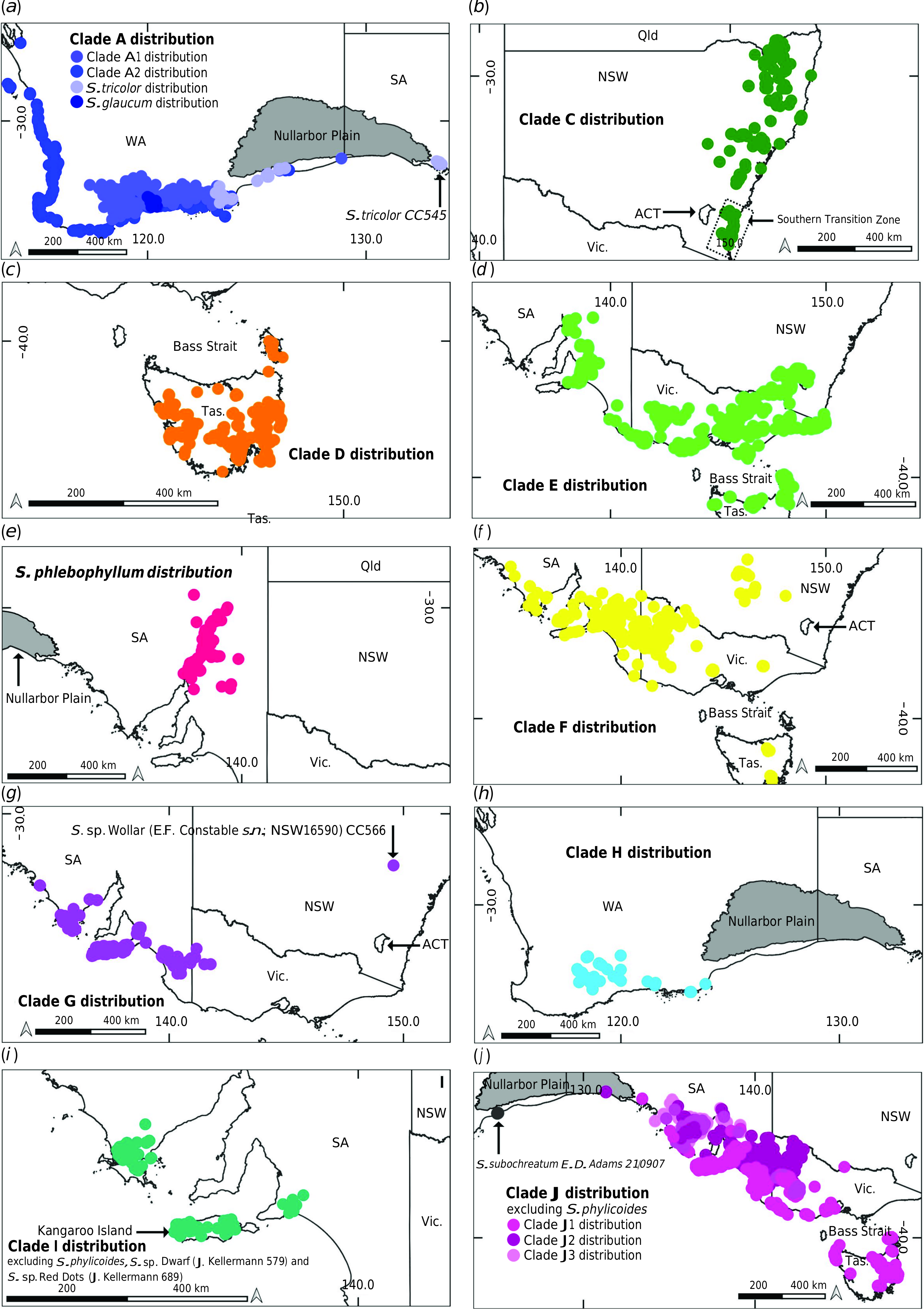
An early split in Spyridium View in CoL has been identified in the nrDNA phylogeny, between the east and west of Australia across the Nullarbor Plain (Clades A and B; Fig. 2 View Fig , 3 a–j View Fig ). There are many examples of east–west divergences in plants distributed across southern Australia, including Phebalium Vent. ( Mole et al. 2004), Eucalyptus L’Hér. subgenus Eucalyptus ( Ladiges et al. 2012) , Goodeniaceae R.Br. ( Jabaily et al. 2014), Xanthorrhoea Sol. ex Sm. ( McLay et al. 2021), Adenanthos Labill. ( Nge et al. 2021 a) and Pomaderris ( Nge et al. 2021 c) . The Nullarbor Plain disjunction in a range of plant groups has been related to vicariance associated with uplifting and climatic cooling in the mid-Miocene ( Crisp and Cook 2007), including in Pomaderris ( Nge et al. 2021 c) , a close relative of Spyridium View in CoL in the tribe Pomaderreae . Nge et al. (2021 c) concluded that Pomaderris was widespread throughout southern and eastern Australia until c. 14 Ma, when the Nullarbor Plain uplift occurred, with subsequent rapid ‘within region’ diversification in eastern Australia from c. 10 Ma, and little movement across biomes since. Although we did not use dated trees to determine diversification dates (as per Nge et al. 2021 c), a similar explanation for the early east–west divergence in Spyridium View in CoL could be inferred from our results.
Assuming the deep east–west divergence in Spyridium View in CoL relates to formation of the Nullarbor Plain, the nrDNA tree suggests that up to three lineages in the genus have potentially dispersed across the plain subsequent to this early east–west divergence ( Fig. 2 View Fig , 3 a, h, j View Fig ). An east-to-west dispersal of S. subochreatum is inferred from both nrDNA and cpDNA trees, because the species is nested within eastern taxa and its distribution extends to just west of the Nullarbor Plain ( Fig. 3 j View Fig ). Conversely, for S. tricolor , a west-to-east dispersal could be inferred, because the SA sample of this species ( CC 545; Fig. 3 a View Fig ) groups with western taxa. However, because it is sister to other western taxa in Clade A in the nrDNA phylogeny ( Fig. 2 View Fig ), a reverse scenario could not be ruled out. Like S. subochreatum , S. tricolor grows on sandy soils and limestone (FloraBase – the Western Australian Flora, see https://florabase.dpaw.wa.gov.au/, accessed 7 May 2021), suggesting it could have made use of land connections south of the Nullarbor Plain that have been exposed at times of lower sea level since the late Pliocene ( Nelson 1974; Wright and Ladiges 1997). An alternative explanation, that S. tricolor was widespread across this region and became disjunct during the Nullarbor Plain uplift, would require retention of morphological resemblance, such that it is recognised as a single species, despite a considerable geographic disjunction, for a substantial period of time since the mid-Miocene. Population-level sampling of S. tricolor using variable genomic markers could provide a greater insight into geographic history of the species and be used to further test its taxonomic circumscription.
The third lineage for which dispersal over the Nullarbor Plain might possibly be inferred is that including S. polycephalum and S. oligocephalum ( Fig. 2 View Fig , 3 h View Fig , 5 View Fig ). A deep east-to-west dispersal event, before the diversification of the two species, is inferred from both the nrDNA and cpDNA trees, because this clade was nested within species from east of the Nullarbor Plain. Evidence of early east–west vicariance across southern Australia, followed by subsequent dispersal events such as these have been inferred in studies of other plant groups, such as, for example, in Eucalyptus subgenus Eucalyptus ( Wright and Ladiges 1997) , Thelymitra J.R.Forst. & G.Forst. ( Nauheimer et al. 2018), Calytrix tetragona Labill. ( Nge et al. 2021 b) and Pomaderris ( Nge et al. 2021 c) . Despite this, an alternative explanation of vicariance to account for the Western Australian distribution of the S. polycephalum – S. oligocephalum clade cannot be immediately discounted on the basis of our data. Although a vicariance explanation is less parsimonious because it would infer extinction of multiple lineages in western Australia, such reasoning assumes that multiple extinctions are less probable than is a single dispersal, which might not be true ( Sanmartín and Meseguer 2016), for example, in the face of substantial climatic change in Australia since the mid-Miocene. A robust time-calibrated phylogeny for Spyridium View in CoL could help corroborate one of these alternative scenarios.
Within the eastern Australian branch of the nrDNA phylogeny (Clade B, Fig. 2 View Fig ), an early NSW divergence is inferred ( Fig. 3 b View Fig ). This deep divergence of NSW endemics from other south-eastern Australian taxa occurs near a broadly defined area that has been termed the southern transition zone ( STZ; Fig. 3 b View Fig ) by Milner et al. (2012). The STZ is found east of the Great Dividing Range ( GDR) and north of the Victoria– NSW border and is identified as a region where genetic or distributional discontinuities are seen in a range of taxa, but with the exact position of the discontinuities being dependent on habitat requirements of individual species and potentially different timescales of divergence ( Milner et al. 2012). Other plant taxa showing genetic breaks across the STZ include Hardenbergia violacea (Schneev.) Stearn ( Larcombe et al. 2011), Lomatia R.Br. ( Milner et al. 2012), Callitris rhomboidea R.Br. ex Rich. & A.Rich. ( Worth et al. 2018) and Xanthorrhoea ( McLay et al. 2021) . Spyridium provides a further example of this pattern, although potential drivers of the divergence are unclear in this case.
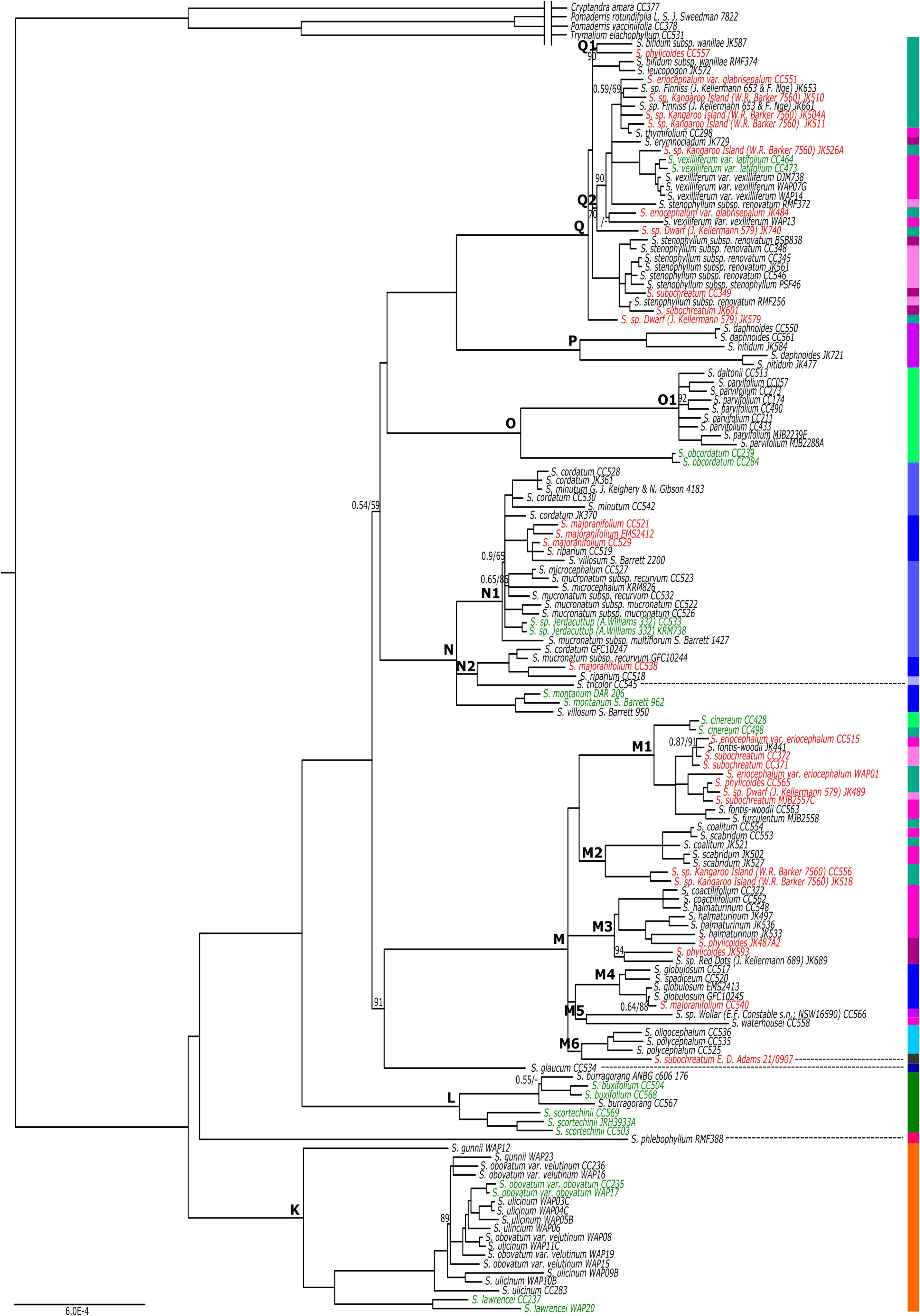
Tasmanian endemics (excluding S. obcordatum ) were found in a single, early diverging clade separate from their mainland counterparts in the nrDNA tree (Clade D, Fig. 2 View Fig , 3 c View Fig ), suggesting early vicariance of Spyridium View in CoL across Bass Strait. This early divergence and diversification of Tasmanian endemics is also supported by the cpDNA phylogeny (Clade K, Fig. 5 View Fig ) and the findings of Kellermann et al. (2005) and Hauenschild et al. (2018). The continued barriers to dispersal and gene-flow are likely to be the inundation of Bass Strait during interglacial periods ( Galloway and Kemp 1981) and the semi-arid climate of the land-bridge exposed during glacial periods ( Kirkpatrick and Fowler 1998). Major glacial and interglacial fluctuations occurred throughout the Quaternary ( c. 2.2 Ma to c. 10 000 years ago; Hope 1994; Quilty 1994) and their resulting climatic extremes have been inferred to contribute to the limited distribution of narrow-range endemism in Spyridium View in CoL in Tasmania ( Coates and Kirkpatrick 1999).
Recent dispersal or gene flow between Victoria and Tasmania are here inferred for the lineage represented by S. obcordatum , the only endemic Tasmanian species not placed in Clades D or K ( Fig. 2 View Fig , 3 d View Fig , 5 View Fig ), and several widespread taxa, including S. eriocephalum var. eriocephalum , S. vexilliferum var. vexilliferum and S. parvifolium ( Fig. 2 View Fig , 3 d, f, j View Fig ). Accessions of each of these widespread taxa collected from Tasmania (and Flinders Island for S. parvifolium ) were found within the same clade as samples of the same taxa from Victoria ( Table 1 View Table 1 ). Similar patterns of recent gene-flow between Victoria and Tasmania have been inferred in other plant groups, including Eucalyptus globulus Labill. ( Freeman et al. 2001), Hardenbergia violacea ( Larcombe et al. 2011) , Correa Andrews ( French et al. 2016), Zieria veronicea Sm. ( Neal et al. 2019), Xanthorrhoea ( McLay et al. 2021) and a range of other species ( Worth et al. 2017). Evidence suggests that at least some areas of the Bassian Plain were covered in eucalypt woodland habitat ( Hope 1978, 1994; Kirkpatrick and Fowler 1998) which could have been suitable for S. parvifolium , S. vexilliferum var. vexilliferum and S. eriocephalum var. eriocephalum (VicFlora 2018) , i.e. potentially facilitating over-land rather than over-water dispersal between Victoria and Tasmania.
Review of circumscriptions of species
The molecular phylogenies support the circumscriptions of several Spyridium View in CoL species, but raise questions about others. A quarter of the taxa represented by more than one accession were identified as monophyletic in the nrDNA phylogeny, with approximately one-third being resolved as polyphyletic, and the remainder being unresolved ( Fig. 2 View Fig , Table 3 View Table 3 ). Of the monophyletic taxa resolved in the nrDNA tree, several were also found to be monophyletic in the cpDNA phylogeny (e.g. S. obcordatum , S. scortechinii and S. montanum ), providing additional support for these circumscriptions of species ( Fig. 5 View Fig , Table 3 View Table 3 ). Of the polyphyletic taxa in the nrDNA phylogeny, two of the most notable were distributed across disparate clades, namely, S. eriocephalum and S. phylicoides ( Fig. 2 View Fig ). Given that both of these species were also resolved in separate clades in the cpDNA tree ( Fig. 5 View Fig ), they are discussed in more detail below, along with several associated phrase-name taxa.
| A |
Harvard University - Arnold Arboretum |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| CC |
CSIRO Canberra Rhizobium Collection |
| NSW |
Royal Botanic Gardens, National Herbarium of New South Wales |
| K |
Royal Botanic Gardens |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |