Eucalyptus odorata, Behr, Behr
|
publication ID |
https://doi.org/ 10.1071/SB21029 |
|
DOI |
https://doi.org/10.5281/zenodo.10974607 |
|
persistent identifier |
https://treatment.plazi.org/id/5261160A-FF8B-FFD4-BB67-401FFEFEB0E3 |
|
treatment provided by |
Felipe |
|
scientific name |
Eucalyptus odorata |
| status |
|
Relationships within the E. odorata View in CoL complex
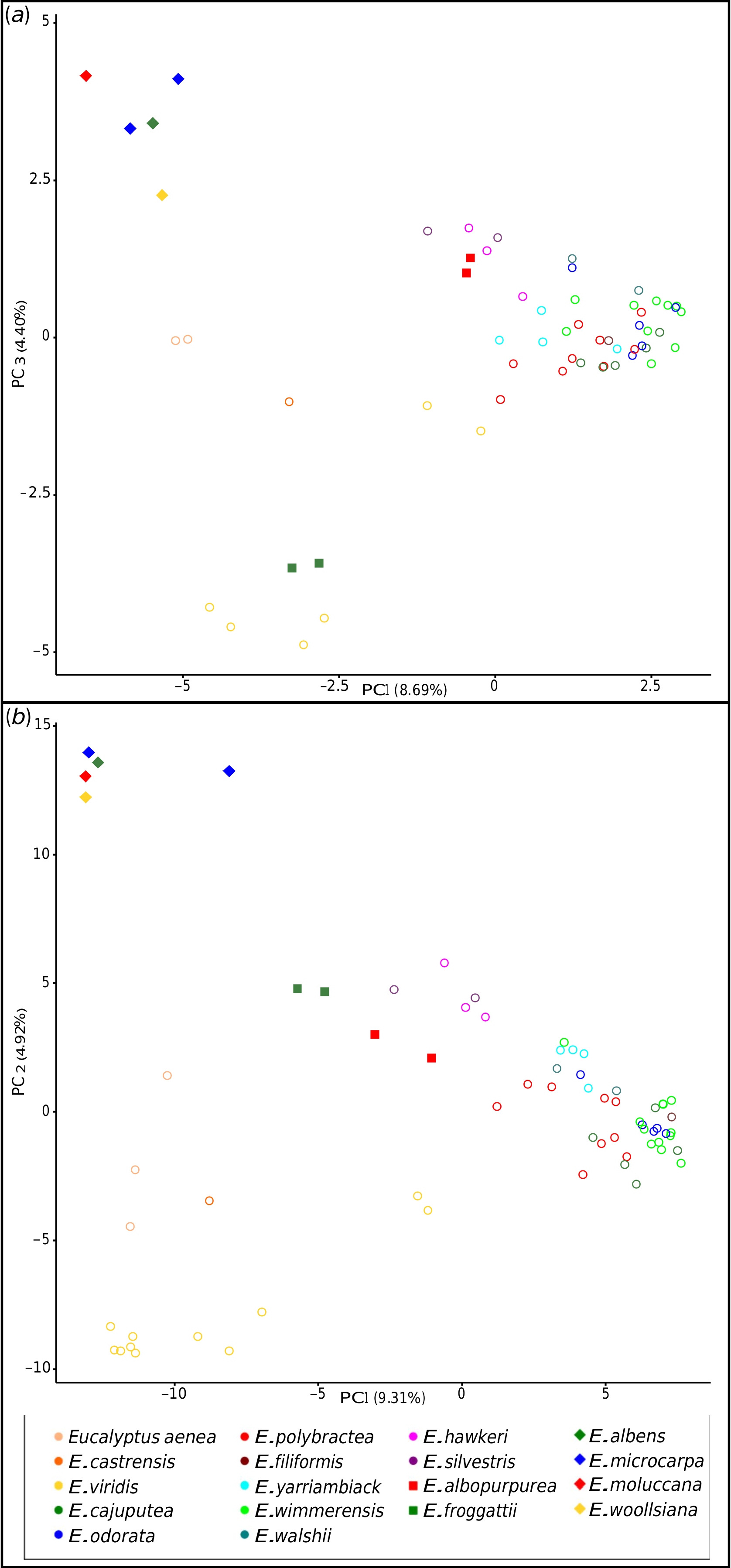
Relationships within the E. odorata complex are largely unresolved in our phylogeny, with very little bootstrap support in both the MP and ML analyses, and no species with multiple populations sampled being resolved as monophyletic. However, our PCA ( Fig. 3 View Fig ) and hybridisation tests ( Tables 4 View Table 4 , 5 View Table 5 ) have shed some light on the possible patterns of relatedness and introgression that explain this lack of resolution. There is strong support for the idea that E. viridis and the two segregate species from the Hunter Valley, E. aenea and E. castrensis , form a clade sister to the rest of the complex if northern (Queensland) populations currently regarded as E. viridis are excluded. Although E. viridis co-occurs with E. polybractea at multiple locations and the two may hybridise on occasion (but no evidence of this was seen in this study), the former is the most morphologically distinct species in the complex, given its linear juvenile leaves and its narrow, green adult leaves, and, at most sites, the two are easily recognisable and morphologically distinct. The two Hunter Valley segregates of E. viridis , E. aenea and E. castrensis , form a sister lineage to E. viridis in our phylogeny. However, the PCA ( Fig. 3 View Fig ) and hybridisation tests ( Tables 4 View Table 4 , 5 View Table 5 ) give weight to the hypothesis that these populations have experienced introgression from a grey-box species, which may account for their morphological distinctness, although given the results of our ABBA-BABA tests ( Table 4 View Table 4 ), E. albens appears the probable parent rather than E. moluccana as was hypothesised by Nicolle (2019). As the NewHybrid analysis assigns these samples to E. viridis rather than to a hybrid generation, this finding of introgression from a grey box is likely to be the result of historic introgression. The genetically distinct sample of E. aenea ( PSF 90 J) was recognised as differing from smooth-barked typical E. aenea in the field because of its stocking of rough bark that reached ~ 3 m up the trunks. Although genetically distinct from the other E. aenea samples in our dataset, this is not due to more substantial genetic input from a grey box as hypothesised. Although grey boxes are the most closely related species to occur in the vicinity of E. aenea , ironbark species of E. section Adnataria ( E. crebra F.Muell. , and E. fibrosa F.Muell. ) dominate the site and may be the culprit for this genetic distinctness, although our dataset does not allow us to test this. As our E. castrensis sample is more referable to the broader application of the name per Hill and Stanberg (2002) that may represent E. aenea , and we have not sampled material from the putative E. aenea × E. microcarpa – E. moluccana hybrids, which match the type material of the species ( Nicolle 2019), we cannot say anything further regarding the distinction of E. castrensis from E. aenea .
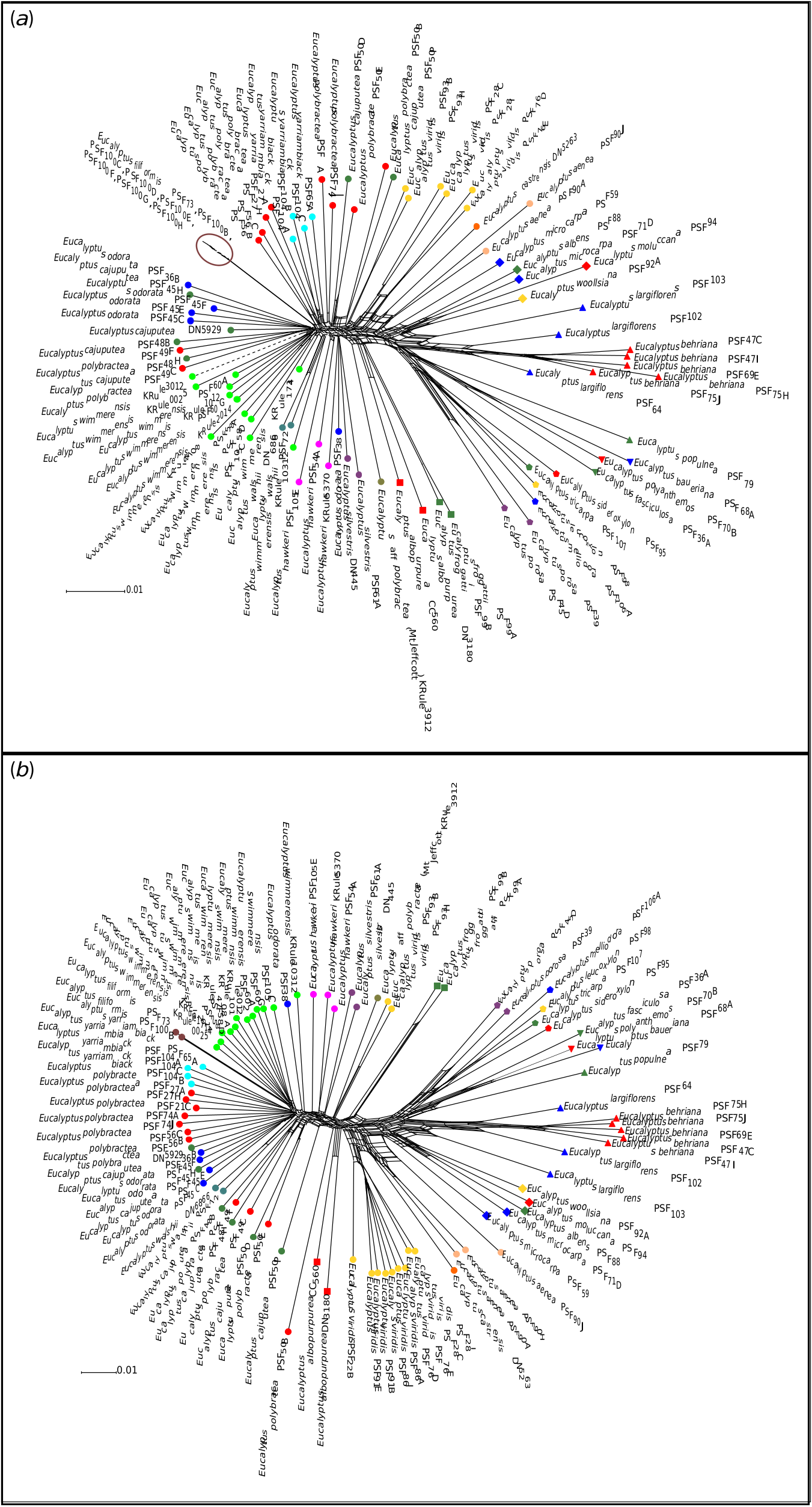
Previous authors have noted that Queensland populations in the vicinity of Inglewood, where the two northern E. viridis samples included in this study were collected, and Durikai State Forest have broader leaves than typical for E. viridis , with Blakely (1934) classifying these populations as E. viridis var. latiuscula Blakely. Chippendale (1988) suggested that these populations may be hybrids of E. viridis and the grey-box E. woollsiana , whereas Brooker et al. (2015) believed that these populations show greater morphological similarities to E. wimmerensis , which they regard as a subspecies of E. viridis , than to the typical form of E. viridis found in the Victorian goldfields and scattered populations in NSW. In the more resolved reduced sampling phylogeny ( Fig. 5 View Fig ), the northern samples are placed as the next clade to diverge in the complex after the main E. viridis clade, with the samples of E. polybractea from the most northerly population diverging next, although the node between these clades is supported only in the ML analysis. The ABBA-BABA tests also do not support introgression from the co-occurring grey-box E. woollsiana into these samples compared with E. viridis and E. polybractea from West Wyalong ( Table 4 View Table 4 ), as was previously hypothesised ( Chippendale 1988). Along with the placement of these samples in the PCA analyses ( Fig. 3 View Fig ), this raises the question of whether rather than an E. viridis × E. woolsiana hybrid, they may be E. viridis × E. polybractea hybrids, although given the lack of E. polybractea populations nearby (the nearest known population at West Wyalong being over 820 km away), they would have to be phantom hybrids, which have been observed in eucalypts previously, albeit without such a large geographic distance to the phantom parent ( Kirkpatrick et al. 1973; Hopper and Wardell-Johnson 2004). However, our ABBA-BABA tests showed no support for this hypothesis ( Table 4 View Table 4 ), with the northern E. viridis samples showing a similar number of shared alleles with typical E. viridis as our samples of E. polybractea from the most northerly population of this species at West Wyalong. This leaves the simplest explanation as the most probable, in that these northern populations previously regarded as E. viridis var. latiuscula represent a currently unrecognised distinct entity that is sister to the core E. odorata complex rather than being closely related to typical E. viridis , which fits with the observation of morphological similarities to E. wimmerensis of seedlings from populations previously ascribed to this variety at Inglewood and Durakai in Queensland by Brooker et al. (2015).
Relationships between the few supported clades and the majority of samples in the core E. odorata complex are unsupported, despite the topology returned broadly reflecting geography. Only the taxa known from a single site, namely E. yarriambiack and E. filiformis , are supported as monophyletic ( Fig. 5 View Fig ), and because we have sampled all known wild individuals of E. filiformis , we can say with confidence on the basis of the lack of genetic differences between them, that the species represents a single clonal colony. Previously it has been noted that E. filiformis does not appear to not readily reproduce ( Rule 2004), although specimens grown at the Royal Botanic Gardens Victoria from seed show differences in adult morphology from the wild individuals ( Rule 2018). We have sampled one of these cultivated individuals ( PSF 73) and shown it to also be clonal, suggesting there may be occasional pollination occurring in the wild population and, for this individual at least, any morphological differences from the wild plants are not due to genetic differences.
Eucalyptus polybractea is polyphyletic in the phylogeny, although relationships among populations are not fully resolved, especially between the type population at West Wyalong (samples PSF 74 A and PSF 74 J) and the Victorian goldfields populations (samples PSF 21 C, PSF 27 A and PSF 27 H). Although our phylogenetic analyses suggest that the two samples from the Wimmera (samples PSF 56 B and PSF 56 C) are potentially conspecific with E. wimmerensis and the West Wyalong population is sister to the rest of the core E. odorata complex ( Fig. 5 View Fig ), in our network analyses collections from the Victorian goldfields and West Wyalong were more genetically similar to one another, and to E. yarriambiack and E. filiformis , than to the Wimmera samples ( Fig. 2 View Fig ). On the basis of the supported Flinders Ranges clade, which contains both E. polybractea and E. cajuputea samples ( Fig. 5 View Fig ), we suggest that E. polybractea should not be considered to occur west of the Murray Basin.
Although relationships among E. wimmerensis samples in the phylogeny remain unclear, they show little genetic differentiation, although the species’ boundaries remain unclear, given the uncertainty regarding the identity of the E. polybractea populations in the Wimmera. Although E. wimmerensis subsp. grata and Eucalyptus walshii were not genetically distinct from E. wimmerensis in our analyses, the PCA ( Fig. 3 View Fig ) and ABBA-BABA tests ( Table 4 View Table 4 ) suggested a low level of introgression from E. microcarpa , which may be responsible for the more robust stature of plants in these populations. The other species found only in the Wimmera and adjacent areas of SA, namely E. silvestris and E. hawkeri , both appear to represent more recent geneflow between E. wimmerensis and the co-occurring E. microcarpa on the basis of our ABBA-BABA and NewHybrid tests ( Table 4 View Table 4 ). This largely fits with the classifications of both Nicolle (2019) and Brooker et al. (2015), although those authors suggested E. odorata as being the E. odorata complex parent in the case of E. silvestris . We have some hesitancy ruling out this hypothesis, given our sampling, because we have shown that there is little genetic distinction between E. odorata and E. wimmerensis , and we cannot rule out there being populations in the Wimmera or adjacent areas of SA that better fit in E. odorata that we have not sampled.
The distinctions between the western taxa, E. cajuputea , E. odorata , and South Australian populations of E. polybractea , are unresolved in our study and require further investigation; however, it is clear what we have called E. polybractea in the Flinders Ranges has minimal genetic links to the typical E. polybractea of Victoria and NSW and may be best considered conspecific with E. cajuputea . Samples from the Flinders Ranges, identified as both E. cajuputea and E. polybractea , form a single clade in our phylogeny when the two aberrant samples are excluded ( Fig. 5 View Fig ), suggesting that there is a single lineage in this region that is distinct from other populations from west of the Murray Basin. The two aberrant samples, one each identified as E. polybractea and E. cajuputea , showed significant negative results for most comparisons in our ABBA-BABA tests ( Table 4 View Table 4 ), suggesting that introgression from a species we have not sampled is possible. The only other E. section Adnataria species that occur at Wilpena Pound and with which hybridisation may be occurring are E. porosa , which we have sampled and can therefore rule out, and E. intertexta R.T.Baker , which we have not sampled as part of this study. Eucalyptus intertexta ( E. series Buxeales ) is related to E. populnea and E. largiflorens , potentially explaining the single positive D -statistic with E. populnea in our ABBA-BABA tests, although the unresolved placement of the E. populnea sample in our phylogenies confounds this because we are unable to establish the relationship between this species and other members of E. series Buxeales . The mallee species E. leptophylla also co-occurs with the population these samples were sourced from and is superficially morphologically similar to members of the E. odorata complex, despite being in E. section Bisectae . This species may be the unknown parent, with its comparatively distant relationship to the E. odorata complex potentially explaining the significant negative D -statistics. Although we have a single sample of this species in our dataset, as part of the most divergent outgroup clade, we were not able to use it as an ingroup in ABBA-BABA tests to test this hypothesis because these require the inclusion of an outgroup with an evolutionary divergence point prior to the divergence of the three ingroup samples ( Durand et al. 2011). The other samples of E. cajuputea and E. odorata formed a polytomy along with the Flinders Ranges clade in the ML analysis, which may support a lack of distinctness of these two species outside the Flinders Ranges.
| MP |
Mohonk Preserve, Inc. |
| ML |
Musee de Lectoure |
| J |
University of the Witwatersrand |
| E |
Royal Botanic Garden Edinburgh |
| NSW |
Royal Botanic Gardens, National Herbarium of New South Wales |
| A |
Harvard University - Arnold Arboretum |
| C |
University of Copenhagen |
| H |
University of Helsinki |
| B |
Botanischer Garten und Botanisches Museum Berlin-Dahlem, Zentraleinrichtung der Freien Universitaet |
| SA |
Museum national d'Histoire Naturelle, Laboratiore de Paleontologie |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.