Coptotriche Walsingham, 1890
|
publication ID |
https://doi.org/10.11646/zootaxa.5333.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:CC8CEE25-A7BD-48B3-B315-B67FB455748C |
|
DOI |
https://doi.org/10.5281/zenodo.8269189 |
|
persistent identifier |
https://treatment.plazi.org/id/601087E8-FFE8-FFAA-989E-5F56FCE99695 |
|
treatment provided by |
Plazi |
|
scientific name |
Coptotriche Walsingham, 1890 |
| status |
|
1. Genus Coptotriche Walsingham, 1890 View in CoL View at ENA
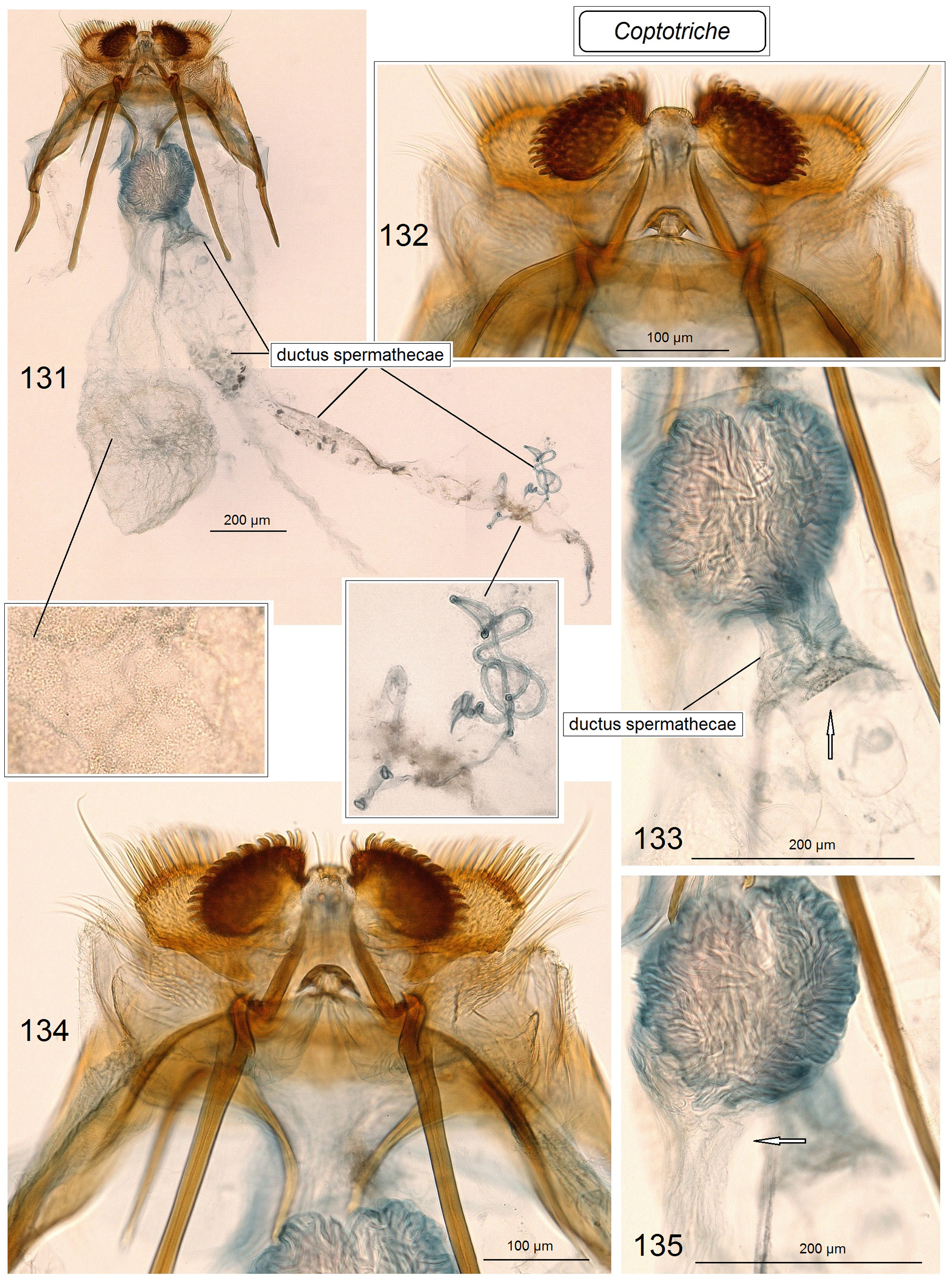
( Figs 80–142 View FIGURES 80–87 View FIGURES 88–98 View FIGURES 99–104 View FIGURES 105–113 View FIGURES 114–120 View FIGURES 121–125 View FIGURES 126–130 View FIGURES 131–135 View FIGURES 136–142 )
Coptotriche Walsingham, 1890: 322–323 View in CoL . Type species: Tischeria complanoides Frey & Boll, 1873: 220–221 View in CoL (junior syn. of T. zelleriella Clemens, 1859 View in CoL ).
Emmetia Leraut, 1993: 64–65 View in CoL .
Type species: Coptotriche marginea (Haworth), 1828: 556 View in CoL .
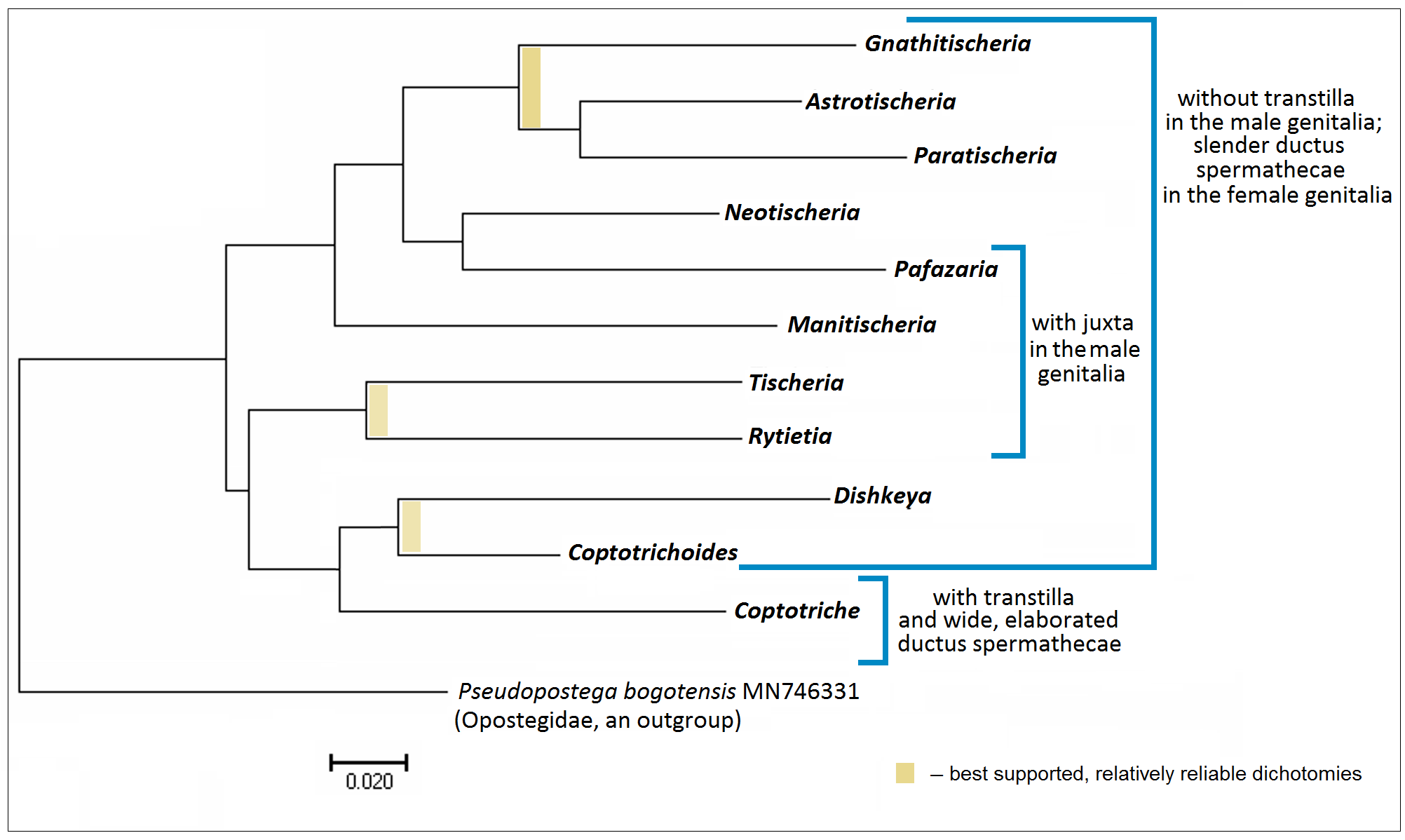
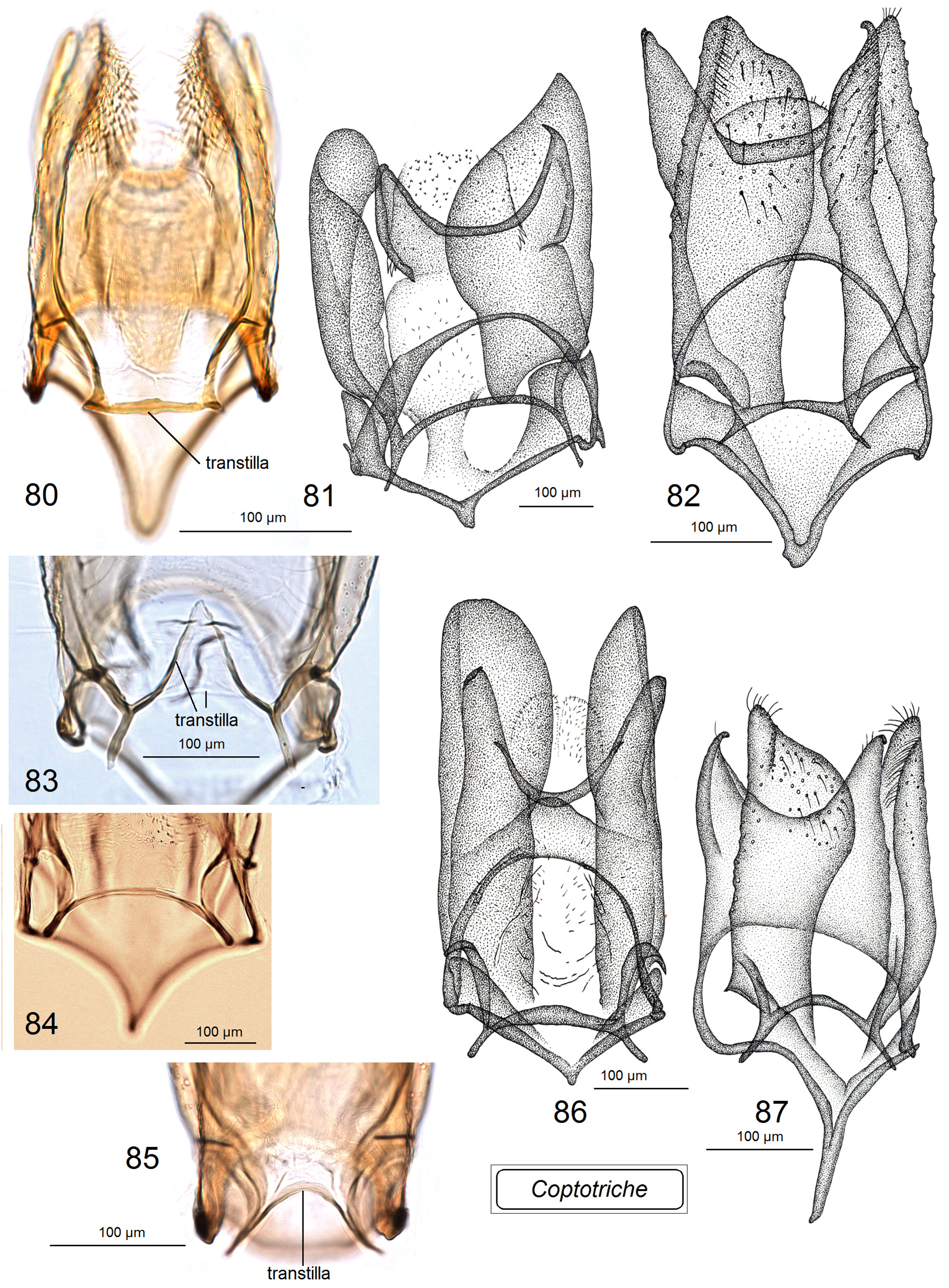
Diagnosis. In the male genitalia, species of Coptotriche are characterized by presence of a transtilla (the only Tischeriidae genus with a transtilla), wide valva, spined anellus, and a spined diaphragm of the tegumen; phallus is either tulip-shaped with numerous small or large spines or with two large apical lobes bearing a few spines. In the female genitalia, the genus is usually characterized by a strongly developed, heavily folded accessory sac, and a proximally wide, usually elaborated (twisted) ductus spermathecae. Fully developed leaf mines of Coptotriche are characterized by the folded margin of the mined leaf (folded leaf mine), except for some East and South East Asian species; a round nidus inside the leaf mine is inconspicuous. See Tabs 2 View TABLE 2 , 3 View TABLE 3 for occurrence of these diagnostic characters in other genera of Tischeriidae .
Notes. External characters of adults, including wing venation are not informative and, therefore, insufficient for differentiation of Coptotriche because of their general uniformity or, in some cases, variability of these characters within the entire family.
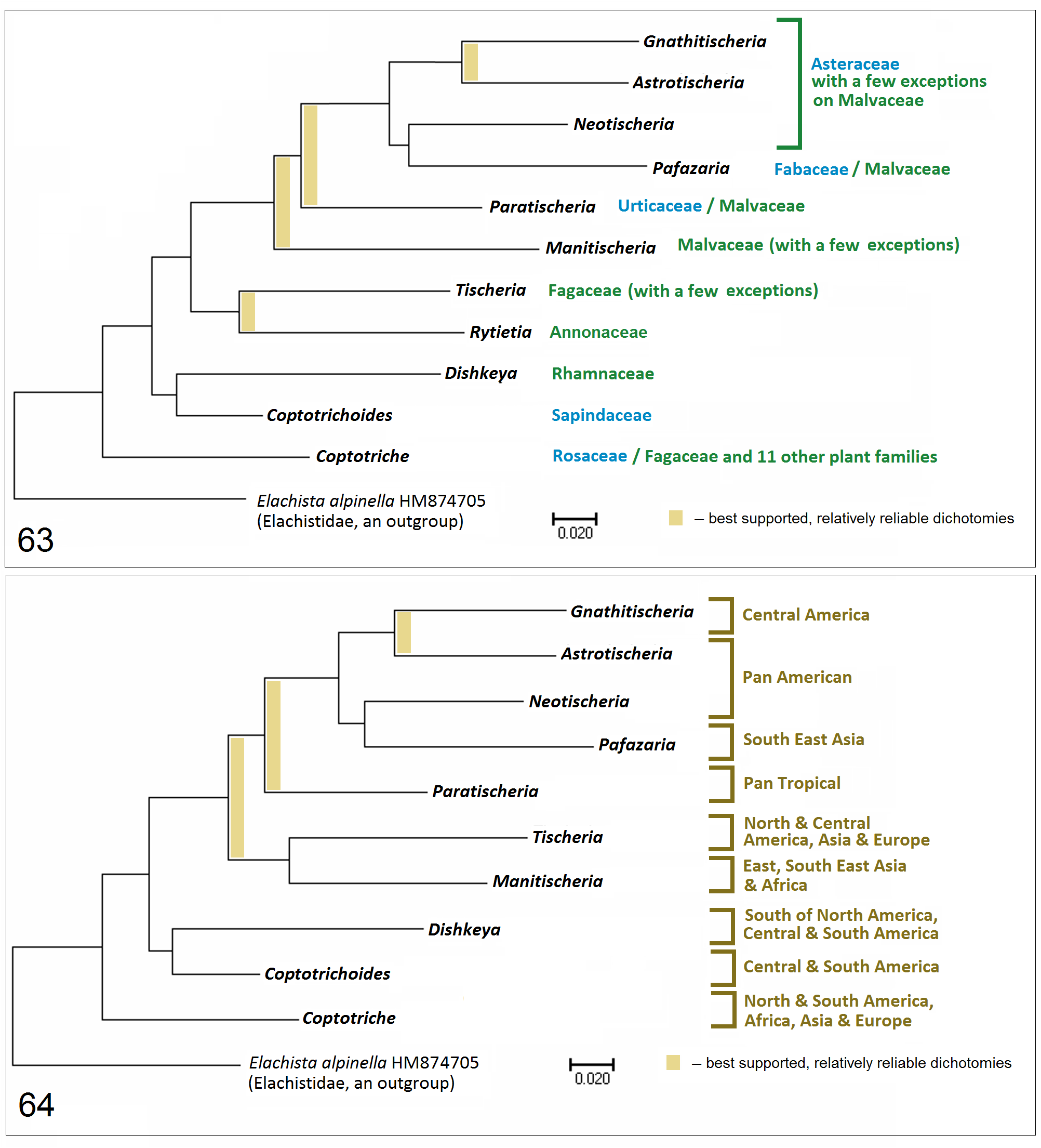
Molecular data provided relatively strong support for distinctness of this genus (see Discussion). Depending on the selected outgroup, Coptotriche is either the sister taxon to Coptotrichoides + Dishkeya ( Fig. 62 View FIGURE 62 ) or the most distinct, basal genus, separate from the rest of the Tischeriidae genera ( Figs 63, 64 View FIGURES 63, 64 ).
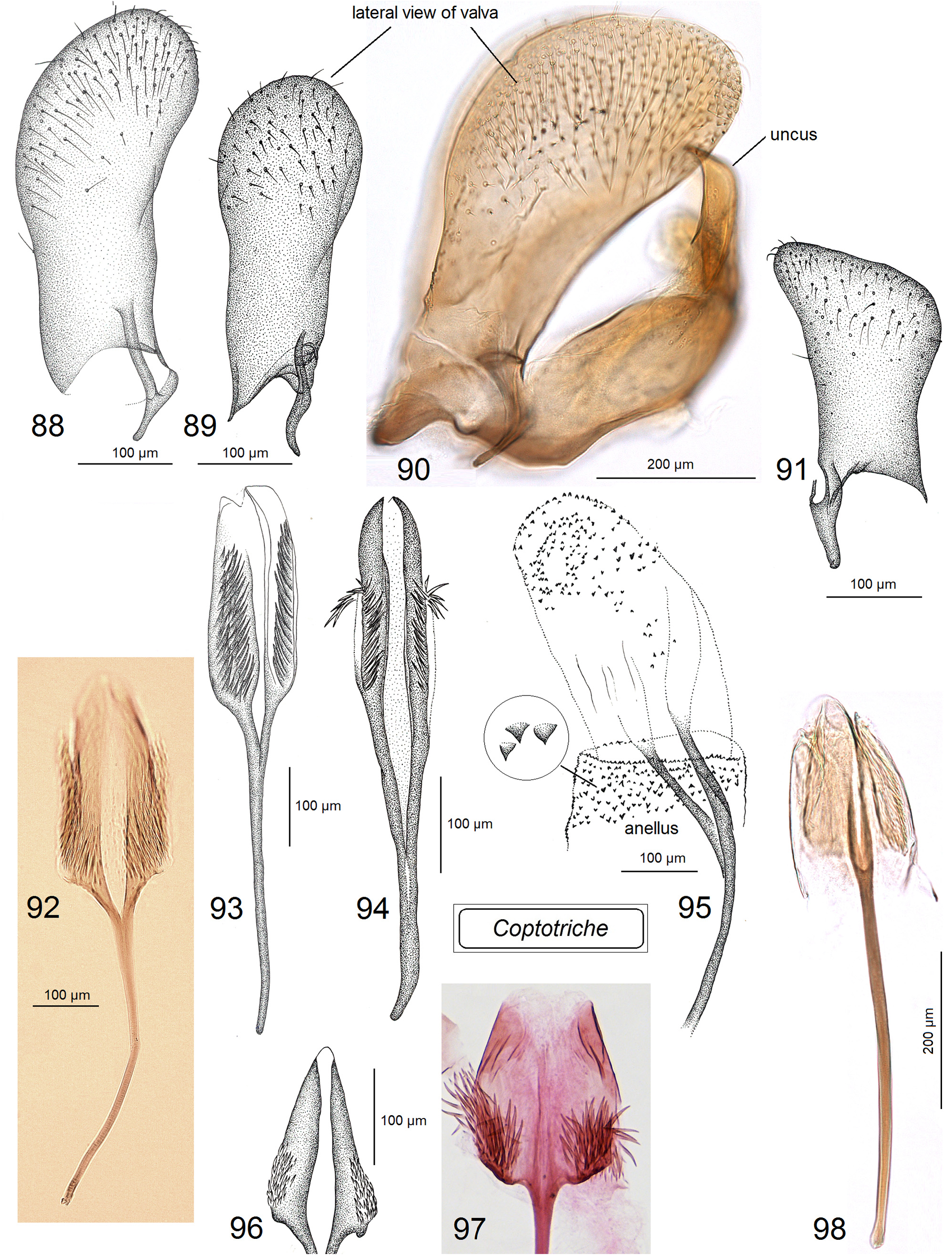
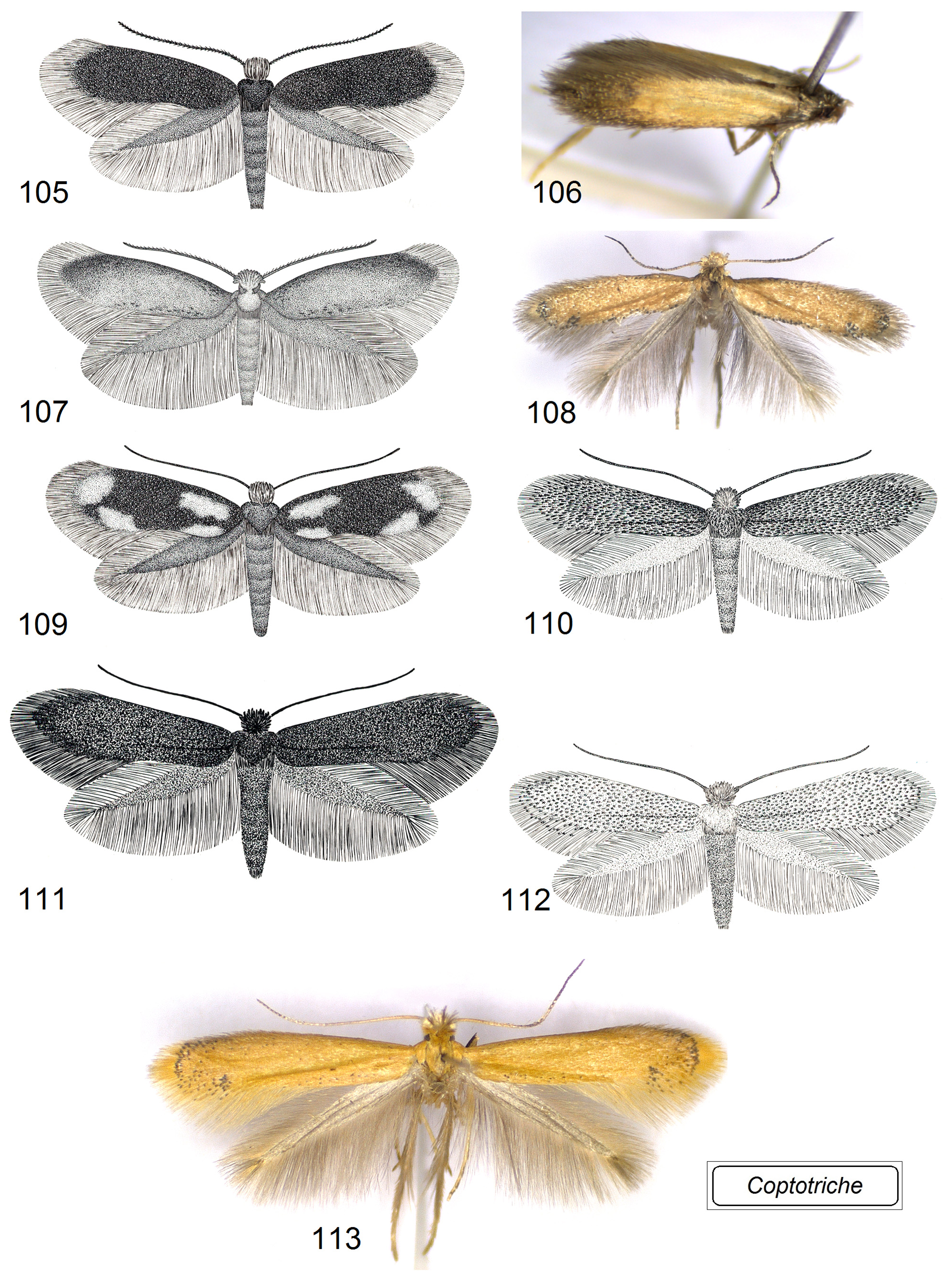
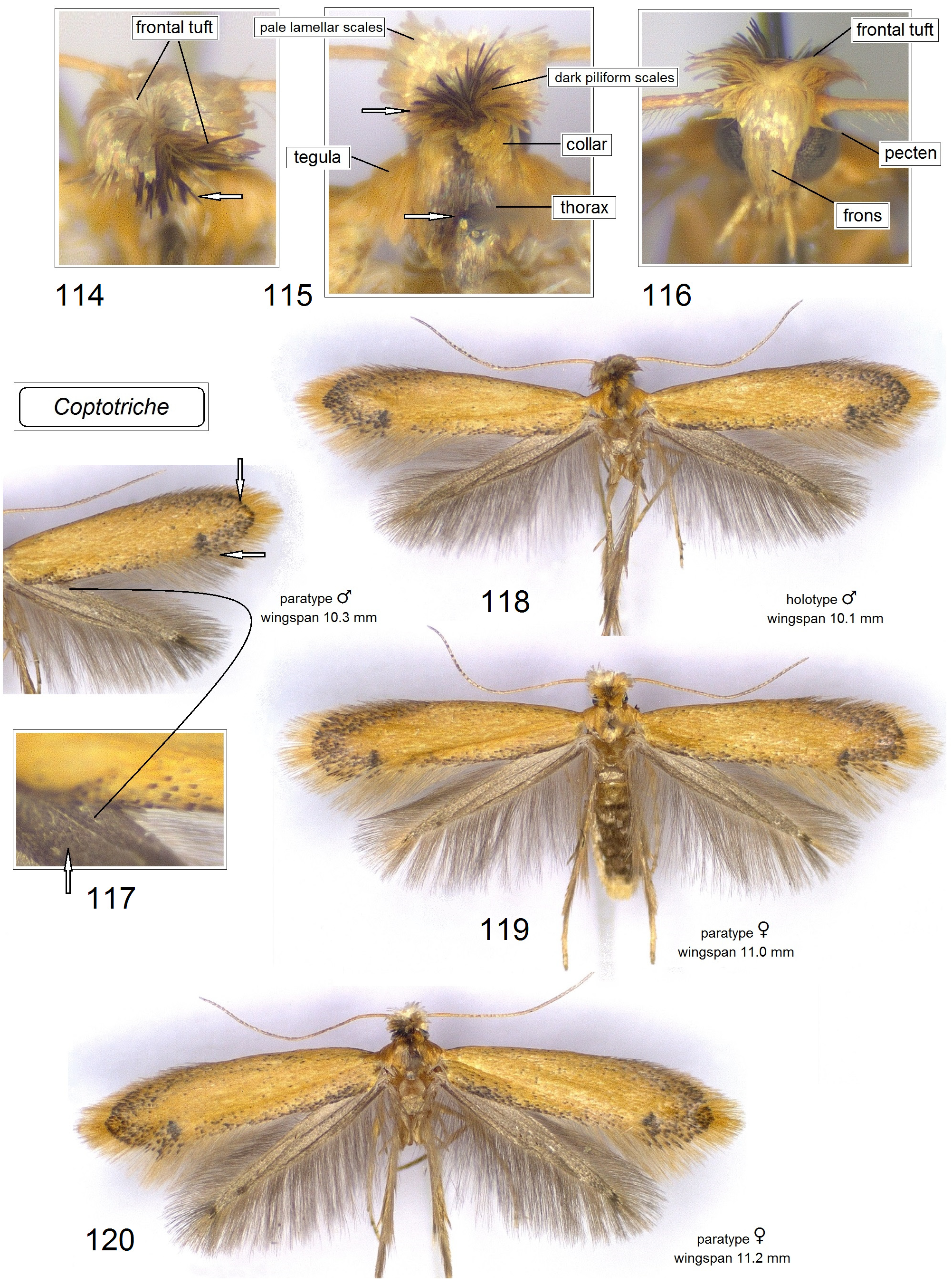
Adult ( Figs 105–120 View FIGURES 105–113 View FIGURES 114–120 ). Head: frontal tuft overlapping the frons, comprised of long, slender lamellar scales; sometimes frontal tuft is comprised of two different types of scales: pale, slender lamellar scales and dark, piliform scales ( Xu et al. 2021); occasionally frontal tuft is extremely long and comprised of slender lamellar scales, e.g., in Coptotriche imperator Puplesis & Diškus ( Diškus & Puplesis 2003) ; collar distinctly paired, comprised of slender lamellar scales. Forewing often with a small dark tornal spot; forewing color varies from almost uniform, yellowish ochre or dark ochre with some grey-black scales to entirely dark brown; occasionally forewing with four large, pale, irregular spots. Hindwing usually slender, occasionally significantly wider in males of a few species; male androconial scales in only a few species.
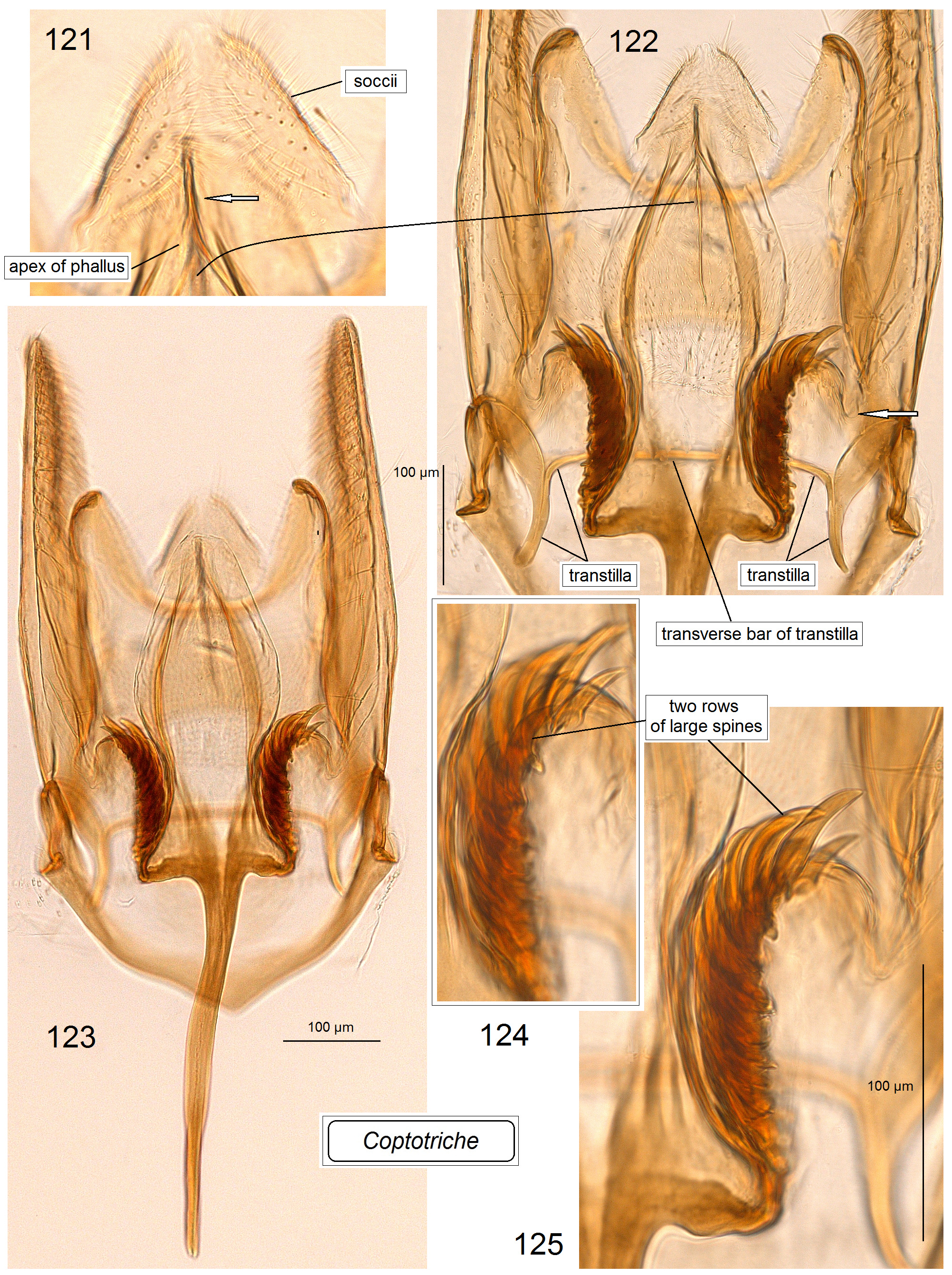
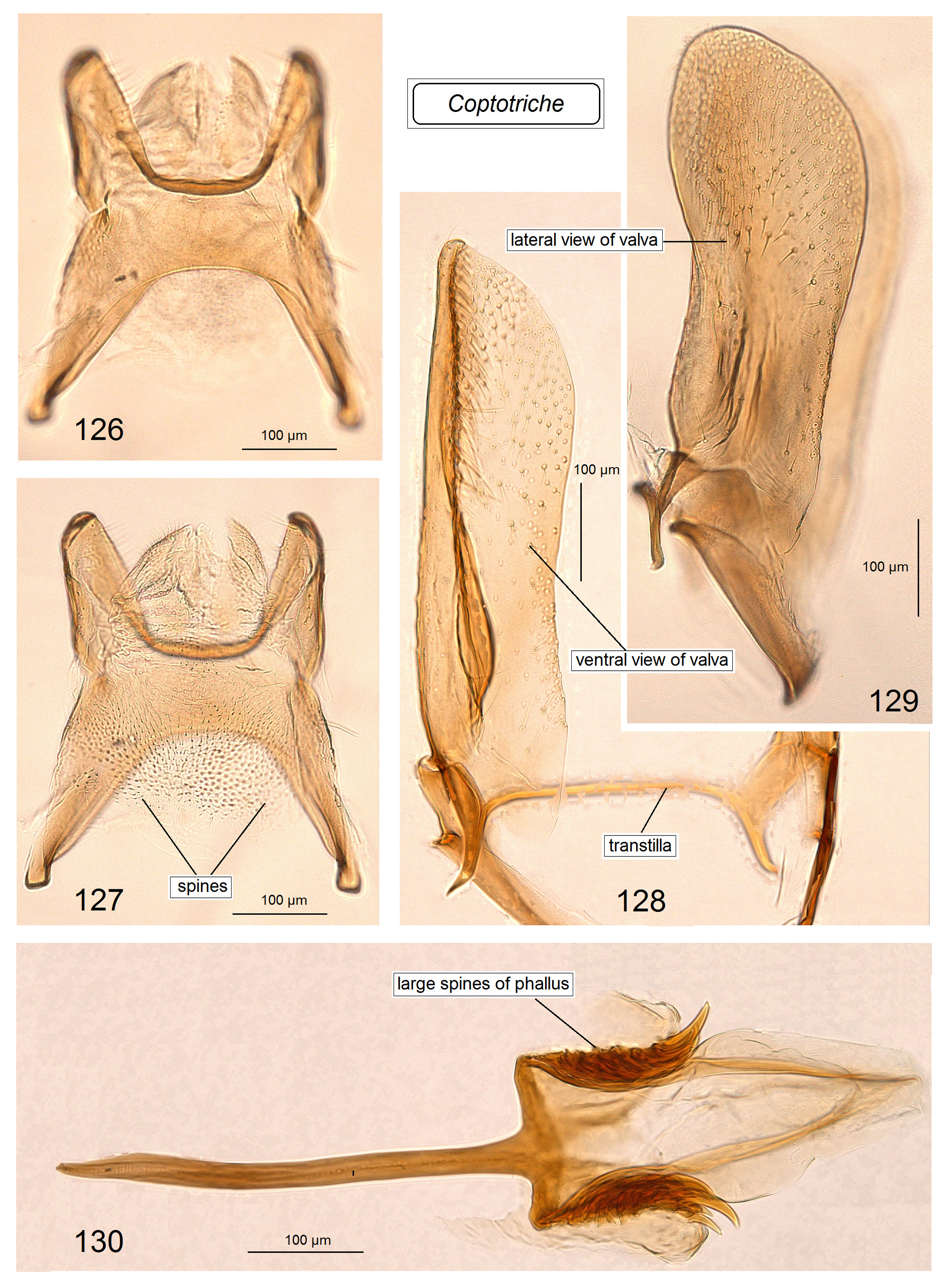
Male genitalia ( Figs 80–98 View FIGURES 80–87 View FIGURES 88–98 ). Uncus with two large (always long and basally wide) lateral lobes which look triangular in ventral view. Socii membranous, often large, usually weakly, occasionally distinctly paired, and always spinose, with numerous tiny spines. Tegumen short or very short, with a unique, distinctly spinose diaphragm; pseudognathos absent. In lateral view, valva usually very wide and rounded or truncated apically; basal process of the valva short; occasionally, e.g., C. basipectinella valva with a few distinctive pectens on basal half ( Puplesis et al. 2004). Transtilla present, always with a distinctive, long, or sometimes short transverse bar; sublateral processes of the transtilla relatively short; occasionally, transverse bar of the valva with a large triangular posterior lobe. Anellus membranous, ring or conus-shaped, usually with numerous small spines; juxta absent. Vinculum usually short or very short, with a band-like or triangular ventral plate; sometimes ventral plate with a short or long anterior process medially. Phallus usually tulip-like, with numerous spines on a wide apical lobe or with two apical lobes possessing two or four large spines; in some two lobed-phalli spines are minute and numerous or absent.
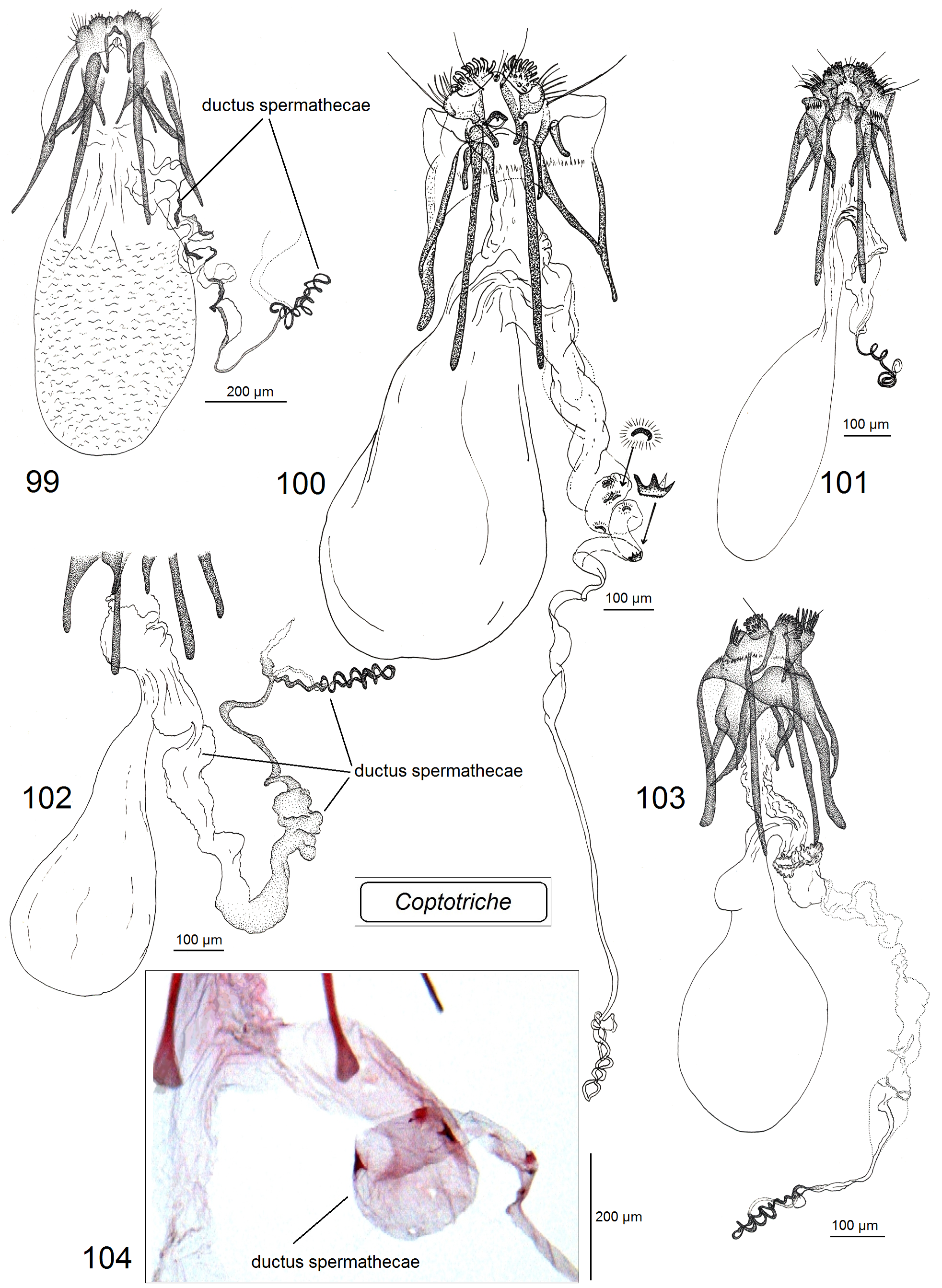
Female genitalia ( Figs 99–104 View FIGURES 99–104 ). Ovipositor lobes usually large or very large and usually covered with relatively large, robust modified setae, peg-setae; the gap between ovipositor lobes varies from relatively broad to narrow; second pair of ovipositor lobes two–three times smaller or equal in size in comparison to main ovipositor lobes; second pair of ovipositor lobes sometimes with long wide setae instead usual piliform chetae. Lateral lobes usually short, rarely long, and wide, occasionally absent. Apophyses often stout (robust), large and heavily chitinized. Anterior apophyses significantly to slightly shorter than posterior apophyses. Prela usually with three pairs of projections; inner pair with a slightly to strongly widened base, sometimes rod-like. Caudal sclerite strongly developed, inverted U-shaped, rounded caudally. Antrum absent. Accessory sac usually folded, slender; ductus spermathecae usually very wide and variously folded, sometimes with distinctive spines (pectinations) in proximal half, slender, usually with 0.5–2 coils (sometimes with many small, occasionally large coils) in distal half; vesicle often small, irregularly-shaped, occasionally large, oval-shaped. Corpus bursae varies from long to very short, gradually narrowing towards caudal end or with a short slender “neck”; occasionally slender part (the “neck”) is of the same length as the wider part; pectinations of corpus bursae absent, only occasionally cover whole corpus bursa.
Bionomics. The genus is characterized by the greatest host-plant diversity among Tischeriidae . However, more than a third of the currently known species have been reared from Rosaceae host plants, and almost a third from Fagaceae host plants; the remaining species are known to be feed on Combretaceae and Symplocaceae , also nine species feed on the following nine families (one species per family): Hypericaceae , Phyllanthaceae , Betulaceae , Staphyleaceae , Anacardiaceae , Nyssaceae , Ericaceae , Theaceae , and Apocynaceae . In total, 13 host-plant families are utilized by Coptotriche . Host-plant relationships of a third Coptotriche species are still unknown. Larvae mine leaves and produce irregular blotch-like (usually elongated) leaf mines; occasionally, initial part of the mine is gallery-like with a few short but slender lateral galleries. Frass is not deposited in leaf mines; occasionally very small amounts of scattered frass can be found in a leaf mine. Usually leaf mines are close to the leaf margin and the mining larva folds a margin of the mined leaf before pupation. The nidus is invisible through the epidermis, so dissection of the mine is necessary for study of the nidus.
Species diversity and geographical distribution. The total number of currently described Coptotriche species is 66. The majority are from the temperate regions: North America, Europe, and non-tropical Asia, including the Russian Far East and Japan. A substantial number of Coptotriche species have been described from South and South East Asia: C. compta (Meyrick) ( Meyrick 1915b) , C. imperator Puplesis & Diškus , C. inclinata Puplesis & Diškus , C. thailandi Puplesis & Diškus , C. terminaliae Puplesis & Diškus , C. bifurcula Puplesis & Diškus ( Diškus & Puplesis 2003) , C. camptotheca Xu & Dai ( Figs 97 View FIGURES 88–98 , 104 View FIGURES 99–104 ), C. turpinia Xu & Dai , C. asiana Diškus & Stonis ( Fig. 113 View FIGURES 105–113 ) ( Xu et al. 2021); one new South East Asian species, C. sapaensis Diškus & Stonis , sp. nov., is described in this publication (see below). Some species were recorded from South Africa: C. zimbabwiensis Puplesis & Diškus , C. africana Puplesis & Diškus ( Fig. 83 View FIGURES 80–87 ), C. basipectinella Puplesis & Diškus , C. versicolor Stonis, Diškus & Mey ( Puplesis & Diškus 2003; Stonis et al. 2019a), and one odd species, C. alavelona Lees & Stonis , from Madagascar ( Lees & Stonis 2007; Lees & Minet 2022). Recently, a few species of Coptotriche were also discovered to occur in Central America: C. forsteroniae Stonis & Diškus (Stonis & Diškus 2008) , and C. pulverea (Walsingham) (Stonis et al. 2008) , a species formerly known only from the Caribbean ( Walsingham 1897). A couple species were also discovered from South America: C. carmencita Stonis & Diškus from Peru and C. parvisacculata Diškus & Stonis ( Figs 85 View FIGURES 80–87 , 90, 98 View FIGURES 88–98 , 108 View FIGURES 105–113 ) from Argentina ( Stonis et al. 2019b).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Coptotriche Walsingham, 1890
| Stonis, Jonas R., Diškus, Arūnas, Remeikis, Andrius, Orlovskytė, Svetlana, Solis, Alma, Paulavičiūtė, Brigita, Xu, Jiasheng & Dai, Xiaohua 2023 |
Emmetia
| Leraut, P. 1993: 65 |
Coptotriche
| Walsingham, T. G. 1890: 323 |
Tischeria complanoides
| Frey, H. & Boll, J. 1873: 221 |