Lasioptera umbelliferarum Kieffer 1909
|
publication ID |
https://doi.org/ 10.5281/zenodo.203443 |
|
DOI |
https://doi.org/10.5281/zenodo.6194101 |
|
persistent identifier |
https://treatment.plazi.org/id/415887DF-2656-423E-FF14-FF4AFABDF94E |
|
treatment provided by |
Plazi |
|
scientific name |
Lasioptera umbelliferarum Kieffer 1909 |
| status |
|
Lasioptera umbelliferarum Kieffer 1909 View in CoL
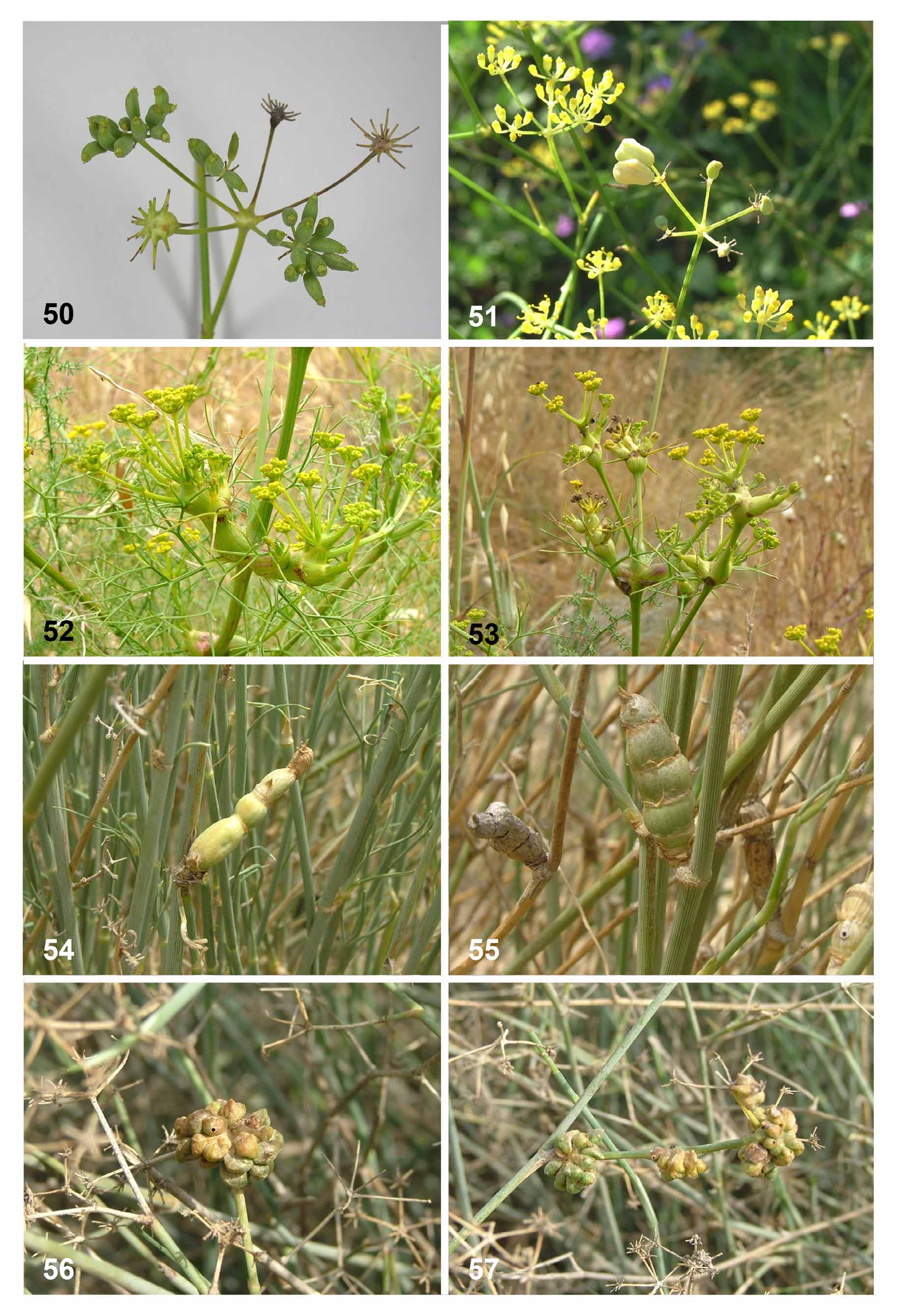
( Figs. 3 View FIGURES 1 – 7 , 12–13 View FIGURES 8 – 15 , 20 View FIGURES 16 – 21 , 23, 26 View FIGURES 22 – 29 , 42–43 View FIGURES 42 – 49 , 52–53 View FIGURES 50 – 57 )
Lasioptera umbelliferarum Kieffer 1909: 28 View in CoL
Neotype of Lasioptera umbelliferarum View in CoL designated here – Ƥ, Israel, Giv’at Olga, 19.III.1996, A. Freidberg, reared from Bilacunaria boissieri View in CoL stem gall. Lasioptera umbelliferarum View in CoL was originally recorded ( Rübsaamen 1895) and subsequently described only from its gall ( Kieffer 1909) from Seseli View in CoL sp. from Russia (near the Caspian Sea) but the host plant could have been misidentified. The type is considered lost together with the majority of Kieffer’s collection. Möhn (1968) later described the larvae of this species in his revision of the Palaearctic species of Lasioptera View in CoL based on material that he recovered from herbarium-preserved galls. He noted that he never found in these herbaria galls of L. umbelliferarum View in CoL on Seseli View in CoL but did find them on three species of the closely related genus Hippomarathrum View in CoL . We found the galls in Israel on Bilacunaria View in CoL , a closely related genus to Seseli View in CoL and Hippomarathrum View in CoL that is sometimes treated under the latter (Mabberley 2008). The recorded distribution of L. umbelliferarum View in CoL fits the distribution of Bilacunaria View in CoL and the published descriptions of the galls and larvae leave little doubt that the species we found in Israel is indeed L. umbelliferarum View in CoL . Pupae and adults of this species are described here for the first time.
The neotype is designated in order to fix the identification of the species and maintain the current usage of its name. It is deposited in the National Collection of Insects, Zoological Museum, Tel Aviv University, Israel (TAUI).
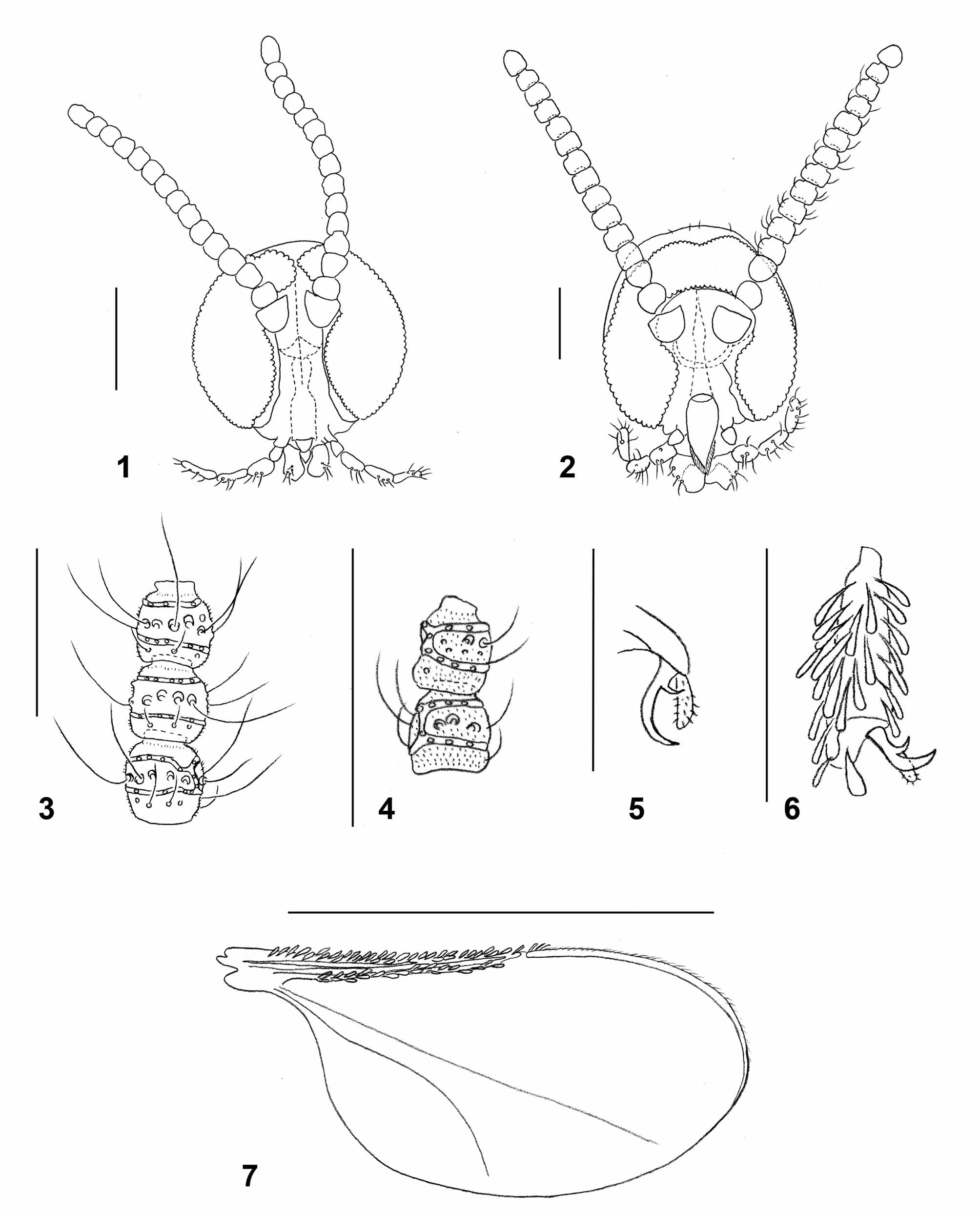
Adult: Head: Frons, gena, scape, pedicel, and area along posterior margins of eyes densely covered by white scales and hairs. Eye facets circular. Gap between eyes on vertex 0–1 facet wide. Palpus 4-segmented; palpiger usually well developed; segments 1-3 successively longer; first segment as long as or slightly longer than wide; fourth segment same length or shorter than third. Labella slightly tapered, with few long setae. Antenna: Dark brown. Scape and pedicel covered by white scales. Flagellomeres: 17–19 in female (n=13), 13–16 in male (n=10), number occasionally varies between antennae of same individual; flagellomeres except for apical as long as wide or slightly wider than long, especially in proximal half of antenna; first two flagellomeres partially fused, last often longer than preceding, consisting of two fused flagellomeres; each flagellomere except for apical with two whorls of circumfila connected by 1–2 longitudinal branches, row of short, curved setae proximal to proximal circumfilum, and several strong and very long setae between circumfila whorls; otherwise evenly covered by microtrichia ( Fig. 3 View FIGURES 1 – 7 ). Apical flagellomere often with 3–4 circumfila whorls. Circumfilar whorls on flagellomeres 1–2 closer to each other and often with more longitudinal connections than those on other flagellomeres.
Thorax: Dark brown, covered laterally by white scales and hairs, dorsally with two longitudinal lines of long white hairs. Wing: hyaline; length 2.00– 2.53 mm in female (n=12), 1.84–2.37 mm in male (n=8); R1 and R5 join C at about 0.4 and 0.6 of wing length, respectively; M present, Cu unforked. R1, R5 and C along anterior wing margin densely covered by dark brown scales except for white spot at merging point of R5 and C. Posterior wing margin with long, fine hairs. Haltere whitish-yellow. Legs: coxae with long white hairs, remaining parts covered by white scales dorsally, short black scales with sparse white scales ventrally. Tarsal claws evenly curved, each with short, thick basal tooth. Empodia longer than bend in claw.
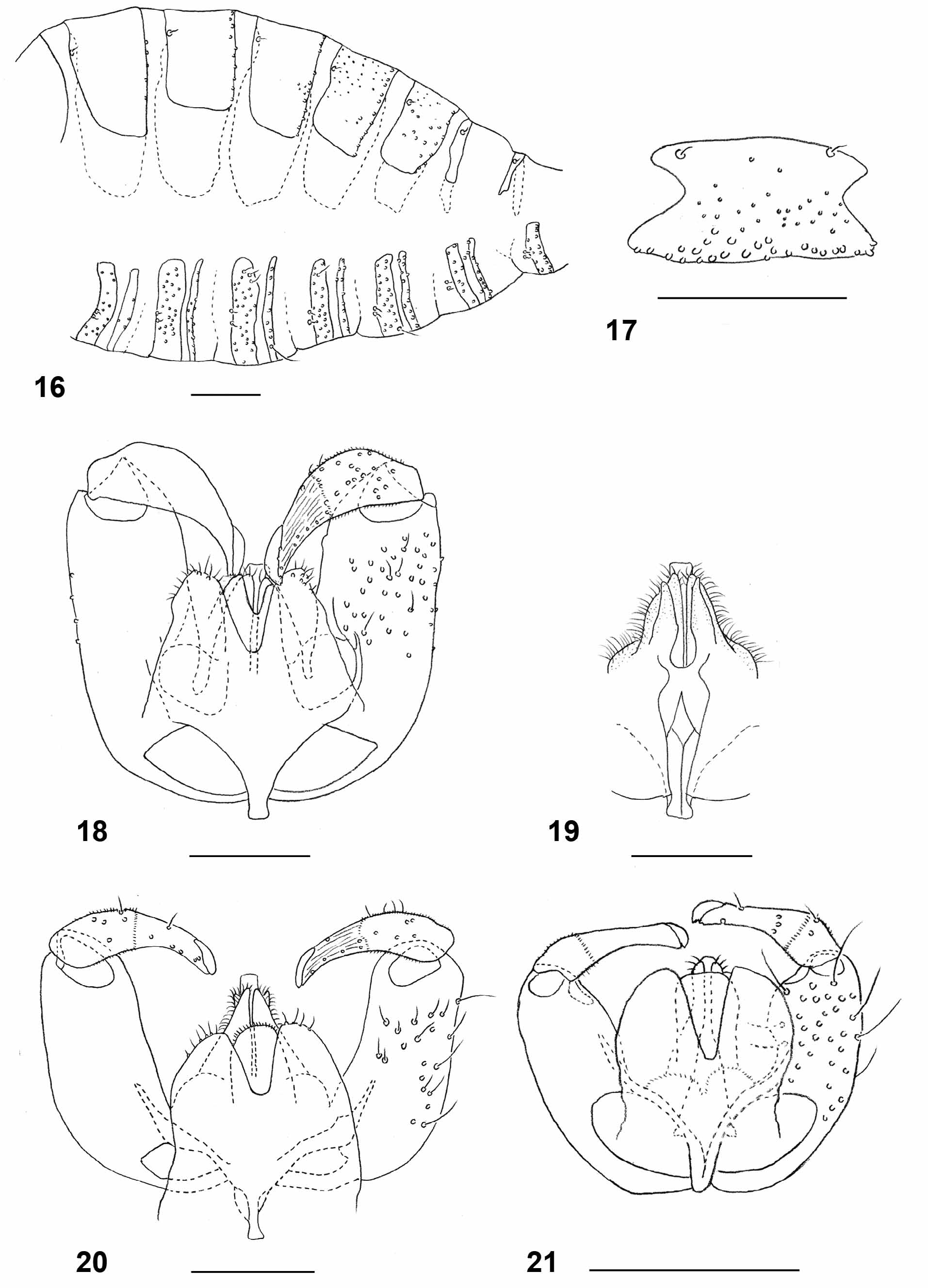
Female abdomen: As in L. carophila except for the following: densely covered by silvery-white scales ventrally and laterally; tergites each with wide band of dense black scales occupying half to two thirds of tergite length, with small extension in mid-posterior part. Tergite 7 as in Fig. 12 View FIGURES 8 – 15 . Sclerites of tergite 8 long and slender, widened anteriorly and posteriorly ( Fig. 13 View FIGURES 8 – 15 ). Sternites evenly sclerotized. Ovipositor: long (segments 9+10 of abdomen 4.29–5.33 times as long as tergite 6 (n=12)).
Male abdomen: as in L. carophila except for the following: color and scale pattern as in female. Terminalia ( Fig. 20 View FIGURES 16 – 21 ): gonostylus evenly narrow. Aedeagus blunt to slightly rounded apically. Hypoproct entire. Cerci separated by deep notch.
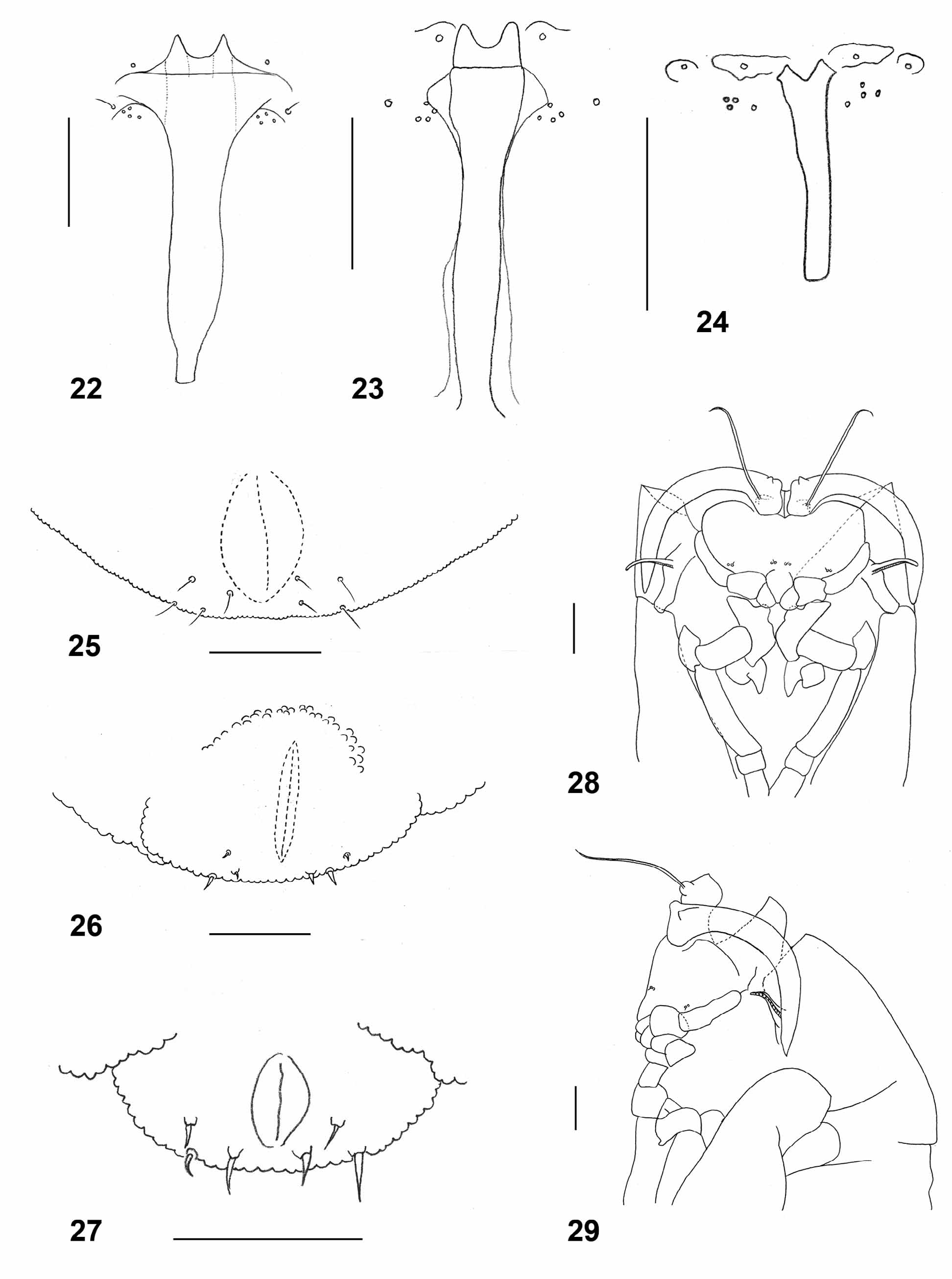
Larva (third instar): bright orange with dark brown spatula; length: 2.36–3.56 mm (n=20). Integument covered entirely by rounded to slightly pointed bumps. Antennae about twice as long as wide. Cephalic apodemes 1.5–2.8 times as long as head capsule (n=11). Spatula ( Fig. 23 View FIGURES 22 – 29 ) with long shaft and two apically rounded anterior teeth separated by evenly rounded notch; shaft conspicuously widened immediately posterior to teeth and slightly widened at posterior end. On each side of spatula one sternal papilla and four lateral papillae, all asetose. Median pleural papilla asetose. Other pleural papillae and dorsal papillae with short setae. Terminal segment with two groups of three or four terminal papillae with short setae ( Fig. 26 View FIGURES 22 – 29 ).
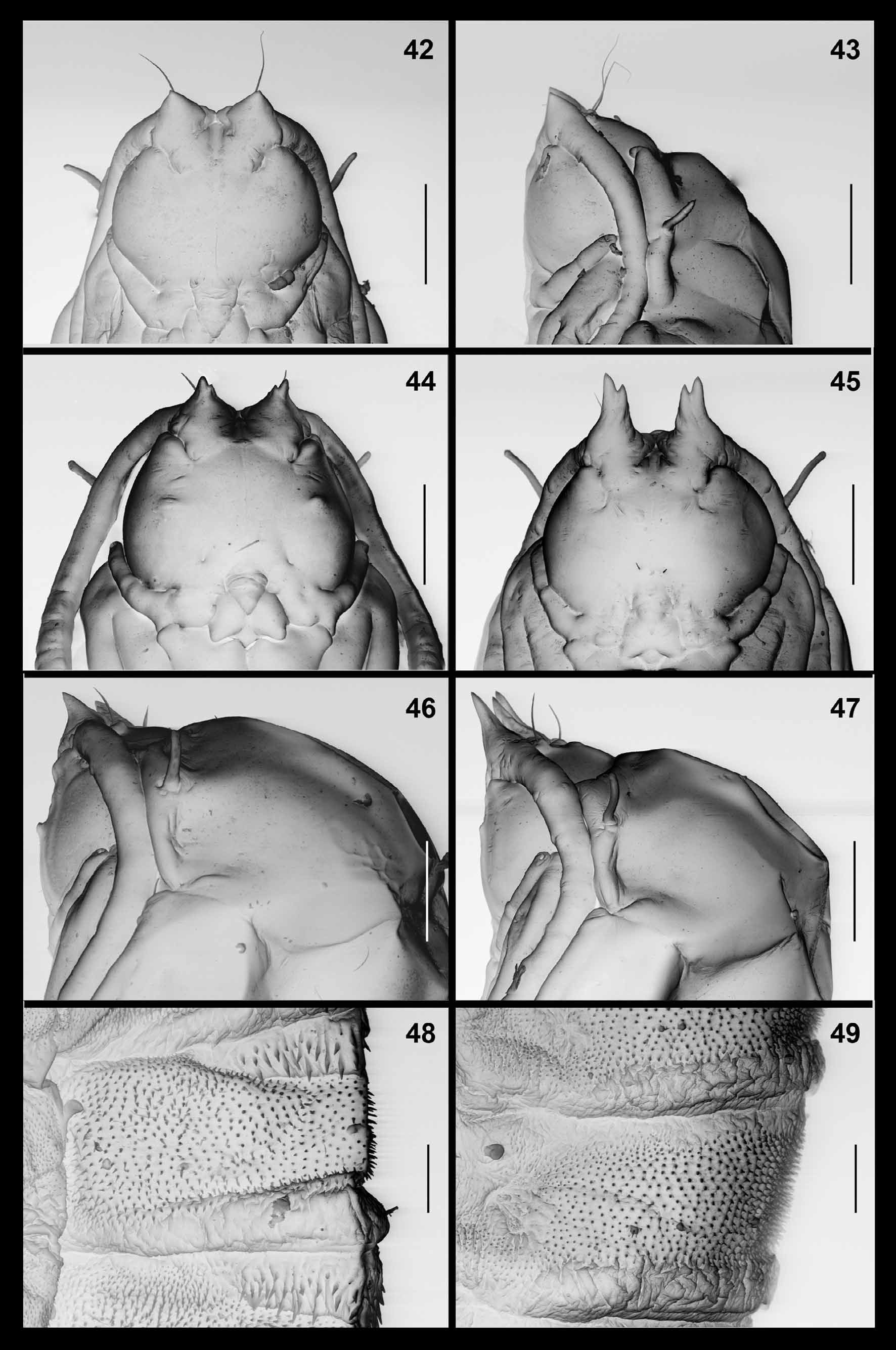
Pupa ( Figs. 42–43 View FIGURES 42 – 49 ): orange. Antennal bases developed into short, pointed horns. Cephalic seta long, situated on elevated base. Frons without papillae. Prothoracic spiracle thin and elongate; trachea ends at apex. Abdominal segments covered by short, pointed spinules except for short crinkled posterior area on each segment.
Neotype: Ƥ, Israel, Giv’at Olga, 19.iii.1996, Freidberg A., reared from Bilacunaria boissieri stem gall. Mounted on permanent microscope slide. Deposited in TAUI.
Other material examined. 3 larvae, Georgia, Elisabethpol, vii–viii.1934, unspecified collector, ex. Hippomarathrum crispum, Möhn collection number 9382C, (mounted on permanent microscope slide by N. Dorchin), SMNS; 2 larvae, Israel [ Palästina], unspecified date and collector, ex. Hippomarathrum crispum, Möhn collection number 9382B, (mounted on permanent microscope slide by N. Dorchin), SMNS; 5 Ƥ, 2 3, same data as Neotype (1Ƥ ZFMK, others TAUI); 2 Ƥ, Giv’at Olga, 12.iii.1996, Dorchin N., ex. Bilacunaria boissier i (1 Ƥ TAUI, 1 Ƥ ZFMK); 4 Ƥ, 6 3, Park Hasharon, 22.i.2000, Freidberg A. and Freidberg P., ex. Bilacunaria boissier i (3 Ƥ, 4 3 TAUI, 1 Ƥ, 2 3 ZFMK); 2 Ƥ, 1 3 (pinned), Park Hasharon, 22.i.2000, Freidberg A., ex. Bilacunaria boissier i TAUI; 12 larvae (on two microscope slides), 3 pupal exuviae (on one microscope slide), Hadera, 20.ii.2010, Freidberg A., ex. Bilacunaria boissieri , TAUI; 1 Ƥ, 2 3, Tel Baruch, 8.iii.2011, Freidberg A., ex. Bilacunaria boissieri (pinned), TAUI.
Distribution. South-west Asia, around the Caspian Sea ( Russia [Petrowsk], Iran [Kurdistan], Georgia, Azerbaijan). The discovery of this species in Israel suggests that it has a continuous distribution in the Irano-Turanian region. In Israel it is distributed along the coastal plain, presumably corresponding to the distribution range of its host plant.
Biology. Lasioptera umbelliferarum is univoltine. Galls develop into conspicuous swellings in stems and flower stalks (umbels) that may reach several centimeters in length and more than 2 cm in diameter ( Figs. 52–53 View FIGURES 50 – 57 ). Galls in umbels often prevent proper development of flowers. In Israel, the galls begin to develop in late winter (February), are green, sometimes with darker longitudinal stripes, and reach their final size in March–April, when the larvae inside them are still first instars. Each gall may contain several dozen larval chambers embedded in woody tissue so that galls are extremely hard and difficult to dissect. At later stages of gall development, a thick layer of spongy tissue develops around the larval chambers. By summer, the galled stems dry out and turn grayishbrown but remain attached to the bases of the green plants. The fully grown larvae remain inactive inside the galls until the following winter. They pupate and emerge in February-March. The inside walls of the larval chambers in fully developed galls are covered by a conspicuous white layer of mycelia. The galls frequently contain inquilinous moth larvae that feed on the gall tissues and dig tunnels in them, thus killing some of the gall inducers indirectly. The gall-midge larvae are also attacked by several species of parasitic wasps.
Collecting the galls while they are still green results in the death of larvae inside them, thus the only way to rear this species is to collect the dried galls that contain pupae or diapausing larvae in February, shortly before adult emergence. This might be the reason why adults of this species have not been described to date. The above described phenology is in agreement with the data given by Rübsaamen (1895), who noted that galls collected in Russia in mid June contained third-instar larvae. By contrast, Möhn (1968), who examined galls from various localities in Western Asia, noted that galls that were collected in July-August contained fully developed pupae. These pupation dates do not match our findings and appear to be erroneous, in particular Möhn’s record from Israel.
Remarks. The closest relative of L. umbelliferarum appears to be L. eryngii , which induces similar galls in several Eryngium species in Europe and the Mediterranean region, but we never found L. eryngii in Israel. According to Möhn (1968), the two species differ in the length of setae on the larval ventral papillae, and the teeth of the larval spatula are usually not as widely separated in L. eryngii as in L. umbelliferarum . Lasioptera umbelliferarum has a longer ovipositor and a significantly longer and slenderer sclerite 8 (compare Figs. 10–11 View FIGURES 8 – 15 to Figs. 12–13 View FIGURES 8 – 15 ). For additional differences between L. umbelliferarum and related species see the remarks under L. carophila above.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Lasioptera umbelliferarum Kieffer 1909
| Dorchin, Netta & Freidberg, Amnon 2011 |
Lasioptera umbelliferarum
| Kieffer 1909: 28 |