Cladoselache, Dean, 1894
|
publication ID |
https://doi.org/10.1206/0003-0090(2007)307[1:TBIPSA]2.0.CO;2 |
|
persistent identifier |
https://treatment.plazi.org/id/7D6087A8-6273-FFFD-98B1-FBF7FEDDFEB4 |
|
treatment provided by |
Tatiana |
|
scientific name |
Cladoselache |
| status |
|
GENERAL REMARKS: A comprehensive revision of the well known late Devonian shark Cladoselache is beyond the scope of the present work; it will merely be noted here that the systematics of Cladoselache are currently in disarray. The late Michael Williams (Cleveland Museum of Natural History) left notebooks in which he considered that Cleveland Shale Cladoselache represents two different but closely related genera, distinguishable mainly on the basis of postcranial features. However, until his unpublished findings can be analyzed and properly documented, Cladoselache is provisionally retained as a single genus.
Some investigators have separated Cladoselache from other sharks at ordinal level (e.g., Dean, 1909; Zangerl, 1981), whereas others have classified it alongside forms that are included here in the Symmoriiformes (e.g., Denaea ; Glikman, 1967). More recently, Coates and Sequeira (2001b) have presented competing phylogenetic analyses in which Cladoselache either is a plesiomorphic sister taxon to all symmoriiforms or is nested within a clade comprising symmoriiforms plus holocephalans. Although some morphological characters of the braincase were included in their analyses, these did not impact the alternative phylogenetic positions of Cladoselache they postulated.
In addition to the Cleveland Shale material, parts of a well-preserved three-dimensional cladoselachian shark have been described from the Chattanooga Shale of Tennessee ( Maisey, 1989). Although that specimen has provided important information about the visceral skeleton and musculature in cladoselachians, its cranium is poorly preserved and relatively uninformative.
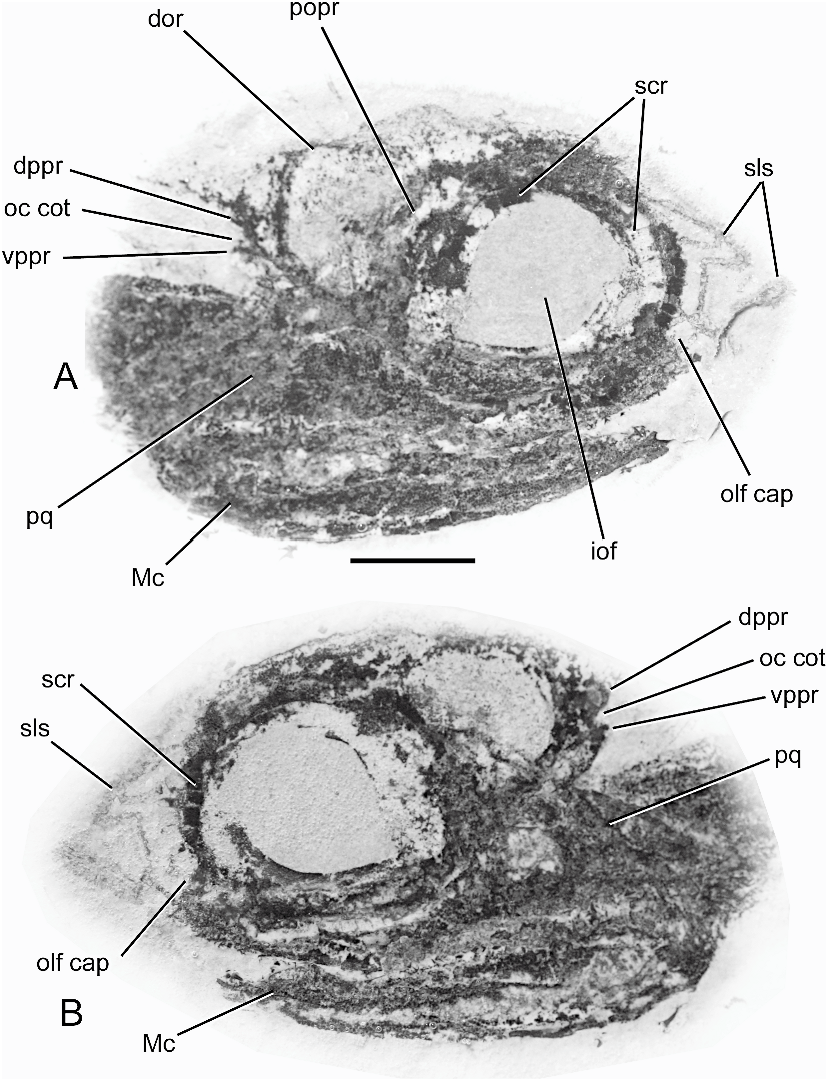
CRANIAL MORPHOLOGY: The braincase in Cladoselache is known mainly from two Cleveland Museum specimens described by Harris (1938), both of which were identified as C. kepleri (figs. 58, 59). These and other specimens were extensively prepared by Williams as part of an intended revision of Cladoselache , and now provide much more information on its cranial morphology. Unfortunately, all the specimens of Cladoselache in which the braincase is exposed are compressed dorsoventrally, and the original depth of the cranium is therefore difficult to estimate (as in Cobelodus aculeatus, Akmonistion , and Stethacanthus ). Well-preserved palatoquadrates of Cladoselache have a shallower otic process than in most symmoriiforms, suggesting that the orbit was not as deep as in those forms.
Several features of the braincase are remarkably similar in Cladoselache and symmoriiforms. One feature of particular interest is the presence of a single median fossa for the dorsal aorta below the occipital region. This fossa can be seen in at least two specimens (CMNH 5769, 5611; figs. 58, 60), and shows that the dorsal aorta was either unpaired as it entered the basicranium, or that paired vessels shared a single opening. Unlike in ‘‘ Cobelo- dus ’’, however, the aorta apparently divided almost immediately on entering the basicranium, since the fossa in CMNH 5769 contains a pair of openings that presumably contained paired aortae. The aortic pattern in Cladoselache and symmoriiforms differs from that in Paleozoic sharks such as Tamiobatis , Cladodoides , and Orthacanthus , in which the aortae had already separated behind the occiput and the paired aortic canals are widely spaced.
The arrangement of basicranial foramina in the floor of the otic region is also very similar in Cladoselache , ‘‘ Cobelodus ’’, and Cobelodus aculeatus . The canals for the lateral aortae emerge a short distance behind the postorbital arcade (at which point each aorta presumably gave rise to an internal carotid, orbital and efferent hyoidean branch, because the foramen for the orbital artery lies but a short distance from the aortic foramen). By contrast, in Paleozoic sharks such as Tamiobatis , Cladodoides , and Orthacanthus , the foramen for the lateral aorta and its orbital artery are farther apart, presumably leaving more of the arteries exposed beneath the braincase.
Other unusual features of the braincase shared by Cladoselache and symmoriiforms include: presence of a distinct ‘‘waist’’, or narrowing of the otic region behind the postorbital arcade; absence of a lateral otic process; an extremely wide but anteroposteriorly short postorbital arcade; an expansive jugular canal (e.g., CMNH 5769; fig. 58); and possibly the presence of a periotic process (e.g., CMNH 5611; fig. 60).
The dorsal surface of the braincase in Cladoselache has not been described previously, but parts of it are visible in CMNH 6233 (fig. 61). Features of interest include the presence of a notch in the lateral margin of the supraorbital shelf, similar to that found in Cobelodus aculeatus and Stethacanthulus meccaensis , a small median opening probably for the pineal organ, and a short occipital arch separated from the otic region by an otico-occipital fissure (the arch is either incomplete or was uncalcified dorsally). In this specimen, the roof of the otic region, the medial capsular walls, and the semicircular canals are either uncalcified or missing. The floor of the medullary region is therefore exposed, but the posterior dorsal fontanelle (which presumably lay above this area) cannot be observed.
A wide hypotic lamina is present (fig. 58), an important similarity with other elasmo-
(opposite side of specimen shown in Fig. 55 View Fig ). Scale bar 5 10 mm.
branchs. There is no evidence of a glossopharyngeal foramen in the basicranium, and the nerve probably left the braincase via the oticooccipital fissure. The otic region in Cladoselache and symmoriiforms is much shorter than in Tamiobatis , Cladodoides , and Orthacanthus . In addition, the occipital cotylus in Cladoselache and symmoriiforms is narrow from side to side, whereas it is much wider in forms such as Tamiobatis , Cladodoides , and Orthacanthus . The phylogenetic significance of these features is unclear because of their wider occurrence in other gnathostomes. Within chondrichthyans, however, a wide occipital cotylus and widely spaced aortic canals are unusual features that may represent apomorphic characters (possibly correlated with elongation of the otic region).
Cladoselache has separate, paired foramina for the anterior and posterior ramules of the palatine ramus. A separate foramen for the posterior ramule is also present in Cladodoides and perhaps in Tamiobatis vetustus ( Maisey, 2005: figs. 5, 35). However, in ‘‘ Cobelodus ’’ the passages for both palatine ramules open into the roof of the groove for the orbital artery (fig. 8), and a similar arrangement is possible in Cobelodus aculeatus , because there is no evidence of separate foramina for these ramules despite the fact that the orbital artery was enclosed by a canal. Both palatine ramules in C aculeatus may have accompanied the orbital artery throughout its canal, and may have accompanied the orbital artery within its groove in ‘‘ Cobelodus ’’. The presence of separate foramina for the palatine ramules therefore distinguishes Cladoselache from symmoriiforms.
A median basicranial fenestra and paired internal carotid foramina are present in Cladoselache , although it is not possible to determine whether these openings communicated with the cranial cavity or opened into the orbit as in ‘‘ Cobelodus ’’. There is a broad suborbital shelf, extending anteriorly as far as the orbital articulation. This articular surface may indicate the posterior end of the embryonic trabecular cartilage, as inferred in ‘‘ Cobelodus ’’ and Cladodoides ( Maisey, 2004a, 2005), suggesting that the polar cartilage made an extensive contribution to the basicranium in Cladoselache .
While Cladoselache appears to share several apomorphic cranial features with symmoriiforms, no definitive evidence of an interorbital septum has been detected (although one could be represented by a faint ridge in CMNH 5769; fig. 58). Some doubt therefore exists whether its braincase was tropibasic or platybasic. Cladoselache has customarily been classified in a separate family or even order (e.g., Dean, 1894, 1909; Zangerl, 1981), but the observations presented here suggest that such a remote classification is unjustified and instead support the phylogenetic hypothesis that Cladoselache is closely related to symmoriiforms ( Coates and Sequeira, 2001b).
DISCUSSION TROPIBASIA IN SYMMORIIFORMS
For many years, the only Paleozoic sharks in which the braincase was well known were Tamiobatis , Cladodoides , and Orthacanthus ( Eastman, 1897; Gross, 1937, 1938; Romer, 1964; Schaeffer, 1981; Maisey, 2005). All these forms have fundamentally identical cranial morphology, with a platybasic chondrocranium, wide ethmoidal and orbital regions, a stout postorbital process surrounding a moderately wide jugular canal, an elongate otic region in which the lateral otic process contains part of the posterior semicircular canal, and a moderately long occipital region separated from the rest of the braincase dorsally by a persistent oticooccipital fissure.
A few previous investigators nevertheless suspected the presence of an interorbital septum in symmoriiforms (e.g., Zangerl and Case, 1976; Williams, 1985). Zangerl and Case (1976: 120) stated that in their original (unpublished) reconstruction of the braincase in Cobelodus aculeatus (based entirely on compression fossils studied by means of stereo X-rays) ‘‘the medial walls of the orbits were assumed to be close to the sagittal plane, or actually formed an interorbital septum.’’ Evidently it was their subsequent misinterpretation of the three-dimensionally preserved specimen found afterward (FMNH PF 3090; figs. 37, 38) that caused them to change their interpretation and to place the orbits on either side of the cranial cavity in a ‘‘conventional’’ platybasic arrangement. Without the almost perfectly preserved three-dimensional ‘‘ Cobelodus ’’ braincase, it would be extremely difficult even now to recognize a deep interorbital septum in compression fossils of C. aculeatus .
Despite the impossibility of making ontogenetic observations in fossils, it can still be inferred on the basis of morphological features that the embryonic orbital cartilage in ‘‘ Cobelodus ’’ probably contributed to the entire upper half of the orbit and had a membranous or blastemic connection with the trabeculae at the approximate level of the optic foramen (fig. 2A). The orbital cartilages were entirely fused at the ventral midline, forming an interorbital septum that was poorly chondrified centrally but strongly calcified where it met the polar cartilage and parachordals. Although the position and extent of the embryonic trabeculae can only be inferred, the fact that the bucco-hypophyseal fenestra is separated from the cranial cavity suggests that the trabeculae were fused along much of the midline, probably forming an extensive trabecula communis to which the orbital cartilages were also fused. Thus, in ‘‘ Cobelodus ’’, the adult tropibasic condition probably arose from a tropitrabic embryonic one. The preoptic pila was incompletely calcified in ‘‘ Cobelodus ’’, but was apparently better developed in Falcatus and Stethacanthulus , where there is broad contact between the septum and postnasal wall. Farther posteriorly in the orbit, cartilage extending between the optic and ophthalmic foramina in part probably represents the metoptic pila. The ophthalmic foramen lies immediately above the optic pedicel centrally in the orbit, and the oculomotor foramen lies farther posteriorly above and even slightly behind the pedicel attachment area (forming the base of the antotic pila in modern elasmobranchs; see De Beer, 1931, 1937; Holmgren, 1940; El-Toubi, 1949).
Cartilage extending behind the pedicel includes the facial and trigeminal foramina, and is therefore regarded here as having formed in tissues derived both from the antotic pila and from secondary chondrification of the embryonic prootic fissure between the pila and the otic capsule (including the prefacial commissure). In ‘‘ Cobelodus ’’, the polar cartilage probably contributed to approximately one-third of the orbit floor, i.e., it was slightly less extensive than in Cladodoides (fig. 2B). The pituitary vein presumably lay within an extracranial subpituitary space between the posterior ends of the trabecular-polar cartilage and the parachordals as in other gnathostomes ( Allis, 1928).
The positions of the efferent pseudobranchial artery, optic pedicel, and pituitary vein in both ‘‘ Cobelodus ’’ and Cladodoides suggest that the polar cartilage made an extensive contribution to the braincase. By contrast, in modern elasmobranchs (and in gnathostomes generally), the polar cartilage is comparatively small and the trabeculae commonly extend into the posterior part of the orbit (fig. 1). Thus, despite many obvious differences between the neurocrania of ‘‘ Cobelodus ’’ and Cladodoides , early cranial development in both forms was apparently characterized by hypertrophy of the polar cartilage and confinement of the trabeculae to the anterior half of the orbit. Features that are typically located in the posterior part of the orbit in modern Notorynchus lie farther anteriorly in Cladodoides (e.g., the oculomotor foramen, bucco-hypophyseal chamber, optic pedicel, efferent pseudobranchial foramen, and palatobasal articulation; Maisey, 2005: fig. 27). ‘‘ Cobelodus ’’ resembles Cladodoides in all these respects, suggesting that hypertrophy of the polar cartilage and related forward displacement of features in the orbit is a derived developmental condition shared by Cladodoides and ‘‘ Cobelodus ’’.
These observations suggest that the unusual morphology of the neurocranium in ‘‘ Cobelodus ’’ resulted from at least two distinct evolutionary modifications to the presumably conserved pattern of cranial development seen in modern elasmobranchs. The first step involved hypertrophy of the polar cartilage, and probably arose in sharks in which the adult braincase was still morphologically platybasic (e.g., Cladodoides , Tamiobatis , Cladodus ; Maisey, 2005; Ginter and Maisey, 2007). The second step (in symmoriiforms such as ‘‘ Cobelodus ’’) involved deepening and expansion of the orbit and development of an interorbital septum (presumably involving extensive fu- sion of the embryonic trabeculae to form a trabecula communis along the entire length of the orbit), accompanied by an anterior shift of the ventral arm of the lateral commissure (at least relative to its position in Cladodoides ). Thus, hypertrophy of the polar cartilage may have been a precursor condition to the tropibasic adult condition in symmoriiforms. The combined effect of these ontogenetic changes apparently resulted in a crowding together of the embryonic metoptic and antotic pilae. Consequently, much of the interorbital septum in symmoriiforms was presumably supported by (or formed in) the polar cartilages on either side of the hypophyseal chamber, and also by cartilage derived from the antotic pila farther dorsally. The anterior part of the interorbital septum either did not chondrify or remained uncalcified, but was probably connected to the trabeculae by membranous tissue.
Comparison with Recent actinopterygians suggests that the tropibasic adult morphology found in symmoriiforms such as ‘‘ Cobelodus ’’ involved altered ontogenetic trajectories of the trabeculae, which became more extensively fused at the ventral midline and formed a trabecula communis that extended to meet the orbital cartilages (tropitrabic condition), resulting in the development of deep orbits that are barely separated medially (probably correlated with relatively large eye size and the related upward displacement of the brain). However, there are significant differences in the inferred extent of the antotic pila and polar cartilage of ‘‘ Cobelodus ’’ and in actinopterygians. In the latter, loss of the antotic pila and reduction or loss of the polar cartilage may be correlated with the development of the posterior myodome. By contrast, in ‘‘ Cobelodus ’’ the antotic pila is apparently retained (though possibly merged with the metoptic pila), and a posterior myodome is not developed. In addition, the polar cartilage in ‘‘ Cobelodus ’’ seems to have been much more extensive than in actinopterygians and osteichthyans generally.
The efferent pseudobranchial artery in osteichthyans does not traverse the space between the polar and trabecular cartilages as in elasmobranchs ( De Beer, 1924, 1937; Holmgren, 1943), and an optic pedicel is absent in all modern osteichthyans. Unlike in elasmobranchs, therefore, these landmarks cannot be used to identify the former extent of the polar cartilage (although the site of the foramen for the pituitary vein may similarly indicate its posterior margin). A pedicel attachment site has been identified in several early osteichthyans (e.g., Ligulalepis , Psarolepis , Achoania , Stylolepis ; Basden et al., 2000; Basden and Young, 2001; Zhu et al., 1999; Zhu et al., 2001; Zhu and Yu, 2002), immediately behind the optic foramen and anterodorsal to the foramen for the pituitary vein (fig. 62). Perhaps significantly, in early osteichthyans where the oculomotor foramen has been identified (e.g., Psarolepis , Ligulalepis ; Yu, 1998; Basden and Young, 2001), it is positioned directly above the presumed pedicel attachment area (i.e., as in sharks). Despite the profoundly different course of the efferent pseudobranchial artery with respect to the polar and trabecular cartilages in elasmobranchs and osteichthyans, the topo- graphic and developmental relationships of the antotic pila and polar cartilage are remarkably similar. If the optic pedicel, polar cartilage, and trabeculae represent evolutionary novelties of the prechordal part of the neurocranium in gnathostomes, then fusion of the pedicel to the embryonic antotic pila just above the dorsal margin of the polar cartilage and immediately anterior to the basisphenoid pillar may represent a conserved gnathostome pattern that arose prior to the divergence of osteichthyans and chondrichthyans.
SCLERAL OSSICLES
Scleral ossicles are present in many osteichthyans (including tetrapods) as well as in placoderms, but ossicles are absent in modern chondrichthyans although the scleral cartilage may form a capsule ( Walls, 1942). The only chondrichthyans in which scleral ossicles have been identified are extinct and all date from the Paleozoic (e.g., Falcatus , Damocles , Cladoselache ), suggesting that these structures were lost early in chondrichthyan evolution and never reappeared. The absence of bone in the scleral capsule of chondrichthyans is in all probability related to the acquisition of tesselated endoskeletal calcification (replacing endoskeletal bone?), since the capsular cartilage is calcified prismatically (like much of the endoskeleton) in modern sharks such as Lamna . By contrast, the absence of capsular bone in most osteichthyans (other than teleosts; Franz- Odendaal and Hall, 2006) probably represents a true reduction in ossification.
In modern amniotes, scleral ossicles develop intramembranously in dermal (neural crest) tissue forming the scleral mesenchyme ( Franz-Odendaal and Hall, 2006 and references therein), and the same developmental history can be postulated for these ossicles in chondrichthyans such as Falcatus . By contrast, the scleral cartilage is formed directly in ectomesenchymal tissue and can be cartilaginous (e.g., sarcopterygians, Lamna ), membranous (e.g., actinopterygians), or bony (e.g., osteostracans, placoderms). The presence of scleral ossicles has been regarded as a gnathostome synapomorphy ( Maisey, 1986; Donoghue et al., 2000), although today these structures occur only in osteichthyans ( Latimeria , tetrapods and teleosts) and are absent in modern dipnoans ( Kemp, 1999; Franz-Odendaal and Hall, 2006). Scleral ossicles are nevertheless present in many extinct basal sarcopterygians ( Jessen, 1966; Schultze, 1973; Janvier, 1996) and actinopterygians (e.g., Edinger, 1929; Walls, 1942; Lund, 2000). Paradoxically, scleral ossicles in acanthodians seem to be restricted to a highly derived clade including Cassidiceps and acanthodiforms ( Hanke and Wilson, 2004: character 2).
In some osteostracans and placoderms, the scleral cartilage forms an ossified cup surrounding the eye, to which a dermal sclerotic component is fused (e.g., Tremataspis , Dicksonosteus ; Janvier, 1981, 1985, 1996; Burrow et al., 2005), but in many arthrodires (as well as in petalichthyids and antiarchs), the scleral cartilage was apparently unossified and separate ossicles are present (e.g., Holonema , Brachydeirus , Dunkleosteus , Lunaspis , Bothriolepis ; Gross, 1932, 1961; Miles, 1971). Fusion between the scleral ossicles and cartilage therefore seems to be restricted phylogenetically to stem-group gnathostomes such as osteostracans and placoderms (it has not been recognized in any living or fossil crown-group gnathostome). This distribution led Franz-Odendaal and Hall (2006: fig. 5) to propose that a fully ossified capsule may represent the phylogenetically primitive gnathostome condition. However, it is far from clear whether the propensity for the optic capsule to be extensively and/or heavily ossified represents a cladistically primitive or derived condition, given its disjunct phylogenetic distribution within craniates (and especially among placoderms). In this regard, it is of interest that a strong developmental relationship exists between the scleral cartilage and the corneal-scleral limbus (where the ossicles are formed), because the scleral cartilages of teleosts and birds and the collagenous sclera of mammals first appear as a ring adjacent to the limbus before spreading posteriorly around the eye ( Coulombre and Coulombre, 1958; Franz-Odendaal and Hall, 2006). A similar development can be postulated for placoderms and osteostracans, except that the scleral and capsular ossifications probably co-ossified at the corneal-scleral limbus. Such co-ossification has a relatively restricted systematic distribution, and could therefore be secondary from both a developmental/ontogenetic and a phylogenetic perpective. Thus, absence of fusion between the scleral ossicles and cartilage may represent a highly conserved condition in crown group gnathostomes.
A RECONSTRUCTION OF THE JAWS IN ‘‘ COBELODUS ’’
The three-dimensional ‘‘ Cobelodus ’’ cranium provides an opportunity to model a hypothetical set of mandibular and hyoid arches and replicate their original shape and orientation, using other symmoriiforms as a guide (fig. 63). Although the visceral skeleton is not preserved in ‘‘ Cobelodus ’’, the palatoquadrates of Cobelodus aculeatus , Stethacanthulus meccaensis , and Symmorium reniforme all have a high postorbital flange, suggesting a deep and round orbit as in ‘‘ Cobelodus ’’. Unfortunately, almost all the comparative material is flattened and distorted, obscuring the original curvature of the jaw ramus as well as the three-dimensional shape of individual cartilages.
The spatial dimensions and orientation of the orbital and postorbital articulations in the three-dimensional cranium provided the principal constraints for the reconstruction presented here. The curvature of the palatine rami and Meckel’s cartilage below the ethmoid region was based on comparison with other elasmobranchs, but also followed the simple assumption that the tooth-bearing regions of both jaws had to be approximately parallel to each other in order for the teeth to occlude properly and provide a continuous biting surface (a factor that is overlooked in many previous reconstructions of extinct elasmobranchs). The outward swing of the jaw rami toward the mandibular joint was also based on comparison with other elasmobranchs, but in addition was constrained by the requirement of having the paired mandibular joints aligned along a single transverse axis of rotation. Flat templates were scaled and shaped according to these criteria, then photographed and digitally superimposed on a lateral view of the braincase.
In the reconstruction, with the postorbital and orbital articulations aligned, it is possible to fit the hyomandibula between the periotic process and mandibular joint (allowing for outward curvature of the jaw ramus), although the absence of a definite hyomandibular facet and variation in the position of the hyomandibular head in articulated symmoriiform fossils makes its position in ‘‘ Cobelodus ’’ highly conjectural (hyomandibular support for the mandibular arch in symmoriiforms is itself a controversial matter; see below). In ‘‘ Cobelodus ’’, the mandibular joint lies some distance behind the occipital region, but the otic region is short and there is little space behind the orbit for the origin of maxillary levator or first dorsal constrictor musculature.
The postorbital articulation is said to be laterally directed in some symmoriiforms, suggesting that it was covered by part of the postorbital arcade ( Zangerl and Case, 1976; Williams, 1985; Coates and Sequeira, 1998). However, in ‘‘ Cobelodus ’’, the postorbital articulation is clearly positioned on the posterior surface of the arcade, and it is difficult to imagine how it could meet the lateral surface of the palatoquadrate. If such a laterally directed surface was present in ‘‘ Cobelodus ’’, it may have formed a secondary articulation or an attachment surface for ligaments connecting the palatoquadrate to the postorbital arcade above their primary articulation, or it may have acted as an insertion for tendons running from an adductor γ muscle (if present). The palatoquadrate in Falcatus and Damocles probably made broad contact with the postorbital arcade and the attachment may have been reinforced by ligaments (figs. 54–57). In Notorynchus , ligaments holding the postorbital articulation preclude any anterior shift- ing of the jaws and no translation is possible, any motion being restricted to the plane of the articular surface ( Luther, 1908; Wolfram, 1984). The same was probably true in Paleozoic sharks with a postorbital articulation, but motion may have been further restricted in ‘‘ Cobelodus ’’ and other symmoriiforms if additional ligamentous connections were present dorsal to the articulation (e.g., extending from the otic process to the vertical platform of the postorbital arcade).
Zangerl and Case (1976) determined that the palatoquadrate flange and the postorbital arcade in Cobelodus aculeatus were in close proximity to each other for a considerable distance above and below their actual articulation. The otic flange of the palatoquadrate therefore extends some distance above the primary articular surface. The articular surface for the palatoquadrate is also situated ventrolaterally in Stethacanthulus meccaensis ( Williams, 1985: fig. 6), and includes a lateral ‘‘pocket’’ where it articulates with the postorbital arcade. The postorbital arcade in Symmorium reniforme is comparatively stout and triangular in dorsal view, with a thickened transverse keel on its posterior border ( Williams, 1985). The arcade is also comparatively long anteroposteriorly in Akmonistion zangerli ( Coates and Sequeira, 1998) . A postorbital articulation was probably present in Denaea fournieri , but no details are known ( Fournier and Pruvost, 1922).
A reconstruction of the main mandibular musculature is presented here (fig. 64). Some parts of the branchiomeric musculature are difficult to reconstruct in extinct sharks because the skeleton lacks muscle insertion sites like those of osteichthyans. Comparison with modern elasmobranchs ( Luther, 1908; Wilga, 2005) is helpful only at a very general level, even in forms that superficially resemble early sharks (e.g., hexanchiforms, Chlamydoselachus , Heterodontus ). In Notorynchus , the palatoquadrate levator and first dorsal constrictor originate at the level of the dorsal otic ridge ( Luther, 1908; Daniel, 1934: fig. 92), and this was probably also the case in ‘‘ Cobelodus ’’. The palatoquadrate levator of Notorynchus originates on the lateral wall of the otic capsule just behind the postorbital process and in front of the spiracular canal. From here, the muscle descends anteriorly and meets the palatoquadrate in the posterior part of the orbit. The anterior extent of the palatoquadrate levator is constrained by the position of the mandibular ramus of the trigeminal nerve. In modern elasmobranchs, the ramus invariably passes in front of the palatoquadrate levator ( Luther, 1908). In ‘‘ Cobelodus ’’, this ramus probably traverses the jugular canal and postorbital arcade, and it is therefore unlikely that the palatoquadrate levator extended into the orbit (the palatoquadrate levator lies also entirely behind the postorbital arcade in Squatina , where the ventral part of the lateral commissure is as strongly chondrified as in many Paleozoic sharks).
The shortness of the otic region in ‘‘ Cobelodus ’’ suggests that the second (hyo- mandibular levator) and subsequent dorsal constrictors probably originated entirely on a fascia of the dorsal epaxial musculature (in modern sharks, these generally arise from a fascia above the gill clefts as well as from the extrabranchial cartilages). A palatoquadrate spiracular notch has been reported in some symmoriiforms ( Lund, 1985b), and it has therefore been assumed in the reconstruction that a spiracular canal was present between the first and second dorsal constrictors, oriented almost vertically between the palatoquadrate and periotic process and opening on the top of the head just behind the postorbital arcade.
The extent of the mandibular adductor musculature is fairly evident from the size and extent of the adductor fossa on the palatoquadrate and Meckel’s cartilage. Most of the dorsal adductor muscle mass in ‘‘ Cobelodus ’’ presumably lay behind the postorbital arcade, and the dorsal (palatoquadrate) insertion area is much larger than the ventral (meckelian) one. Unfortunately, in ‘‘ Cobelodus ’’ there is no way to determine the presence or the extent of smaller muscles usually associated with the mandibular adductor in modern elasmobranchs (e.g., adductor γ or superficial adductor, preorbital/ suborbital, labial levator). The inclusion of an adductor γ in the reconstruction is highly speculative. In Notorynchus , this muscle originates near the mandibular joint and is connected to the tip of the postorbital process by a tendon ( Luther, 1908). According to Allis (1923), Chlamydoselachus has an adductor γ, but this is absent according to both Luther (1908) and Shirai (1992); the latter found only tendons and connective tissue in its expected position.
The preorbital muscle has been omitted from the reconstruction presented here although one may have been present. In primitive modern sharks, the preorbital muscle is not usually strong and commonly arises on the postnasal wall or ectethmoid process, inserting on the fascia of the main adductor muscle near the corner of the mouth (e.g., Chlamydoselachus ). By contrast, in many dalatiiforms with a cutting-type dentition (sensu Cappetta, 1987), the muscle is massive and its origin has shifted posteriorly into the orbit (e.g., Etmopterus , Squaliolus ). However, the preorbital muscle is absent in the highly specialized dalatiid Trigonognathus (which has an unusual clutching-type dentition and highly protrusible jaws; fig. 4C). In advanced modern lamniforms, the preorbital muscle has separate dorsal and ventral heads ( Wilga, 2005). If a preorbital muscle was present in ‘‘ Cobelodus ’’, it is unlikely to have arisen on the interorbital region as in modern Squaliolus , or to have extended below the postorbital arcade to insert on the mandibular adductor or lower jaw, because such a course would have been obstructed by the ventral part of the postorbital arcade. Probably, any preorbital muscle would have been rather weak.
Other symmoriiforms display a slightly different postorbital arcade morphology from ‘‘ Cobelodus ’’, and may therefore have differed in the orientation and position of the postorbital articulation. In some forms, the postorbital articular fossa for the palatoquadrate has been described as being approximately midway along the arcade (e.g., Damocles, Akmonistion ; Lund, 1986; Coates and Sequeira, 2001a: fig. 3). In others, the facet is apparently closer to the dorsal margin of the otic flange (e.g., CM 23654, referred to Stethacanthus cf. S. productus ; Lund, 1985b: fig. 1). If such variation can be confirmed, it would suggest considerable variation in the origin, insertion, and distribution of the mandibular musculature that may reflect different feeding strategies and preferences in symmoriiforms.
THE ANTERIOR PALATOQUADRATE ARTICULATION
A detailed discussion of the anterior palatoquadrate articulation has already been presented elsewhere ( Maisey, 2005), and only a few points will be added here. Gegenbaur (1872) considered that the anterior articular surface in modern elasmobranchs corresponded to the palatobasal articulation in osteichthyans. However, Goodrich (1909: 414) suggested that the ‘‘orbito-palatine’’ articulation in modern hexanchiform sharks could either represent a palatobasal articulation (5 ‘‘basal’’ articulation sensu Huxley, 1876) that had migrated anteriorly, or an anterior (palatine) articulation that had migrated posteriorly. While he considered the former view more plausible (‘‘the fact that it articulates really with the basal or trabecular and not antorbital region of the skull is strong evidence that it is a true basal process’’), he also noted: ‘‘the anterior articulation of the palatoquadrate seems to be very far forward in such early forms as the Acanthodii and Pleuracanthini …. Further study of the fossils may enable this point to be decided.’’
Watson’s (1937) subsequent study seemed to settle the issue (at least for acanthodians). He considered that no basal process was present in sharks, and that the absence of a palatobasal articulation in elasmobranchs was an important difference from Acanthodes . He therefore distinguished the anterior process of elasmobranchs as an orbital process. Miles (1965, 1973) reached essentially the same conclusion and interpreted Acanthodes with an osteichthyan-like palatobasal (basipterygoid) process. However, Holmgren (1943) and Jarvik (1977, 1980) argued that the articulation in Acanthodes is homologous with the orbital articulation in squaloids and hexanchiforms, but not to the palatobasal articulation of osteichthyans. On the other hand, De Beer (1931) and Gardiner (1984a) have both argued that the elasmobranch orbital and osteichthyan palatobasal articulations are morphological homologs (a view originally espoused by Huxley, 1876) because they have the same topographical relationship to the lateral head vein, the palatine ramus of the facial nerve, and the embryonic polar cartilage.
El-Toubi (1949: 262), defended Watson’s (1937) position that no palatobasal articulation is present in sharks, and argued that the orbital process in Squalus changes its position relative to the trabecular cartilage during ontogeny and ‘‘ does not articulate with a fixed point in all developmental stages’’. However, during earlier developmental stages when the palatoquadrate orbital process is located farther anteriorly, it has only blastemic connection to the cranium and lacks any real articulation. Although the palatoquadrate in Squalus and Etmopterus shifts posteriorly in ontogeny, the articular surface formed for it on the trabecular cartilage does not shift ( Holmgren, 1940: figs. 55, 67, 79, 81), and is located at the posterior end of the trabeculae from its first inception (like the basitrabecular process in osteichthyans; Goodrich, 1930).
By contrast, the anterior palatoquadrate attachment in Heterodontus and galeomorphs is associated with the lateral or anterolateral part of the trabecular cartilage, and the palatoquadrate connects with the ethmoidal region ( Holmgren, 1940). There is no corresponding articular surface in more advanced galeomorphs although ethmopalatine ligaments are often present ( Wilga, 2005). In Mitsukurina and Carcharias these ligaments are paired, and in Alopias they are accompanied by an unpaired palatonasal ligament, but in other lamnids only a median ligament is present. However, these ethmopalatine and palatonasal ligaments are always connected to the anterior or antrolateral part of the trabecular region, farther anteriorly than the palatobasal articulation in other gnathostomes but corresponding topographically to the anterior articulation in Heterodontus and galeomorphs. Nevertheless, Wilga (2005: 114, fig. 6) inferred that the ethmopalatine ligament in galeomorphs is homologous to the sleeve-like ligament associated with the orbital articulation in hexanchiforms, despite the different positions of these attachments. The implied homology between these ligaments suggests that the orbital articulation was secondarily lost, but that the ligamentous part of the attachment become relocated farther anteriorly on the lateral margin of the trabeculae. However, even if the ethmopalatine attachment in galeomorphs is homologous only to the ligamentous part of the orbital articulation in orbitostylic sharks (and to the corresponding part of the palatobasal articulation in other gnathostomes), the ethmoidal articulation in Heterodontus and orectoloboids is most parsimoniously interpreted as a novel feature, perhaps correlated with their unusually massive and divided preorbital muscle that is oriented vertically and inserts onto Meckel’s cartilage rather than on the mandibular adductor as in other sharks ( Smith, 1942; Compagno, 1977; Motta and Wilga, 1999).
THE POSTORBITAL ARTICULATION
In modern hexanchiform elasmobranchs, there is a postorbital articular surface on the primary postorbital process (fig. 65A–C). However, there is no continuous postorbital arcade and hexanchiforms lack a chondrified lateral commissure ( Holmgren, 1941). By contrast, the postorbital articulation in many Paleozoic sharks is located farther ventrolaterally than in hexanchiforms, presumably on the distal part of the chondrified lateral commissure (fig. 65D, E). Furthermore, the articulation in hexanchiforms is lateral to the exit of the otic lateral line nerve (5 classical ‘‘otic ramus of the trigeminal nerve’’) and dorsal to the lateral head vein whereas, in Paleozoic sharks such as ‘‘ Cobelodus ’’ and Cladodoides , the articulation is ventrolateral to the presumed position of the otic lateral line nerve and the lateral head vein.
Despite strong disagreement between phylogenetic hypotheses based on molecular and morphological analyses of crown group elasmobranchs (mainly involving the placement of batoids; Maisey et al., 2004), there is consensus that hexanchiforms (the only extant elasmobranchs with a postorbital articulation) do not lie at the base of the group. A postorbital articulation is also absent in hybodonts, the putative extinct sister group to modern elasmobranchs ( Maisey et al., 2004). From a phylogenetic perspective, therefore, amphistyly (sensu Goodrich, 1909) in hexanchiforms appears to be a cladistically derived condition. The alternative possibility (that a postorbital articulation was primitively present in crown-group elasmobranchs and was lost independently numerous times in nonhexanchiforms) is considerably less parsimonious.
The postorbital articulation in hexanchiforms cannot therefore be considered primitive, based on morphological and phylogenetic evidence. This conclusion has important implications for those evolutionary hypotheses of jaw suspension in elasmobranchs in which the amphistylic condition in hexanchiforms has been regarded as a cladistically primitive condition from which other patterns supposedly arose (e.g., Wilga, 2005: fig. 7).
HYOMANDIBULAR INVOLVEMENT IN JAW SUSPENSION
In modern batoids and orbitostylic sharks (sensu Maisey, 1980), the hyomandibular facet is located on the posterolateral wall of the otic capsule, immediately in front of the exit of the glossopharyngeal nerve (in batoids and Pristiophorus , there are frequently two articular fossae for the hyomandibula; Holmgren, 1941). The hyomandibular facet sometimes extends farther anteriorly (e.g., Squatina ), and occasionally extends along the entire otic region (e.g., Orectolobus ). Howev- er, in Heterodontus , Chiloscyllium , and advanced galeomorphs, the facet occupies only the anterior part of the otic region ( Holmgren, 1941).
The hyomandibular articulation in extinct sharks is usually located on the posterior part of the capsule and is often associated with the lateral otic process (e.g., Orthacanthus , Tamiobatis vetustus , ‘‘ Tamiobatis sp. ’’, Egertonodus basanus , Tribodus limae ; Schaeffer, 1981; Maisey, 1983; Maisey and Carvalho, 1997). Akmonistion is so far the only symmoriiform in which a lateral otic process has been identified ( Coates and Sequeira, 2001a). A more anterior position for the articulation has been suggested in Tristychius arcuatus ( Dick, 1978: figs. 7, 9) and Synechodus dubrisiensis ( Maisey, 1985) .
‘‘ Cobelodus ’’ lacks a distinct hyomandibular facet, suggesting that the hyomandibula may only have been attached by ligaments (possibly to the periotic process). In other symmoriiforms, a comparatively weak ligamentous connection is also suggested by the extremely variable position of the hyomandibular head. For example, in Falcatus falcatus and Damocles serratus , the hyomandibula extends as far as the postorbital arcade and is mesial to the palatoquadrate postorbital articulation ( Lund, 1985a, 1986), a quite different configuration from that described in Akmonistion ( Coates and Sequeira, 2001a). In Cobelodus aculeatus , the hyomandibular head has been described in various positions; according to Zangerl and Williams (1975), it reached the postorbital arcade, but according to Zangerl and Case (1976) it only extended about halfway along the side of the otic capsule.
There is considerable variation in the relative proportions of the hyomandibula and ceratohyal in symmoriiforms. It is only 50% as long as the ceratohyal in Akmonistion zangerli ( Coates and Sequeira, 2001a: 443) , but in Cobelodus aculeatus it is approximately 60% as long. However, according to Williams (1985: 98), the hyomandibula in Stethacanthulus meccaensis is much shorter (around 33% of ceratohyal length). The hyomandibula in both Falcatus falcatus and Damocles serratus is at least as long as the otic ramus of the palatoquadrate (Lund, 1885a: fig. 8; 1986: fig. 4). In D. serratus , the hyomandibula is considerably longer than the ceratohyal (the length of the ceratohyal has not been determined in F. falcatus ). The hyomandibula extends at least halfway along the otic region in the Pennsylvanian Arkansas symmoriiforms (i.e., approximately as far as the periotic process in ‘‘ Cobelodus ’’), but CT scans show that the hyomandibular head lies some distance from the neurocranium.
Collectively, these observations suggest that the hyomandibula in symmoriiforms was only attached loosely to the neurocranium and that the attachment position was more variable than in most elasmobranchs. A short hyomandibula obviously will not extend as far along the otic region as a longer one, but its attachment to the neurocranium is also governed in vivo by the angular relationship of the hyomandibula to the neurocranium, and by the width of the jaw ramus at the mandibular joint. If the jaws curved outward posteriorly (as in most modern elasmobranchs), even a comparatively long hyomadibula might reach only the posterior part of the otic region. Furthermore, if the hyomandibula was held in place by ligaments, it need not have extended the full distance between the mandibular joint and the ligamentous attachment site, and would easily become displaced during fossilization, possibly accounting for much of the positional variation.
It has been suggested that Cobelodus aculeatus was aphetohyoidean (with a full gill slit opened between the mandibular and the hyoid arches, as had been postulated earlier for Acanthodes by Watson, 1937), supposedly providing empirical support for Gegenbaur’s (1872) theory that a fully developed hyoidean gill cleft represents a primitive evolutionary stage for gnathostomes ( Zangerl and Williams, 1975; Zangerl and Case, 1976; Zangerl, 1981). However, the interpretations on which those proposals were based have been challenged both in Acanthodes ( Miles, 1968) and in Paleozoic sharks ( Maisey, 1984, 1989). Zangerl and Williams (1975) claimed that there was no connection between the epihyal and the mandibular joint in C. aculeatus ; however, such a connection is also absent in modern hexanchiforms, which are clearly not aphetohyoidean and have a spiracle as well as a ligamentous connection between Meckel’s cartilage and the ceratohyal ( Devillers, 1958). Zangerl and Williams (1975) also considered that the relative dimensions of the mandibular and hyoid arch elements supported their aphetohyoid interpretation, but that argument has also been refuted ( Maisey, 1989: 185). Nevertheless, a hyomandibular articulation has not been recognized in Cobelodus aculeatus ( Zangerl and Williams, 1975; Zangerl and Case, 1976) and no strong evidence for one exists in ‘‘ Cobelodus ’’, although one may be present in other symmoriiforms (e.g., Falcatus, Akmonistion ; Lund, 1985a; Coates and Sequeira, 1998). Furthermore, if the symmoriiform palatoquadrate referred to Stethacanthus by Lund (1985b: fig. 5) has a spiracular notch as he suggested, the hyoidean gill cleft was presumably reduced as in modern elasmobranchs.
It has also been argued that the hyoid arch in chimaeroids is ‘‘unmodified’’ (as interpreted by De Beer and Moy-Thomas, 1935) and that it provides neontological support for the aphetohyoid condition. In fact, this arch displays several specialized features ( Maisey, 1984), including: the lateral rather than medial position of the ‘‘pharyngohyal’’ relative to the efferent artery (corresponding to the batoid pseudohyal; Watson, 1937); the ‘‘pharyngobranchial’’ is bypassed by the subspinalis muscle and has no insertion for any interpharyngobranchial muscle ( Daniel, 1934); closure of the hyoidean gill slit and absence of a spiracular pseudobranch; absence of epihyal rays; and absence of hyoid adductor muscles. Additionally, the afferent vascular supply (and water flow) through the pseudobranch of gnathostomes is ‘‘reversed’’ ( Laurent and Dunel-Erb, 1984), providing increased oxygenation of arterial blood flowing to the brain via the efferent pseudobranchial artery. Although the pseudobranch is absent in modern chimaeroids, the ‘‘pseudobranchial’’ artery provides the principal blood supply to the brain because the internal carotids are aborted during ontogeny; De Beer and Moy- Thomas, 1935). The hyoid arch in modern chimaeroids therefore displays a suite of apomorphic features contradicting its interpretation as ‘‘unmodified’’, and in some respects the hyoid arch of elasmobranchs is actually more primitive (e.g., presence of a pseudobranch, absence of a ‘‘pharyngohyal’’ lateral to the efferent artery). The relationship of the chimaeroid ‘‘pharyngohyal’’ to surrounding vessels and muscles strongly supports its interpretation as a morphological novelty. No conclusive evidence for an ‘‘unmodified’’ hyoid arch has been found in gnathostomes, and the aphetohyoid condition is entirely conjectural.
The posterior postorbital process in placoderms (e.g., Dicksonosteus ; Goujet, 1984) is typically associated with attachment areas for the branchial arches, but the hyomandibular facet is positioned much farther anteriorly (on the anterior postorbital process). The osteichthyan parampullary process typically develops on the lateral wall of the posterior ampulla rather than over the external semicircular canal ( Gardiner, 1984a). In modern and extinct osteichthyans, the first branchial arch often articulates with (or is attached by ligaments to) the parampullary process or the opisthotic (e.g., Polypterus , Polyodon , Latimeria , Eusthenopteron , Kansasiella , Mimia , Styloichthys ; Jarvik, 1954; Poplin, 1974; Gardiner, 1984a; Zhu and Yu, 2002). The osteichthyan opisthotic forms in the ventrolateral wall of the otic capsule, surrounding the external semicircular canal as well as the lower part of the posterior canal (including the ampulla), and it also forms the posterior part of the hyomandibular facet. The posterior postorbital process in placoderms such as Dicksonosteus may be homologous to the parampullary process in osteichthyans, and it is also possible that this process (plus the area extending anteriorly to the hyomandibular facet) corresponds to the opisthotic in osteichthyans ( Gardiner, 1984a). However, the posterior postorbital process in placoderms and the parampullary process in osteichthyans are not involved in hyomandibular support, and there is no compelling evidence to suggest homology between either of them and the periotic process in ‘‘ Cobelodus ’’, nor with the lateral otic process in Tamiobatis and Orthacanthus .
CONCLUSIONS
1. Tropitrabia and platytrabia are regarded as ontogenetic conditions that are frequently the precursors to the corresponding adult state (tropibasia and platybasia), but there are important exceptions. Ontogenetic data are unavailable for fossils and the majority of extant species of gnathostomes, but morphological features associated with platybasia and/or tropibasia may provide clues about the precursor ontogenetic condition.
2. The braincase in ‘‘ Cobelodus ’’ and some other Paleozoic symmoriiform sharks is morphologically tropibasic, but the extent to which this characterizes symmoriiforms generally is uncertain. The braincase of Cobelodus aculeatus was misinterpreted as platybasic by Zangerl and Case (1976). The braincase in most modern elasmobranchs is morphologically platybasic (although ventral downgrowths of cartilage below the embryonic trabeculae are present in some etmopterids), but may be tropibasic in Squaliolus . The extensive ‘‘medial area’’ of the anterior basicranium develops early in ontogeny; therefore, if it is regarded as part of the trabeculae, modern sharks may be characterized as tropitrabic. No morphological evidence of an interorbital septum can be recognized in placoderms, but the presence of separate paired ethmoidal ossifications in ptyctodonts suggests that there was a median preorbital cartilaginous area that may have formed in the trabecula communis.
3. The embryonic polar cartilage in ‘‘ Cobelodus ’’ appears to have contributed extensively to the prechordal basicranium, as in Cladodoides . Tropibasia in ‘‘ Cobelodus ’’ may therefore have been superimposed on an already unusual pattern of cranial morphology. Although tropibasia in osteichthyans (especially actinopterygians) is very similar to the pattern found in ‘‘ Cobelodus ’’, their polar cartilage is generally small or even absent.
4. The postorbital arcade in ‘‘ Cobelodus ’’ is unusual in several respects. The lateral commissure is extremely wide and surrounds a much larger jugular canal than in most other elasmobranchs. The ventral attachment of the arcade apparently extended to the ventrolateral border of the polar cartilage, instead of meeting the ventrolateral wall of the otic capsule as in modern elasmobranchs.
5. The basicranial arterial circuit in ‘‘ Cobelodus ’’ must have been considerably modified, because the internal carotids could not have communicated with the cranial cavity via the bucco-hypophyseal chamber. These arteries either were absent or they met the efferent pseudobranchials within the orbits before entering the cranium. Nevertheless, the arterial circuit inferred in ‘‘ Cobelodus ’’ represents a modification of the primitive elasmobranch pattern and differs profoundly from that of osteichthyans, in which the combined internal carotid/efferent pseudobranchial enters the cranial cavity via the basisphenoid pillar and not via the orbit.
6. The epiotic process in ‘‘ Cobelodus ’’ is located farther anteriorly than the lateral otic process of many other Paleozoic sharks. It is uncertain whether these processes are homologous to each other, although both may be associated with the hyomandibular attachment.
7. The labyrinth of the inner ear is similar to that of Cladodoides , modern chimaeroids, and osteichthyans, and lacks the specializations found in modern elasmobranchs and extinct hybodont sharks. In this respect, the inner ear in ‘‘ Cobelodus ’’ conforms to the generalized crown-group gnathostome condition.
8. The occipital region in ‘‘ Cobelodus ’’ is short, but contains the same number of spino-occipital canals (five) as in Cladodoides , where the occipital region is much longer. The number of canals is probably underreported in most fossil sharks, but there is no obvious correlation between the length of the occipital region and the number of spino-occipital canals. The configuration of the occipital region in ‘‘ Cobelodus ’’ differs from that in Cladodoides and other Paleozoic sharks in several respects. Its occipital cotylus is comparatively small and is elevated above surrounding structures. The cotylus is also reinforced by paired dorsal and ventral paroccipital processes. Nevertheless, in ‘‘ Cobelodus ’’ there is a persistent otico-occipital fissure, regarded as a conserved crown-group gnathostome feature shared with several other Paleozoic chondrichthyans and with primitive osteichthyans.
9. Cranial endocasts of ‘‘ Cobelodus ’’ and Cladodoides suggest that the tegmentum was elongated above the dorsum sellae and the hypothalamus was displaced anteriorly. A comparatively straight mesencephalon is also inferred. Presence of a tight ‘‘S’’ in the mesencephalon around the posterior commissure is a potentially apomorphic character of modern elasmobranchs that may be shared with hybodonts and xenacanths.
10. The braincase in Cladoselache shares some apomorphic characters with ‘‘ Cobelodus ’’, although their phylogenetic significance is still uncertain, including: division of the dorsal aorta below or in front of the occipital region; comparatively anterior location of foramina for the lateral aortae between the postorbital processes; a distinct narrowing of the otic region behind the postorbital arcade; absence of a lateral otic process and (possibly) presence of a periotic process; an extremely wide but anteroposteriorly short postorbital arcade; and an expansive jugular canal. In addition, Cladoselache , Cobelodus aculeatus , and Stethacanthulus meccaensis possess an unusual notch in the lateral margin of the supraorbital shelf, although this seems to be absent in ‘‘ Cobelodus ’’ and several other symmoriiforms. However it has not been determined whether Cladoselache was tropibasic. Cladoselache has a wide hypotic lamina, an important but previously unrecognized character shared with other elasmobranchs. Cladoselache and symmorii- forms primitively share a comparatively short otic region and a narrow occipital cotylus. The wide occipital cotylus and widely spaced aortic canals in Tamiobatis , Cladodoides , and Orthacanthus may be apomorphic features of the otic region related to hypertrophy in width as well as length.
11. The postorbital articulation in hexanchiforms is probably a derived feature. The postorbital articulation in Paleozoic sharks is not located on the primary postorbital process as in hexanchiforms, but on cartilage presumably formed in the lateral commissure. In ‘‘ Cobelodus ’’, there is evidence of an additional attachment area (possibly for ligaments) on the posterolateral surface of the postorbital arcade, above the actual postorbital articulation. This may help explain previous reports of a laterally directed postorbital ‘‘articulation’’ in other symmoriiforms. Such extra bracing of the symmoriiform postorbital articulation is envisaged as a modification of the primitive postorbital articulation.
12. No hyomandibular facet has been identified in ‘‘ Cobelodus ’’, but this does not constitute evidence that it was aphetohyoidean (with a complete hyoidean gill slit and ‘‘unmodified’’ hyoid arch). Previous suggestions that symmoriiforms were aphetohyoidean are probably unfounded, although the epihyal in ‘‘ Cobelodus ’’ may have been only loosely bound to the cranium by ligaments (possibly attached to the periotic process).
13. The systematics and classification of symmoriiform sharks are in disarray. Symmoriiforms collectively are probably monophyletic, but the only group to present clearly apomorphic characters within the order is the family Falcatidae . The traditional distinction between ‘‘symmoriids’’ and ‘‘stethacanthids’’ is probably invalid, because it has never been convincingly demonstrated that ‘‘brushless’’ (presumably female) ‘‘stethacanthids’’ existed, or that ‘‘symmoriids’’ are anything but female ‘‘stethacanthids’’. Sex-linked dimorphism of the spine-brush complex has been demonstrated convincingly only in falcatids. The enigmatic Permian shark Dwykaselachus may be a symmoriiform rather than a close relative of modern sharks as suggested by Oelofsen (1986). The braincase in Cladoselache shares some unusual features with ‘‘ Cobelodus ’’ that suggest a relationship with symmoriiforms.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Cladoselache
| Maisey, John G. 2007 |
Stethacanthulus meccaensis
| FMNH PF 2621 |
Damocles serratus
| Lund 1986 |
Damocles serratus
| Lund 1986 |
D. serratus
| Lund 1986 |
Cobelodus
| Zangerl 1973 |
Cobelodus
| Zangerl 1973 |
Cladoselache
| Dean 1894 |
Cladoselache
| Dean 1894 |
Cladoselache
| Dean 1894 |
Falcatus falcatus
| falcatus (St. John and Worthen 1883 |
Falcatus falcatus
| falcatus (St. John and Worthen 1883 |
F. falcatus
| falcatus (St. John and Worthen 1883 |