Tasmanicosa Roewer, 1959
|
publication ID |
https://doi.org/10.11646/zootaxa.4213.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:9C76B987-3897-4666-87EF-62EB5BF5CF04 |
|
DOI |
https://doi.org/10.5281/zenodo.5676921 |
|
persistent identifier |
https://treatment.plazi.org/id/0B32B23C-7B11-9F70-BEF8-3E29FEDCFA8F |
|
treatment provided by |
Plazi |
|
scientific name |
Tasmanicosa Roewer, 1959 |
| status |
|
Genus Tasmanicosa Roewer, 1959 View in CoL
Australian Union-Jack wolf spiders
Tasmanicosa Roewer, 1959: 351 View in CoL .
Orthocosa Roewer, 1960: 774 . New synonymy.
Type species. Lycosa tasmanica Hogg, 1905 , by original designation ( Roewer, 1959). Lycosa tasmanica is here considered a junior synonym of Tasmanicosa godeffroyi (L. Koch, 1965) .
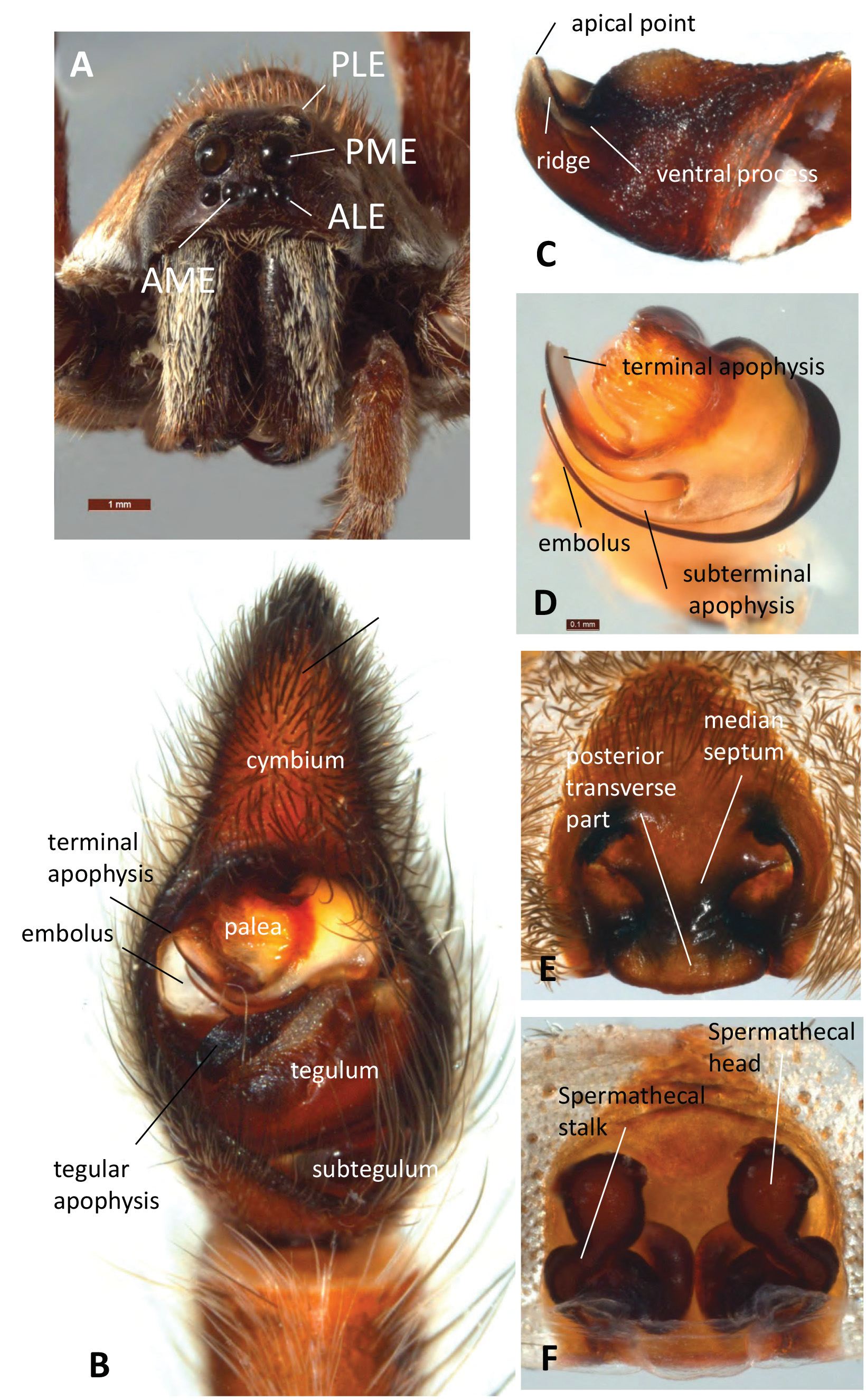
Diagnosis. The most conspicuous character of Tasmanicosa is the Union-Jack pattern of the carapace that consists of dark and light radiating lines, including an undulating line under the lateral eyes to the front of the carapace (e.g. Figs 1A–B, D–E, H View FIGURE 1 ). Median and marginal light bands are present, but often poorly developed. Dingosa is the only Australian wolf spider genus in which spiders also have a Union-Jack pattern on the carapace, but males of this genus differ from those of Tasmanicosa by the heavily enlarged palea and elongated tegular apophysis of the male pedipalp ( Framenau & Baehr 2007). Amongst other morphological characters, Dingosa also differs by the behaviour of constructing turrets around the entrance to the burrow, not observed in Tasmanicosa . Tasmanicosa differs from Venator by the lack of a retrolateral incision in the tegular apophysis of the male pedipalp (absent in V. immansuetus (Simon, 1909)) and the lack of sharp lateral edges of the epigyne atrium in females ( Framenau 2015). It differs from Knoelle by the lack of a large patch of apical setae on the male pedipalp cymbium ( Framenau 2006a). Tasmanicosa (with the exception of T. musgravei ) differs from Hoggicosa by the shape of the epigyne, as the distance between the anterior pockets is smaller than the width of the posterior transverse part ( Langlands & Framenau 2010). In addition, males of Hoggicosa have a patch of dorsally bent setae on the tip of the cymbium, which is lacking in Tasmanicosa . Tasmanicosa distinctly differs from the monotypic Tapetosa , as the spiders are not dorso-ventrally flattened and the legs are not laterigrade ( Framenau et al. 2009). Tasmanicosa differs from Mainosa by a very different body colouration, i.e. the opisthosoma is not black with transverse light bars on the opisthosoma, and by the lack of turret-building behaviour around its burrow entrance ( Framenau 2006b). Tasmanicosa differs from Hogna by the shape of the male and female genitalia as Hogna ¸ as represented by its type species H. radiata (Latreille, 1817) , have a very simple, triangular tegular apophysis in the male pedipalp and a simple, inverted T-shaped median septum of the female epigyne (Framenau et al. 2006), and from Costacosa in the very different shape of the tegular apophysis ( Framenau & Leung 2013). Tasmanicosa differs from Venatrix and Tuberculosa by the lack of an apical cymbial hook-like macroseta in males ( Framenau & Vink 2001; Framenau & Yoo 2006).
Description. Large and robust wolf spiders, total length ca. 12.0–25.0 in males and ca. 14.0–30.0 in females. Prosoma longer than wide with rounded postero-lateral corners. Prominent Union-Jack pattern dorsally, consisting of black and light radiating lines; the most anterior line reaches under the eyes and approaches the front of the carapace. The row of the anterior eyes is slightly procurved, the PME are larger than PLE; the row of the AE slightly narrower than the row of PME; the row of PME is narrower than that of the PLE ( Fig. 2A View FIGURE 2 ). The labium is subquadrate, i.e. about as long as wide, and the endites about twice as long as the labium; both generally dark brown to black, sometimes with lighter anterior rim. The chelicerae are dark brown to black with an elongated patch of white, silvery, yellow or golden setae ( Figs. 1D, F View FIGURE 1 ; 2A). They have three promarginal teeth, the middle largest and its base is often fused with a smaller apical one, the proximal one is more distant. They also have three large retromarginal teeth of equal size.
The leg formula of males and females is IV> I> II> III. Legs are uniform greyish-brown with the coxal venter of the same colour as the sternum, generally dark brown to black, with the exception of T. semicincta with light brown sternum and ventral coxae. The femora are generally the lightest, ventral patellae and apical tibiae are often darker than the remainder of the legs. Legs I and II have entire ventral scopulae on tarsi and metatarsi, which also cover parts of the tibiae in females. Legs III and IV have scopulae on tarsi and half of metatarsi in males and all of tarsi and metatarsi in females.
The sternum is generally dark brown to black, except in T. semicincta , in which it is light brown. The opisthosoma has a dorsal, dark folium pattern that is laterally bordered by light lines or patches ( Figs 1A–B, D–E, H View FIGURE 1 ). The venter is either uniformly dark brown to black, but this patch is often reduced in some species displaying a species-specific pattern (e.g. T. leuckartii , T. semicincta ).
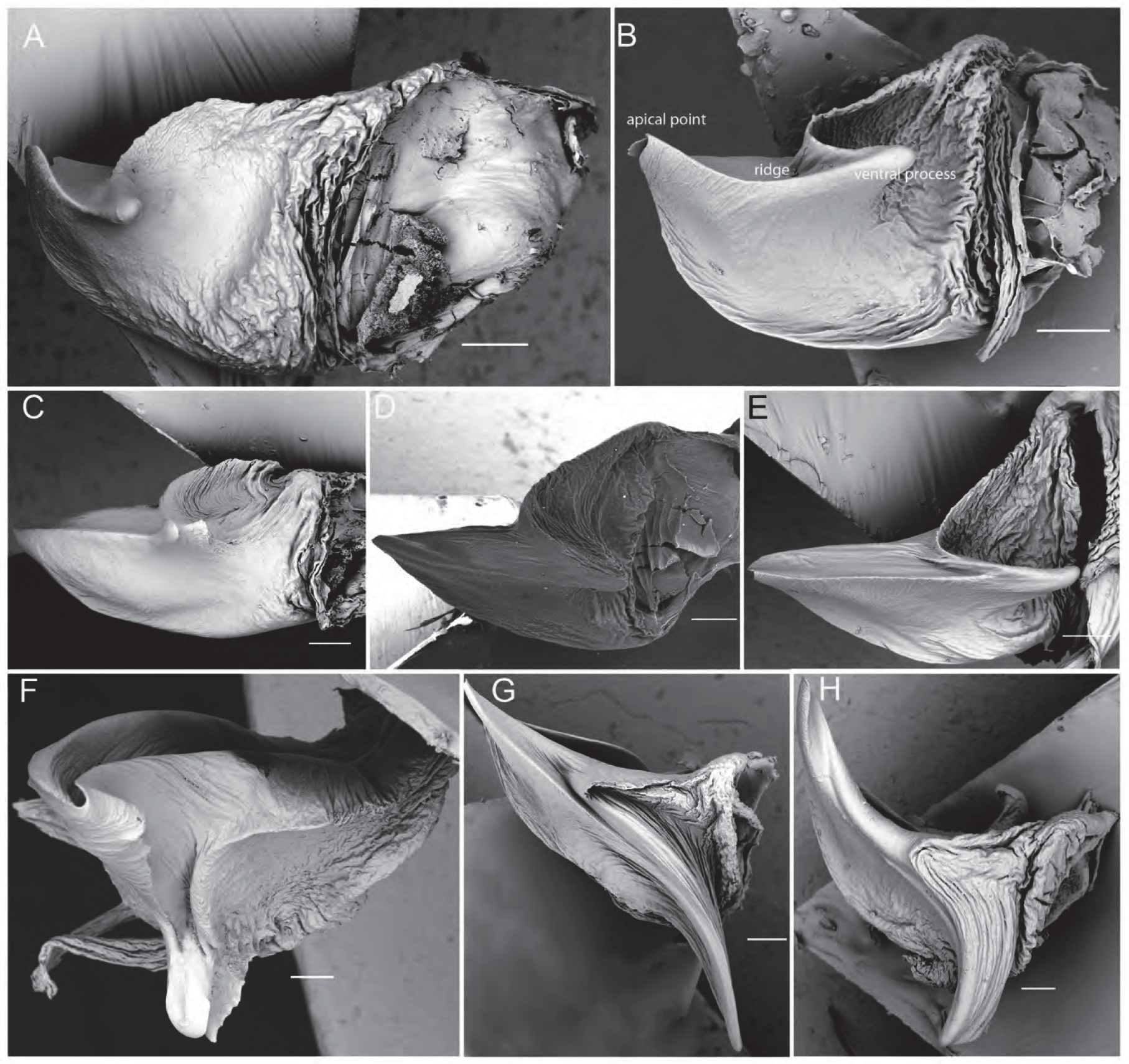
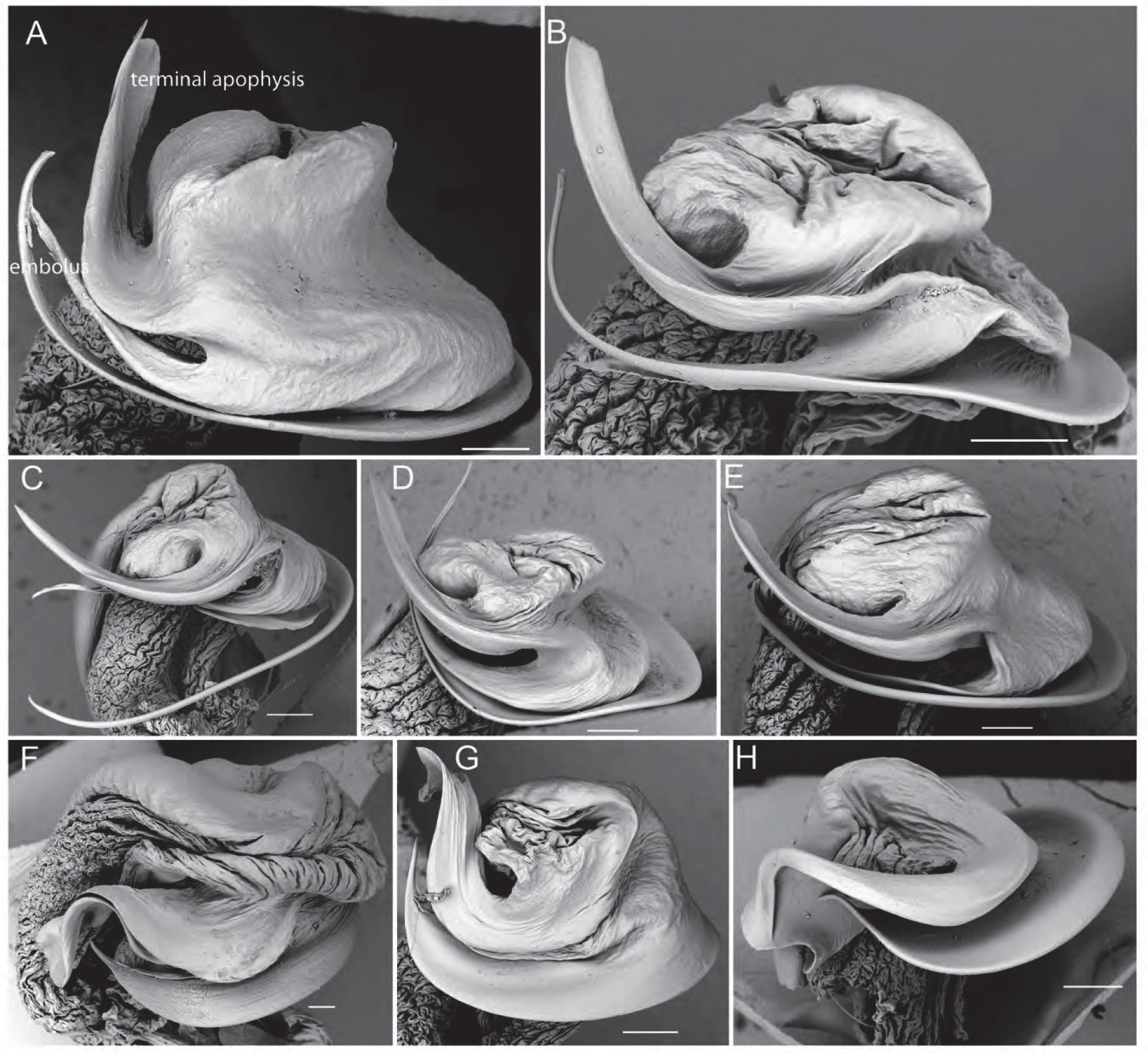
The male pedipalp cymbium is dorsally covered with a dense layer of silvery setae. The tip of the cymbium carries ca. 2–10 macrosetae that are slightly bent ventrally. The tegular apophysis is directed retrolaterally ( Fig. 2B View FIGURE 2 ) and has a ridge of species-specific shape between its apical point and its ventral process (e.g. Figs 2C View FIGURE 2 , 6B View FIGURE 6 ). The ventral process is well-developed. The length of this ridge corresponds to the length of the median septum in females (see Zyuzin, 1993 and Sadana, 1972 for a functional explanation of male and female genitalia in the Lycosinae). The embolus is long and generally sickle-shaped as characteristic for most Lycosinae ( Fig. 2D View FIGURE 2 ). The terminal apophysis is generally broad and sickle-shaped ( Fig. 2D View FIGURE 2 ) but apically modified in a number of species aiding in their identification (e.g. L. leuckartii , L. musgravei , L. gilberta ) ( Figs 7F–H View FIGURE 7 , 11I –J View FIGURE 11 , 15I View FIGURE 15 , 18I View FIGURE 18 ). A subterminal apophysis is present and generally sickle-shaped ( Fig. 2D View FIGURE 2 ).
The median septum of the female epigyne is inverted T-shaped, i.e. with a wide posterior transverse part ( Fig. 2E View FIGURE 2 ) and within this general configuration is variable between species, in particular in reference to it widening anteriorly. The spermathecal heads ( Fig. 2F View FIGURE 2 ) are of varying shape (i.e. spherical, elongated or kidney-shaped) and generally wider than the spermathecal stalks ( Fig. 2F View FIGURE 2 ). The latter are generally twisted, S-shaped or convoluted.
Remarks. Roewer’s (1959: 351) justification of the genus Tasmanicosa was insufficient by modern taxonomic standards and did not include an accurate description of the genus. The diagnosis was limited to a single character referring to the distance between the PME (from German): “ Lycosa tasmanica Hogg, 1905 (in the catalogue, 2a, 240, sub Dingosa ) is better assigned to a new genus (for which we here propose Tasmanicosa n. g.), which differs from Lycosella (see the following genus) in the distance between the PME which is smaller than one diameter of the PME.” The problems associated with this poor diagnosis and much of Roewer’s taxonomic work on the Lycosidae are reflected in the fact that Lycosella Thorell, 1890 belongs to a different subfamily altogether, the Artoriinae Framenau, 2007 (Framenau 2007). The genus Tasmanicosa is here included in the subfamily Lycosinae, as it conforms to the subfamily diagnosis given by Dondale (1986), i.e. the tegular apophysis of the male pedipalp is transverse with a ventrally directed spur and has a sinuous channel on the dorsal surface. Within the Lycosinae, molecular sequence data places the genus in a Gondwanan clade, together with Hoggicosa , Knoelle and some Hogna , albeit with low support ( Murphy et al. 2006).
The transfer of Orthocosa semicincta ( L. Koch, 1877) , the type species of Orthocosa , places Orthocosa into junior synonymy of Tasmanicosa . Other non-Australian species currently listed in Orthocosa are here morphologically assessed based on their original descriptions and are here transferred to more appropriate genera (Appendix A).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Tasmanicosa Roewer, 1959
| Framenau, Volker W. & Baehr, Barbara C. 2016 |
Orthocosa
| Roewer 1960: 774 |
Tasmanicosa
| Roewer 1959: 351 |