Lasioptera Meigen
|
publication ID |
https://doi.org/10.5281/zenodo.203443 |
|
DOI |
https://doi.org/10.5281/zenodo.6194099 |
|
persistent identifier |
https://treatment.plazi.org/id/415887DF-265F-4230-FF14-F9EBFD4BF889 |
|
treatment provided by |
Plazi |
|
scientific name |
Lasioptera Meigen |
| status |
|
Genus Lasioptera Meigen View in CoL View at ENA
Type species: Lasioptera picta Meigen 1818: 88
Microlasioptera Skuhravá and Skuhravý 1981 View in CoL , new synonym Type species: Microlasioptera flexuosa ( Winnertz) 1853 View in CoL : 308
Lasioptera View in CoL is a chiefly Old World genus in the tribe Lasiopterini View in CoL , currently with 128 described species, 38 of them in the Palearctic region ( Gagné 2010). Most species are gall inducers in stems of plants from various families but some are inquilines or successors in galls of other insects. This is a morphologically diverse genus that serves to accommodate lasiopterine species with a 3–4-segmented palpus, a very short R5 vein, a long protrusible ovipositor with strong, modified setae basally and on the fused cerci, and larvae with well developed, 2 or 3-toothed spatula with usually 4 lateral papillae on each side, and 3–4 setose terminal papillae on each side. The genus Microlasioptera View in CoL falls within the continuum of morphological diversity in Lasioptera View in CoL and is therefore synonymized here with Lasioptera View in CoL (see remarks below under L. foeniculi View in CoL n. sp.). Lasioptera carophila Löw 1874 View in CoL
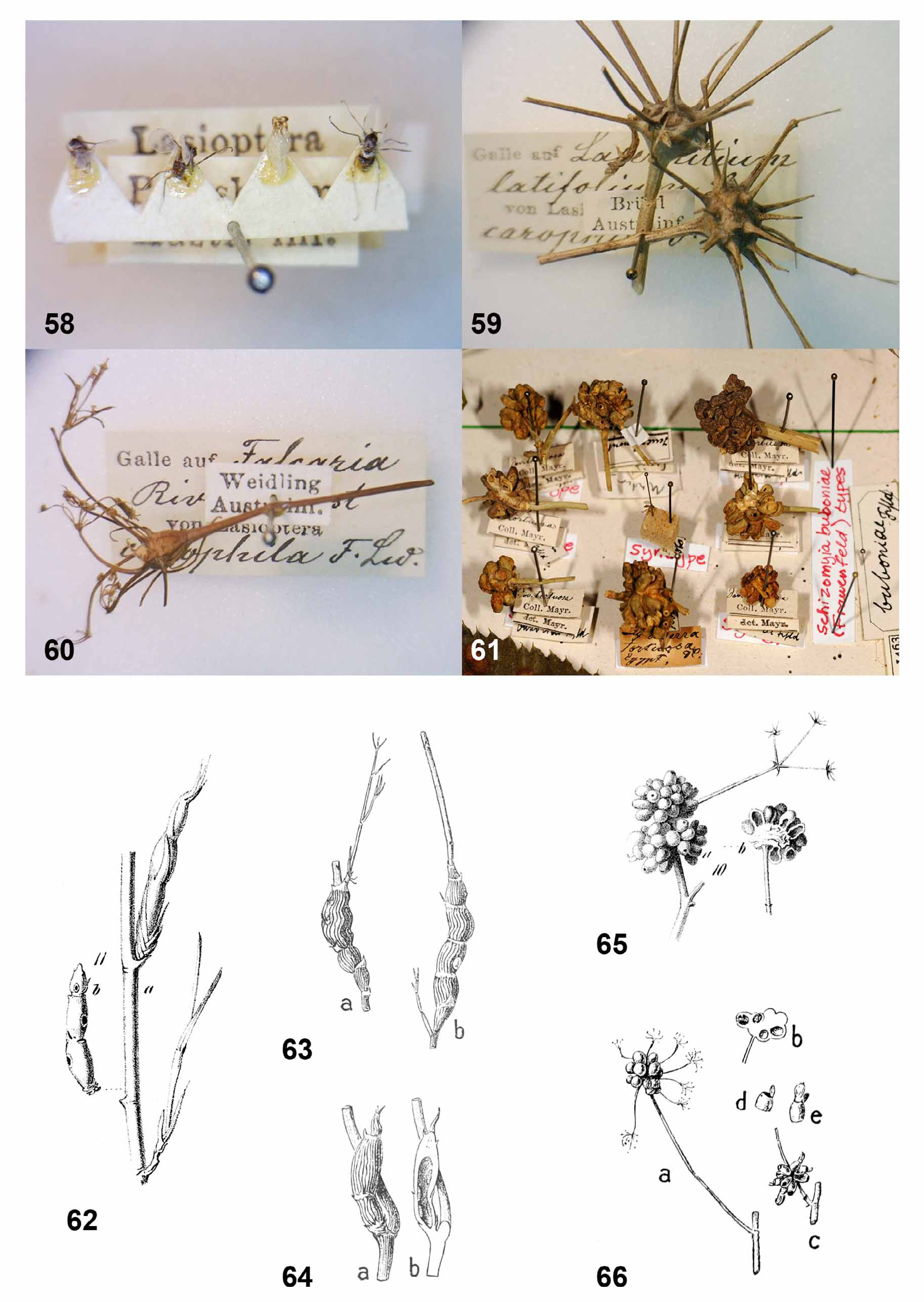
( Figs. 1, 5 View FIGURES 1 – 7 , 8–9, 14 View FIGURES 8 – 15 , 18–19 View FIGURES 16 – 21 , 22, 25 View FIGURES 22 – 29 , 50 View FIGURES 50 – 57 , 58–60 View FIGURES 58 – 66 )
Lasioptera carophila Löw 1874: 149 View in CoL Lasioptera thapsiae Kieffer 1898: 58 View in CoL
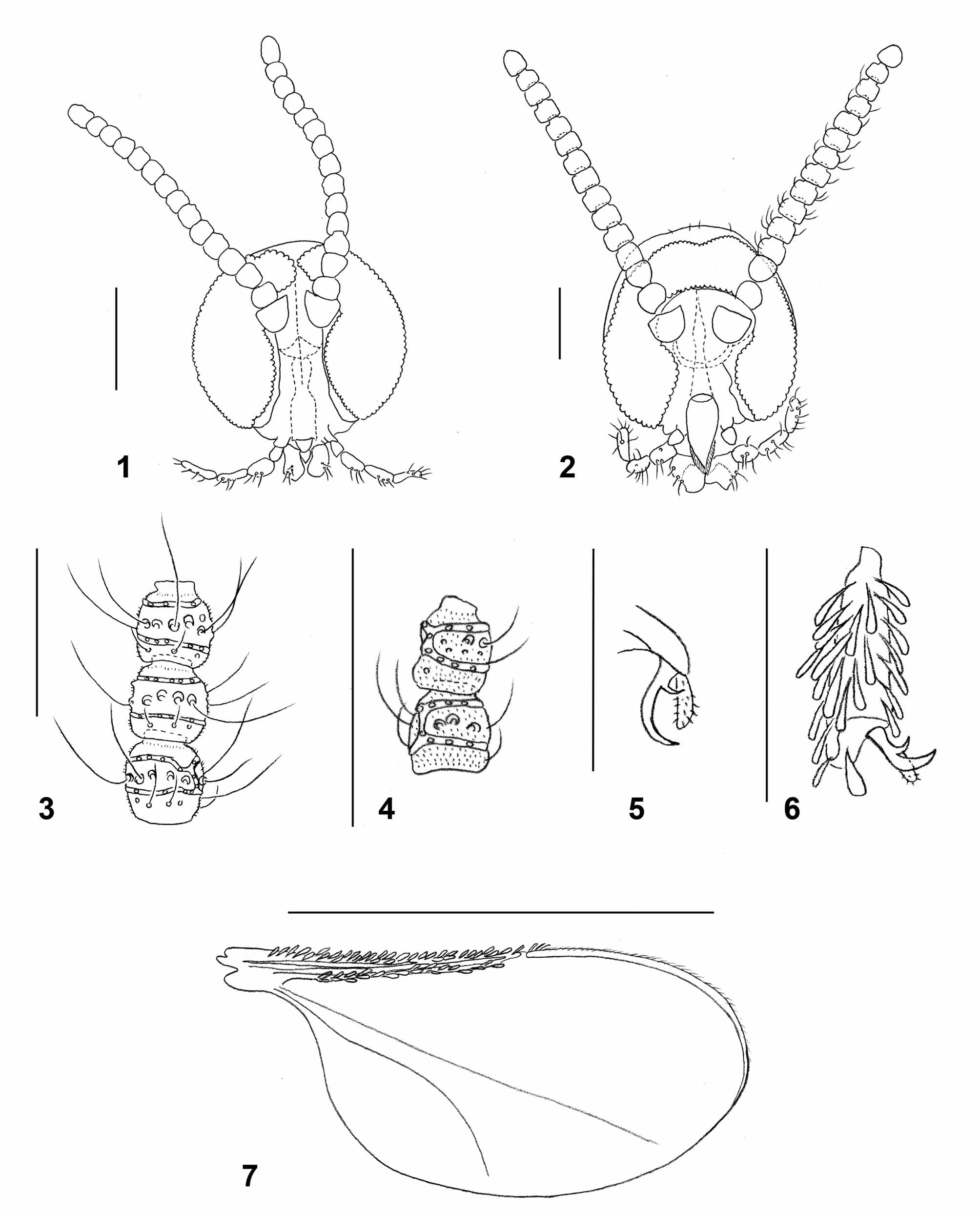
Adult: Head ( Fig. 1 View FIGURES 1 – 7 ): Densely covered by white scales except for area of dark scales on frons. Eye facets circular, gap between eyes on vertex 0–0.5 facet wide. Palpus 4-segmented, segments successively longer or segments 3 and 4 same length. Antenna: 13 flagellomeres in female (n=3), 11 in male (n=3); proximal flagellomeres slightly longer than wide, distal flagellomeres except for apical about as long as wide; first two flagellomeres partially fused, last flagellomere longer than preceding, consisting of 2–3 fused flagellomeres; each flagellomere except apical with two whorls of circumfila connected by 1–2 longitudinal branches, row of short, curved setae proximal to proximal circumfilum, and two rows of strong setae between circumfila whorls; otherwise evenly covered by microtrichia. Apical flagellomere often with 3–4 circumfila whorls.
Thorax: General color dark brown, densely covered by white scales. Wing: length 1.86–1.94 mm in female (n=2), 1.93–2.03 mm in male (n=2); Wing: whitish-hyaline with dense white scales along veins C, R1 and R5, except for patch of dark scales at the merging point of C and R5. M present, Cu unforked. Haltere light brown, knob covered by white scales. Legs: general color brown, covered by dense white scales dorsally, dark scales ventrally. Tarsal claws toothed; teeth strongly curved near base. Empodia longer than bend in claws ( Fig. 5 View FIGURES 1 – 7 ).
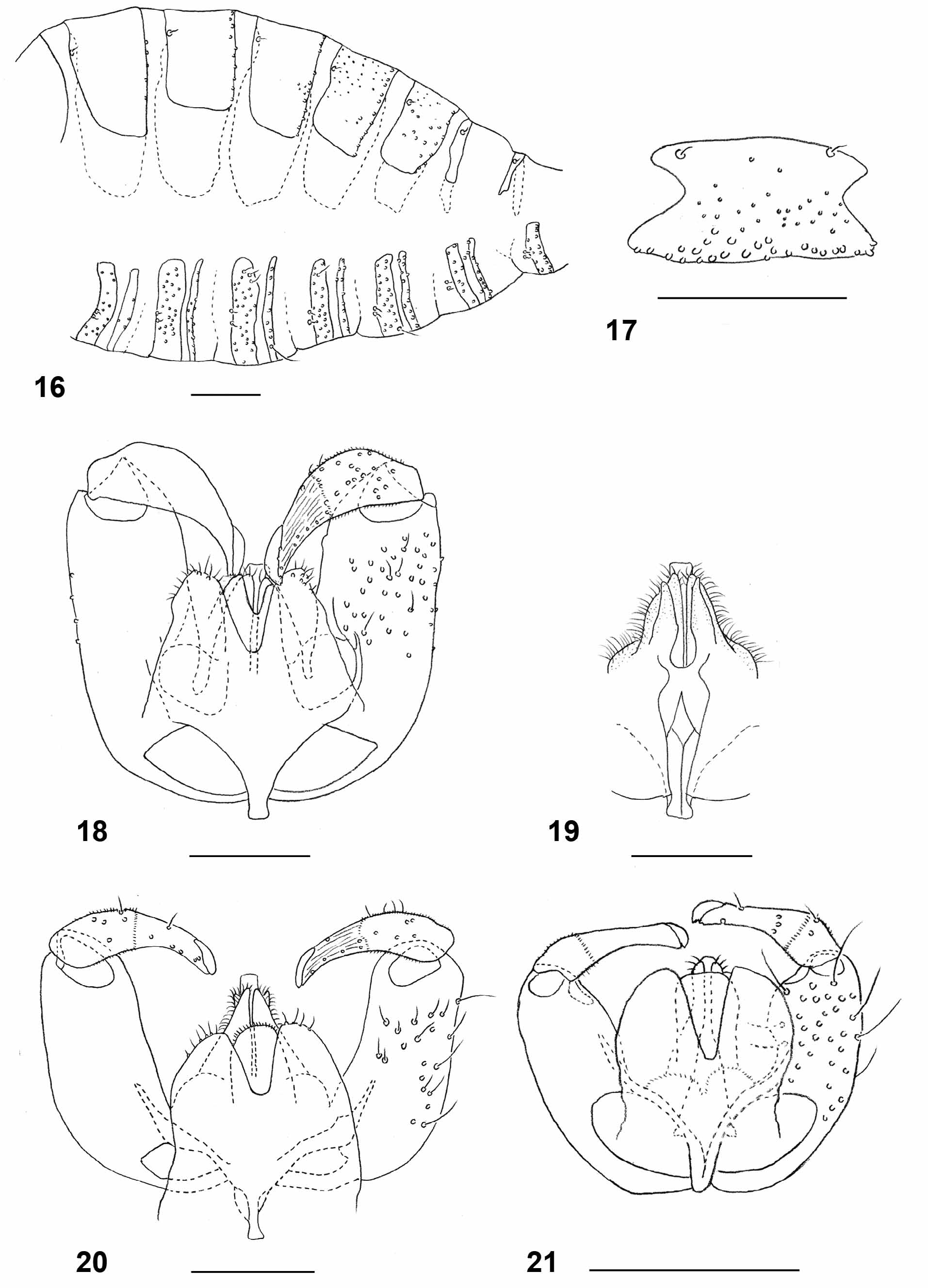
Female abdomen: General color dark brown; tergites each covered by dense white scales except for narrow posterior band of black scales. Tergites 1–6 strongly sclerotized, with two sensory setae at mid-anterior part and row of setae along posterior margin; tergite 7 ( Fig. 8 View FIGURES 8 – 15 ) trapezoid, with two sensory setae anteriorly and 2–3 rows of strong setae along posterior margin; tergite 8 consists of two elongate sclerites, each widened anteriorly and posteriorly, with one sensory seta at proximal third and several strong setae posteriorly ( Fig. 9 View FIGURES 8 – 15 ). Sternites with narrow, weakly sclerotized transverse band around mid length, two closely adjacent sensory setae anteriorly, and row of strong setae along posterior margin; sternite 7 less sclerotized laterally and more setose than preceding; sternite 8 undifferentiated from surrounding membrane. Ovipositor ( Fig. 14 View FIGURES 8 – 15 ): of intermediate length; segments 9+10 of abdomen 4.00–4.08 times as long as tergite 6 (n=2); lateral group of setae on segment 8 comprising about 60 long, curved setae; lateral plate well developed, bearing numerous mostly straight setae laterally and several pairs of long, erect and hooked setae dorsally; aculeus short and blunt; apical lamella setulose, with numerous strong setae on entire surface.
Male abdomen: Scale pattern as in female. Tergites 1–7 with two sensory setae anteriorly and row of setae posteriorly. Sclerotization of tergite 8 reduced to narrow band, with two anterior sensory setae and no posterior setae. Sternites 2–7 with two closely adjacent sensory setae anteriorly, row of strong setae posteriorly and few strong setae mesally; sclerotization of sternite 8 greatly reduced anteriorly, without anterior sensory setae, with numerous strong setae mesally and posteriorly. Terminalia ( Figs.18–19 View FIGURES 16 – 21 ): gonocoxite with numerous strong setae evenly distributed on entire ventral part, restricted to distal two thirds on dorsal part; mediobasal lobe slightly shorter than aedeagus, divided into two longitudinal lobes to base ( Fig. 19 View FIGURES 16 – 21 ), covered by long curved setae. Gonostylus narrowed abruptly at distal third, setulose and setose on proximal half, ridged and with few setae on distal half; tooth well developed. Aedeagus blunt apically. Hypoproct strongly setose, with deep apical notch. Cerci separated by deep notch almost to base, setose and setulose, with several strong setae apically.
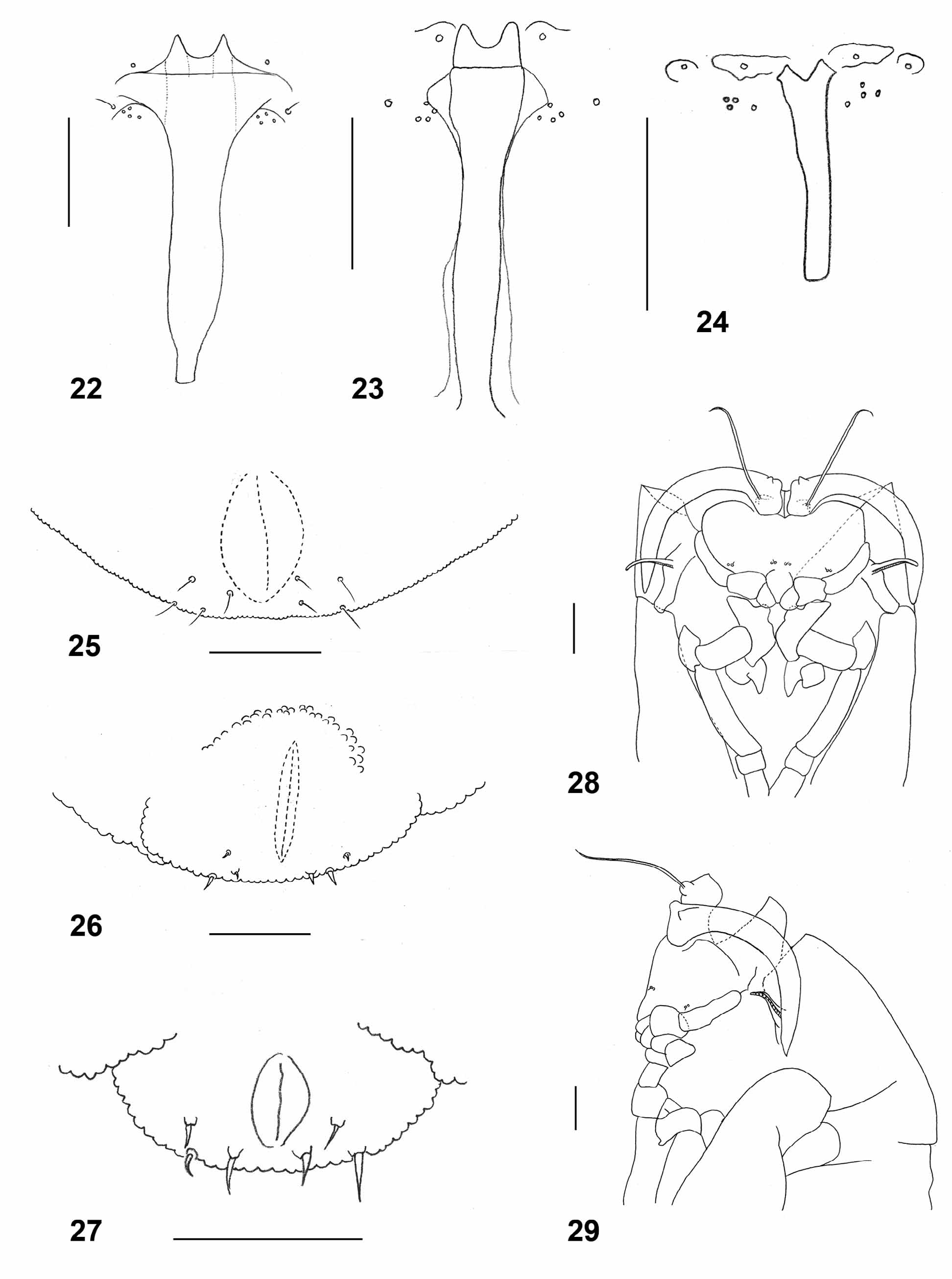
Larva (third instar): Obovate, bright orange, with dark brown spatula; length: 2.5–3.5 mm (n=9). Integument evenly covered by tiny bumps. Antenna about twice as long as wide. Cephalic apodemes about twice as long as head capsule (n=6). Spatula ( Fig. 22 View FIGURES 22 – 29 ) with long shaft and two pointed anterior teeth separated by wide notch; shaft very wide immediately posterior to teeth. On each side of spatula one sternal papilla and four lateral papillae, three of which closely approximated, all asetose. Median pleural papilla with strong seta. Other pleural papillae and dorsal papillae with short setae. Terminal segment ( Fig. 25 View FIGURES 22 – 29 ) with two groups of four terminal papillae with conspicuous setae.
Pupa: Dark brown (when mature). Antennal bases developed into short, pointed horns. Cephalic seta slightly longer than antennal horn, situated on elevated base. Prothoracic spiracle thin and elongate, trachea ends at apex. Proximal two thirds of each abdominal segment covered by tiny, pointed spinules; distal third smooth.
Material examined (all material from Israel reared from galls on Foeniculum vulgare and deposited in TAUI): 3 Ƥ, 1 pupa, 2 pupal exuviae, Austria, Pressbaum, 7.vii.1873, F. Löw, ex. Carum carvi ( syntypes, pinned—Fig. 58) NHMW; 1 3, 1 Ƥ, 1 pupal exuviae, Germany, Remagen, 27.iv-9.v.1907, E.H. Rübsaamen, ex. Daucus (mounted on permanent microscope slide by N. Dorchin) ZMHB; 6 larvae (on one microscope slide), Israel, Kefar Hahoresh, 9.x.1997, N. Dorchin; 1 Ƥ, Israel, Kefar Hahoresh, 11.x. 1997, N. Dorchin; 3 3, Israel, Kefar Hahoresh, 3.x.2008, N. Dorchin and N. Dorchin; 6 larvae, 1 Ƥ, Israel, Kefar Hahoresh 12.x.2009; 1 3, Israel, Kefar Hahoresh, 19.x.2009, N. Dorchin.
Distribution. Europe, western Asia, northern Africa. In Israel, galls were abundant in several locations in the Upper and Lower Galilee and the Mount Karmel area (Nahal Amud, E’n Alva, Kefar Hahoresh, Sede Ya’acov, Shfeya).
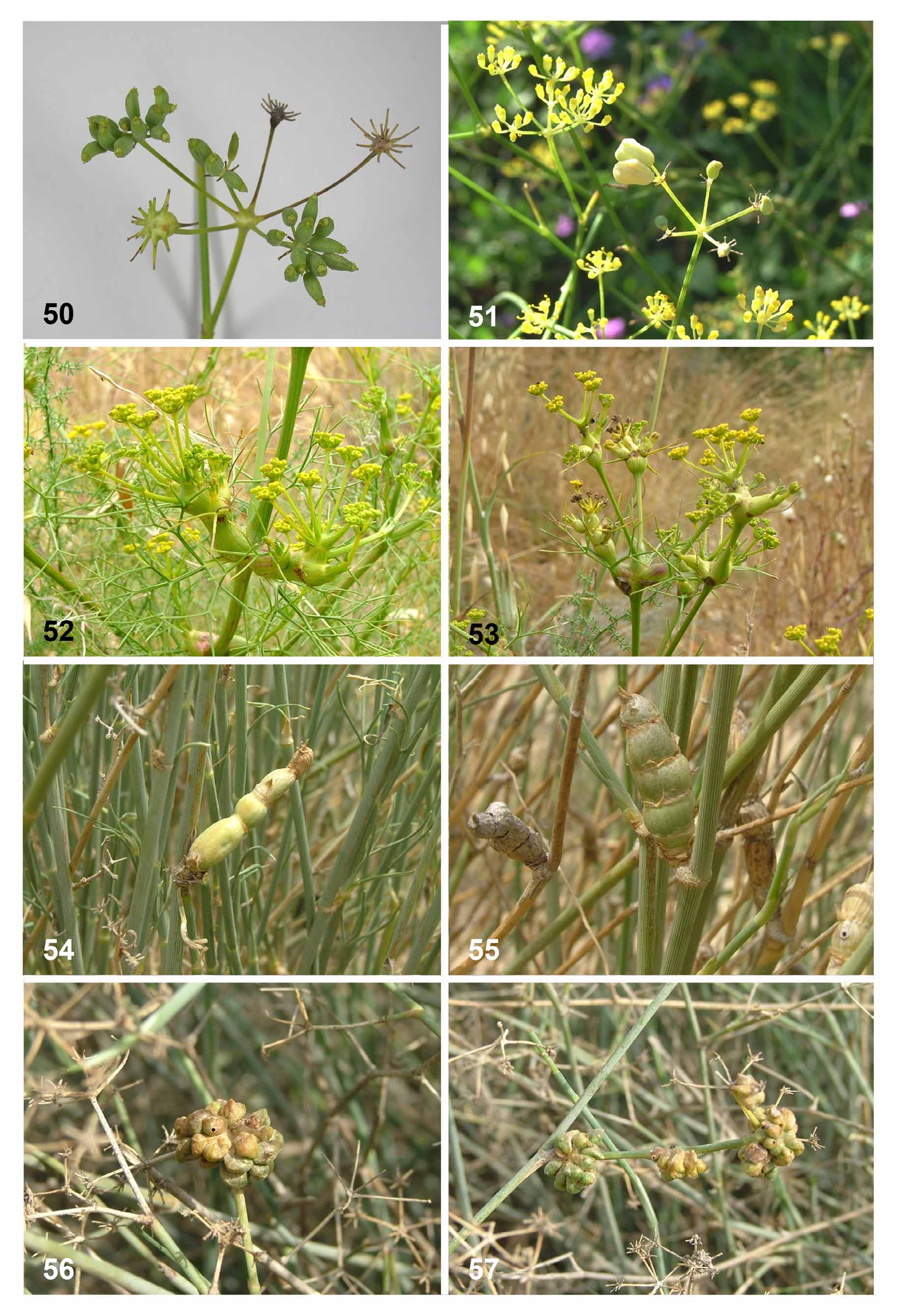
Biology. Lasioptera carophila causes swellings in umbels, umbellules, and sometimes stems of numerous Apiaceae species (e.g., Figs. 50 View FIGURES 50 – 57 , 59, 60 View FIGURES 58 – 66 ). It was originally described from galls on Carum carvi ( Löw 1874) and later recorded by Möhn (1968, as L. argentata ) from more than 30 Apiaceae genera, including Bupleurum , Daucus , Foeniculum , Heracleum and Pimpinella . In Israel we found this species only on Foeniculum vulgare , in which it causes swellings of up to 5 mm in diameter in umbels and umbellules ( Fig. 50 View FIGURES 50 – 57 ). The galls are accompanied by fungal mycelia that line the internal walls of the larval chamber. On most of the known host plants the galls are singlechambered but on some, multi-chambered galls were recorded, and galls on Echinophora spinosa were either single or multi-chambered (Möhn 1968). Stem galls and multi-chambered galls are much more common in woody Apiaceae in southern Palearctic areas than in more northern and western parts of the distribution range (Möhn 1968). All the galls we found on Foeniculum vulgare in Israel were single-chambered. Further study is needed in order to establish whether the records of L. carophila from all of its host plants indeed belong to the same species, in particular because Möhn’s study was based entirely on larval morphology.
Möhn (1968) found that the woodier the host-plant the larger and better developed the larval spatula is. Pupation takes place inside the gall. The larvae prepare an escape route before pupation, leaving only a thin layer of plant material for the pupae to break through before adult emergence. In Europe, the species is bivoltine, with adults emerging in March and in July-August ( Löw 1874, Möhn 1968). In Israel only one generation was recorded, with adults emerging in October. Although the galls were very common, adults were extremely difficult to rear. Most galls contained third instar larvae in October while others contained first or second instars. This observation suggests either a) that the species is univoltine with a certain proportion of larvae taking two (or more) years to develop but adults emerging only in October, or b) that the species has two generations a year, with adults emerging in spring and in fall, but we failed to find the galls of the spring generation. If the second option is correct, then the young larvae observed in the galls in October may be those that will emerge in spring.
Remarks. Kieffer (1904) synonymized L. carophila under L. argentata without providing a reason. Möhn (1968) adopted that synonymy and treated L. carophila as L. argentata in his revision of the genus from the Palearctic region. In that work, Möhn included L. carophila together with L. eryngii , L. turcica and L. umbelliferarum under the argentata group based on larval morphology and the association of all species with host plants of the Apiaceae . This is despite the fact that L. carophila (and supposedly L. argentata ) differs from all other species in this group by having a setose median pleural papilla ( Fig. 22 View FIGURES 22 – 29 ). All species in the group lack setae on the lateral papillae. Möhn further synonymized L. thapsiae Kieffer under L. argentata , presumably because the differences between these species given by Kieffer (1898) were unconvincing and based on Kieffer’s note that adults of both species were found together. It is noteworthy, though, that the galls of L. thapsiae are multi-chambered whereas prior to Möhn’s work, the galls of L. carophila were reported to be single chambered.
Based on larval morphology, L. carophila is most closely related to L. turcica Möhn , as the species share a similarly shaped spatula that is conspicuously widened immediately posterior to the teeth. Adults of L. turcica were not described. Lasioptera carophila is somewhat smaller than L. eryngii and L. umbelliferarum and has fewer antennal flagellomeres than these species, although these are highly variable characters, and our sample size for L. carophila is small. The ovipositor in all three species is similar and we could not find any morphological differences in the cercal segment. Nevertheless, the species do differ consistently in the relative length of the ovipositor, which is shortest in L. eryngii and longest in L. umbelliferarum , with no overlap among the species. The shape of tergite 8 constitutes a good difference and its relative length correlates with that of the ovipositor ( Figs. 9, 11, 13 View FIGURES 8 – 15 ). The original description of L. carophila included only females and pupae, whereas Möhn’s work (1968) was based entirely on larvae. The present work is therefore the first to describe the male of this species. The main difference between this and the male of L. eryngii and L. umbelliferarum is the deeply notched hypoproct in L. carophila compared to the entire hypoproct in the two other species.
| NHMW |
Naturhistorisches Museum, Wien |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Lasioptera Meigen
| Dorchin, Netta & Freidberg, Amnon 2011 |
Lasioptera carophila Löw 1874 : 149
| Kieffer 1898: 58 |
| Low 1874: 149 |
Microlasioptera Skuhravá and Skuhravý 1981
| Winnertz 1853: 308 |