Lamprima Latreille, 1804
|
publication ID |
https://doi.org/ 10.11646/zootaxa.4446.2.1 |
|
publication LSID |
lsid:zoobank.org:pub:4B7A9974-CD3A-4BF5-9062-E48D73F3CADF |
|
DOI |
https://doi.org/10.5281/zenodo.5963652 |
|
persistent identifier |
https://treatment.plazi.org/id/9A0C87C8-FFDB-FF98-FF4B-FF3BFE63FE48 |
|
treatment provided by |
Plazi |
|
scientific name |
Lamprima Latreille, 1804 |
| status |
|
Lamprima Latreille, 1804 View in CoL
Lamprima Latreille, 1804a: 150 View in CoL .
Type species. Lethrus aeneus Fabricius, 1792 , by monotypy.
Neolamprima Gestro, 1875: 997 View in CoL ; Nagel 1922: 16 (synonymy).
Type species. Neolamprima adolphinae Gestro, 1875 , by monotypy.
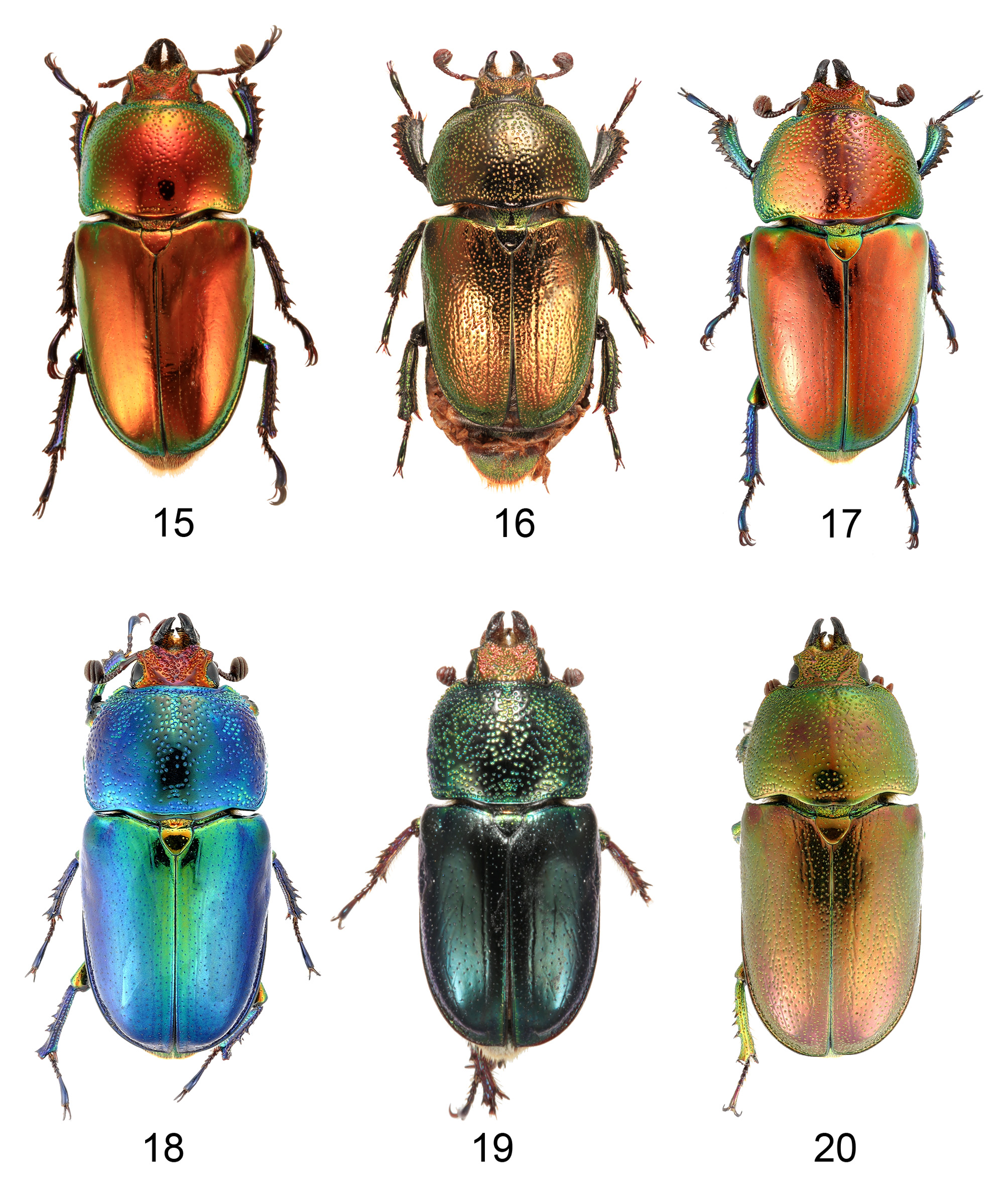
Description. Length. Male 13–60 mm (usually 20–45 mm) including mandibles, mandibles 10–38% of overall length ( Figs 1–14 View FIGURES 1–4 View FIGURES 5–14 ); female 13–27 mm including mandibles, mandibles 5–9% of overall length ( Figs 15–20 View FIGURES 15–20 ). Dorsal surface without scales or visible setae; venter setose, setae simple.
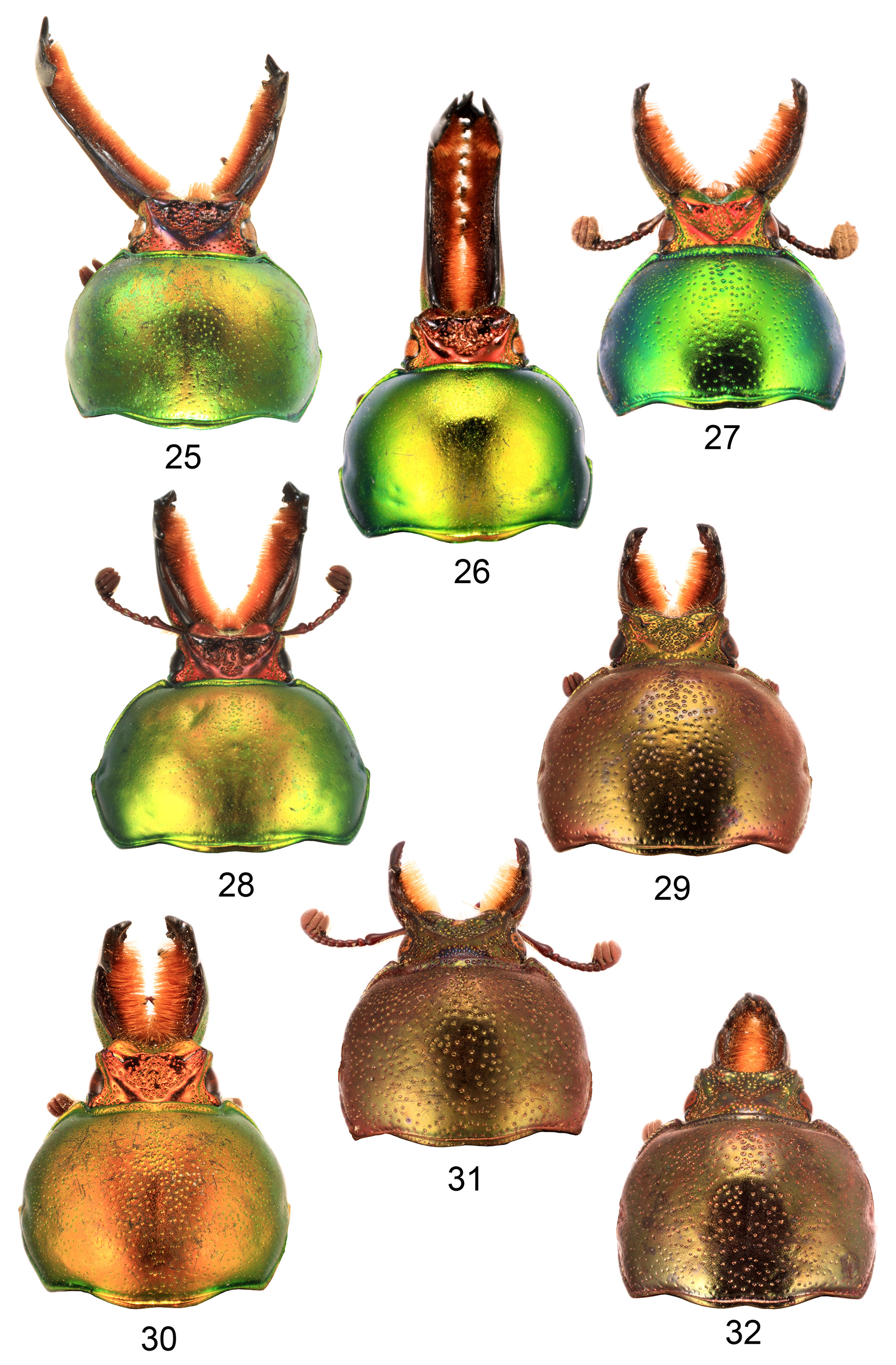
Head. Male without dorsal tubercles on head, anterior of head concave or truncate ( Figs 21–32 View FIGURES 21–24 View FIGURES 25–32 ); genae welldeveloped anterior to eyes but not laterally expanded, at most slightly anteriorly angulate in males; female head not narrowed compared with male; temples short but slightly angulate, shallowly grooved or notched to accommodate anterior angles of pronotum; head deeply inserted into pronotum which almost reaches eyes ( Figs 33–44 View FIGURES 33–35 View FIGURES 36–44 ); eyes undivided, reniform, with shallowly concave anterior and posterior margins, the anteroventral margin sharp and linear, forming the outer edge of the antennal groove; antennae not geniculate, with 10 antennomeres, with 3 antennomere club, the club antennomeres entirely densely setose and often closely appressed; antennomere 7 cupuliform, with thin lateral lobe and 6 slightly asymmetric (male) or with thin lobe (female); male mandibles as long as or longer than head and usually densely internally setose in male (not L. imberbis ); male mandible without basal dorsal tooth; each female mandible with only one dorsal cusp, with large strongly incurved basal ventral tooth, overlapping at tips; penultimate labial palpomere angulate on inner margin in male, not angulate in female; labium broad, approximately one third width of head; mentum solid and punctate, apex truncate; pregula flat; lateroventral grooves present between eyes and sides of buccal cavity for retention of scape.
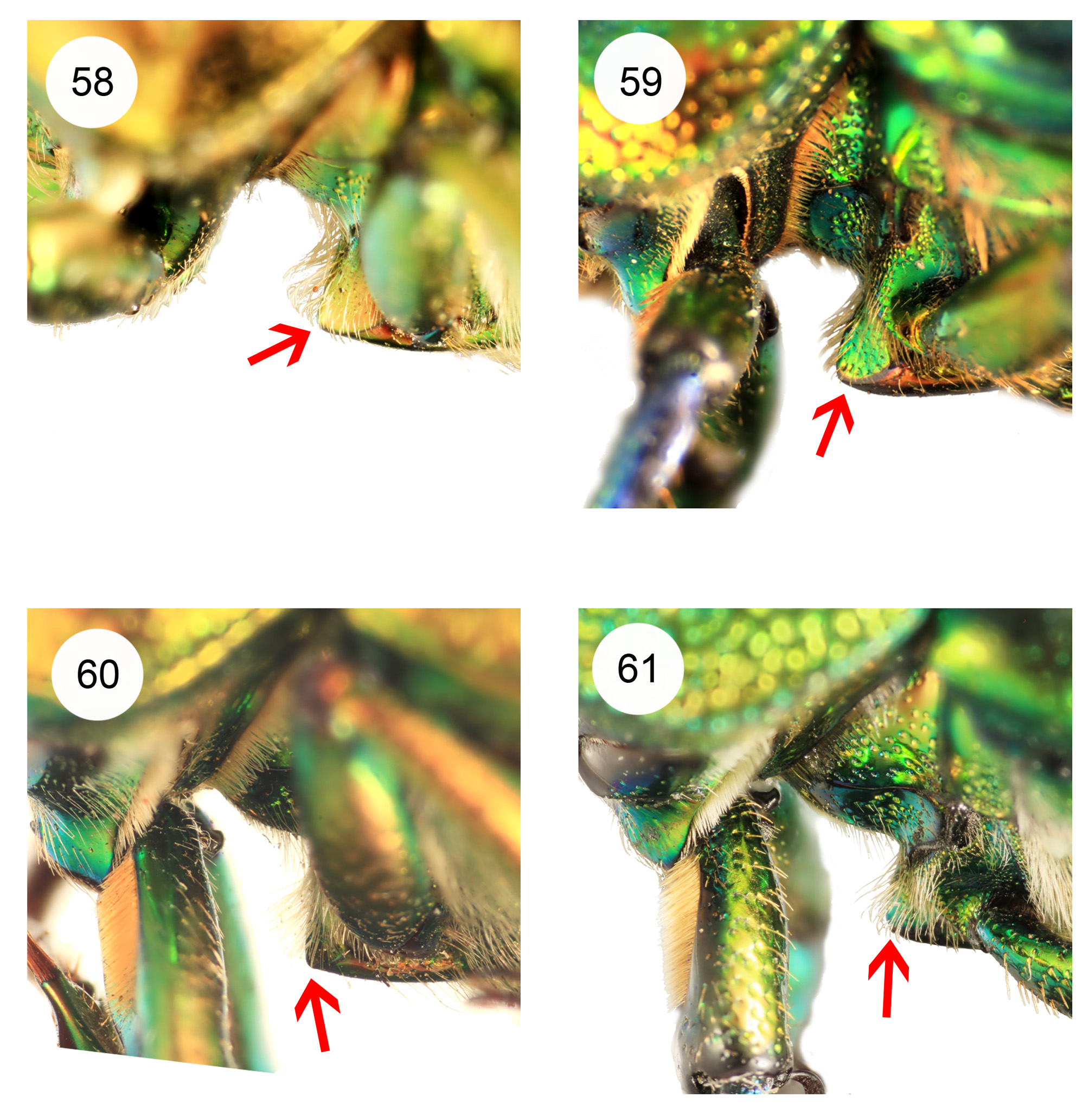
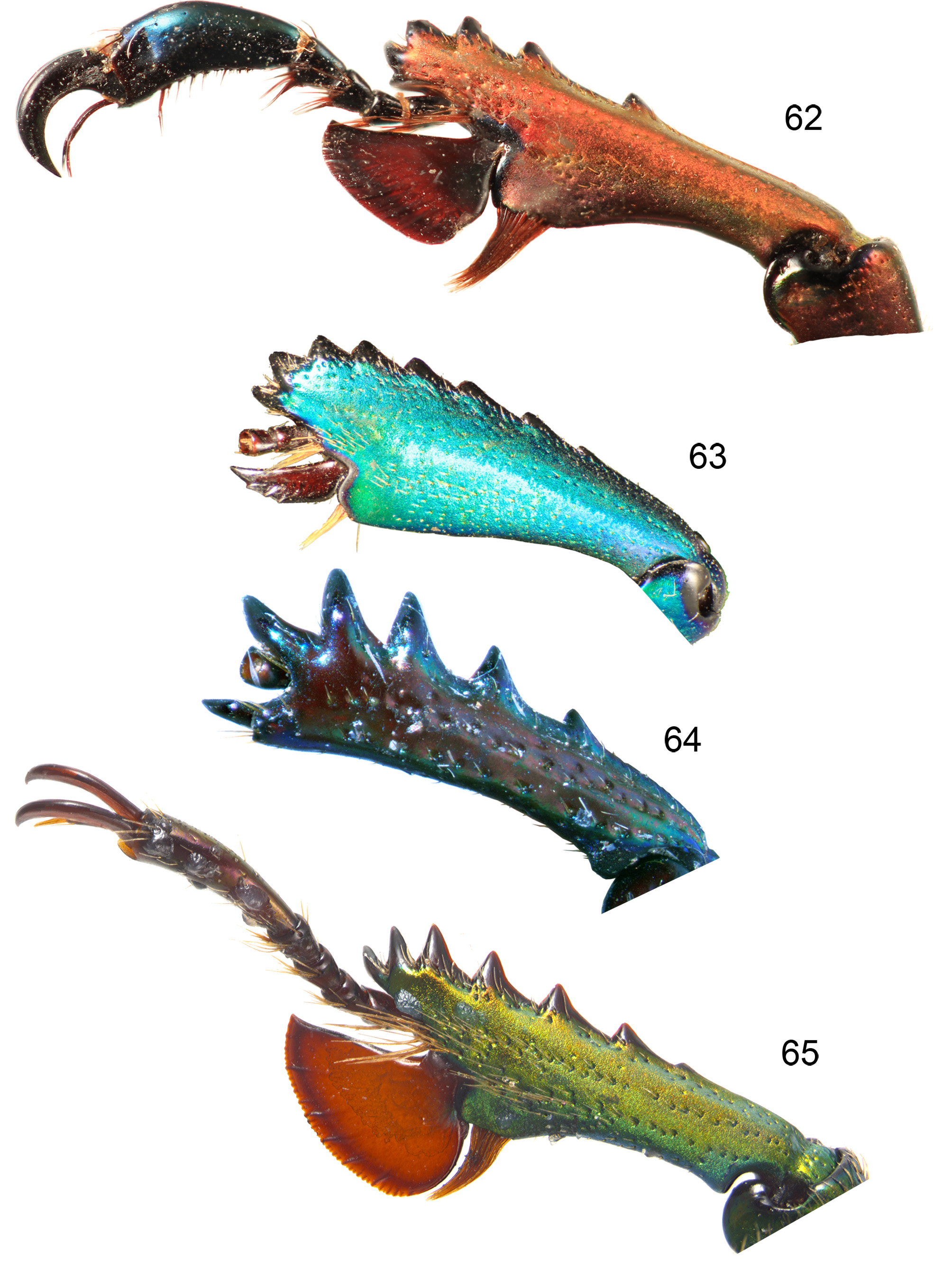
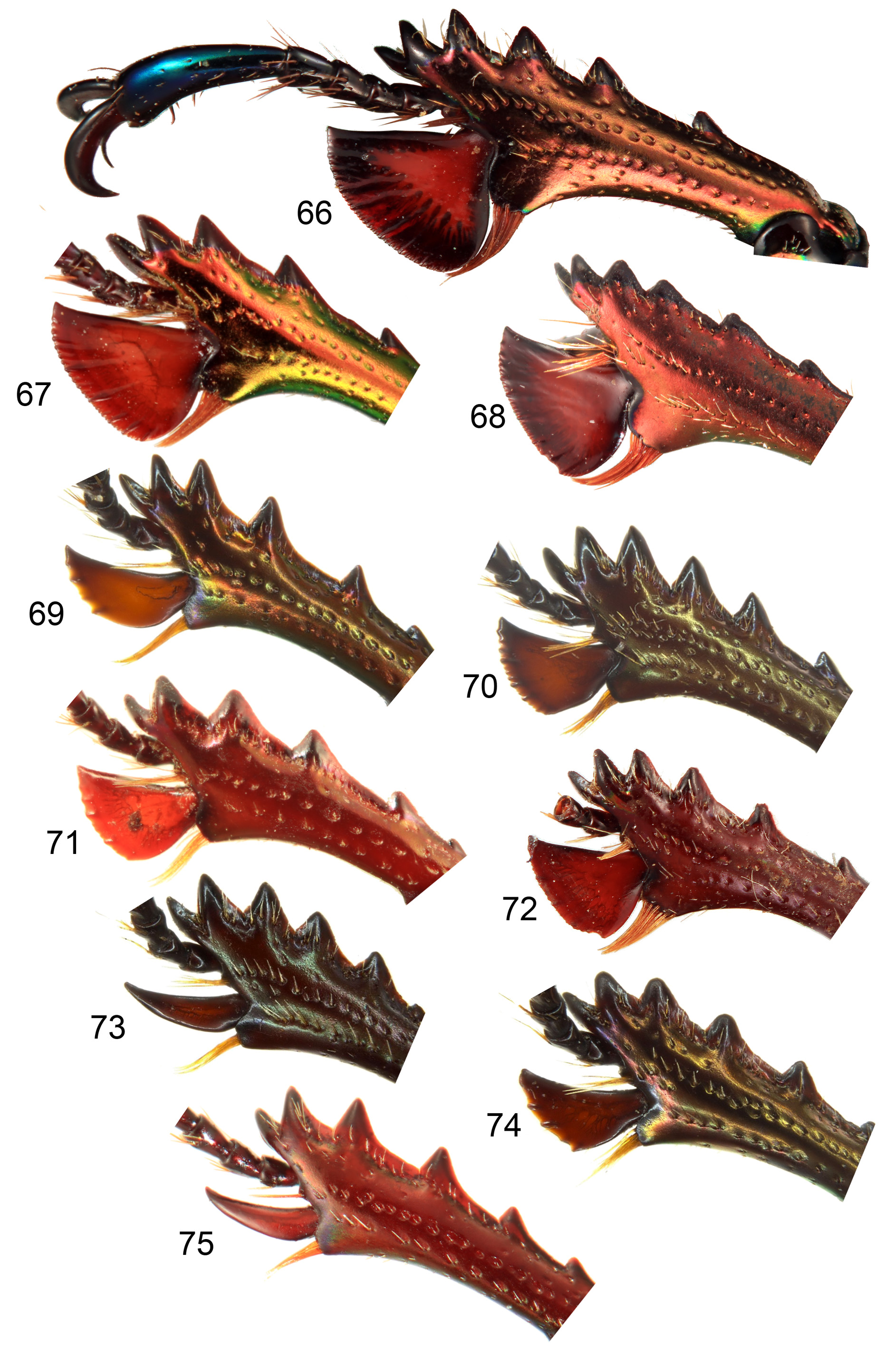
Thorax. Pronotum ( Figs 1–44 View FIGURES 1–4 View FIGURES 5–14 View FIGURES 15–20 View FIGURES 21–24 View FIGURES 25–32 View FIGURES 33–35 View FIGURES 36–44 ) convex with angulate (most males) to rounded sides, broadest slightly posterior to middle, without prominent anterior or posterior angles; anterior of pronotum broadly margined; middle of prosternal process concealed between procoxae, apex flat. Scutellum almost equilateral triangular. Elytra nonstriate but may have irregular, shallow grooves, with scattered shallow simple concave punctures, surface of interspaces smooth and shiny to dull and wrinkled but always with finely microreticulate microsculpture, usually slightly granulose in males ( Figs 54–57 View FIGURES 54–57 ). Elytral humeri not spined. Elytral epipleurae hidden from above, transversely strigose. Hindwings fully developed. Anterior field of mesoscutum strongly and closely punctate; scutellum semicircular to heart shaped; mesanepisternum with sparse, large punctures; posterior half mesepimeron, visible parts of metanepisternum, metepimeron and sides of metaventrite with dense, small punctures (partly coalescent, interspaces less than diameters) and setae; mesoventral process almost parallel-sided to junction with metaventrite, abruptly elevated anterior to this ( Figs 58–61 View FIGURES 58–61 ); metanepisternum with elevated lobe at anterior angle locking into small depression on elytral epipleuron. Profemur without anterior ridge; male protibia ( Figs 62–75 View FIGURES 62–65 View FIGURES 66–75 ) with expanded blade- or fan-like flat spur, except L. imberbis with narrowly elongate, triangular, curved spur; female protibial spur narrowly elongate triangular; male protibia without secondary teeth between major teeth; female protibia without subsidiary teeth between large teeth on outer edge; male metatibia without setose excavation on inner edge, usually with spines on outer edge; tarsal empodium prominent, with paired divergent thin tufts of apical setae.
Abdomen. Ventrites not laterally ridged and without basal transverse grooves.
Male genitalia ( Figs 76–88 View FIGURES 76–83 View FIGURES 84–88 ; unique male of L. imberbis undissected). Phallobase fusiform or spindle-shaped and uniformly sclerotised, with the apical margins rounded laterally and with V-shaped excavations dorsally (shallower) and ventrally (deeper); apical half of dorsal surface with at least a few small raised spicules, which may form irregular oblique ridges. Parameres about 2/5 length phallobase, symmetrical, with preapical setal tuft on ventral surface and incurved pointed tip; ventral inner edge of parameres soft, irregularly ridged, with the surface either inflated towards the penis or collapsed laterally, depending on preservation of the specimen; penis symmetrical, in two visible parts: basal 2/3 darkly sclerotised, rigid and narrowly conical between parameres, obliquely ridged at base; apical third thinly sclerotised and usually translucent, as a thin straight cylinder; endophallus, if everted, of similar width to apex of penis but soft and flexible, apically contracting to long, thin flagellum.
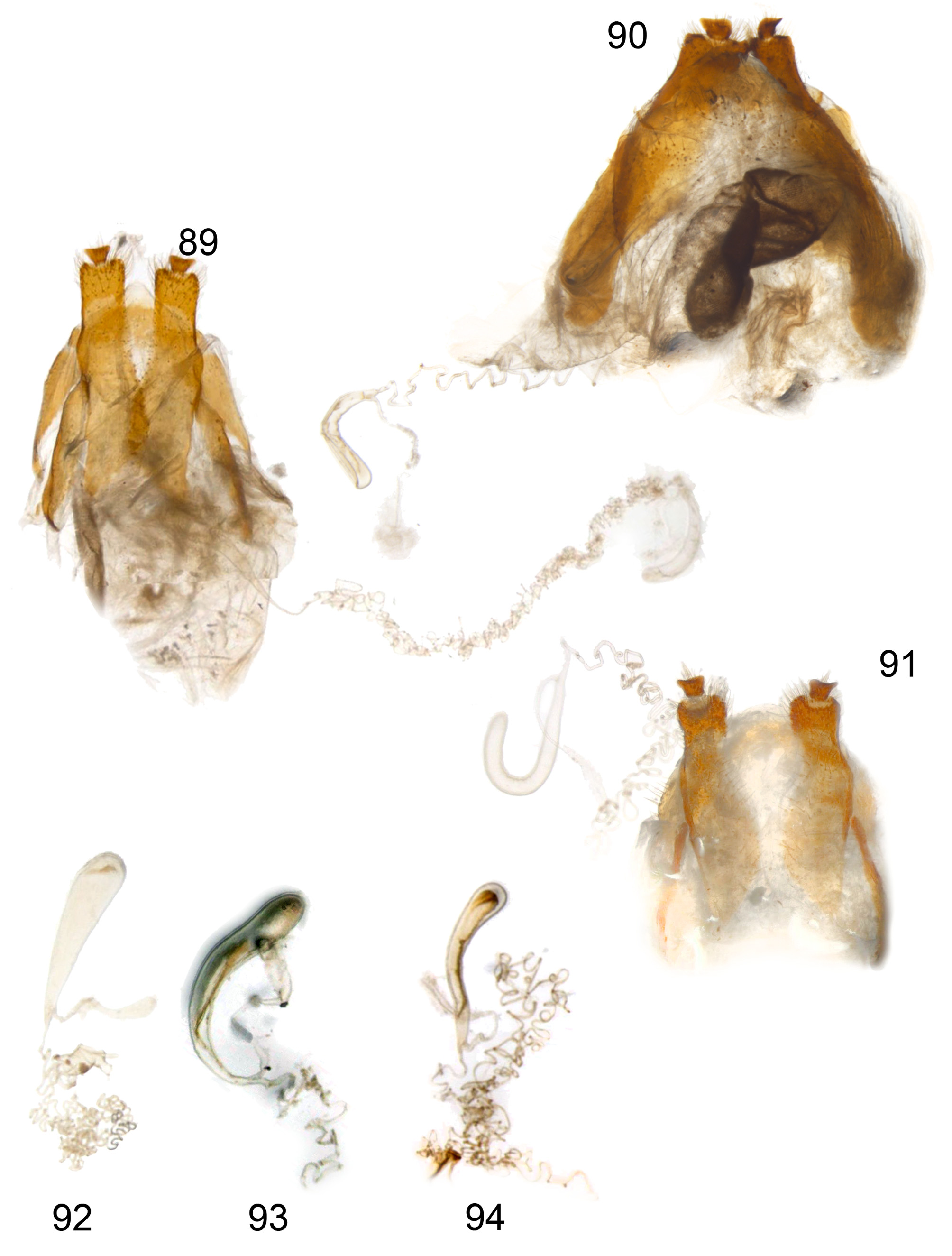
Female genitalia ( Figs 89–94 View FIGURES 89–94 ; L. imberbis unknown). Tergite IX with acute to broadly rounded translucent apex; pleurite IX and sternite IX well developed as elongate sclerites; hemisternites flat and apical halves elongate rectangular with truncate apices, gonostyli flat, inverted-trapezoidal in shape, inserted on middle of membranous apex of hemisternites; spermathecal duct coiled and convoluted, spermatheca sclerotised, variable in shape from tear-drop to hook; spermathecal gland smaller than spermatheca, glandular duct longer than spermatheca.
Larval diagnosis. The following diagnosis is based on published descriptions of L. aurata larvae ( Alderson 1975 [as L. varians ]; Lawrence 1981). The larva is similar to the lamprimine Phalacrognathus W.J. Macleay, 1885 ( Wood et al. 1996).
Head: antenna with 3 antennomeres; second antennomere without setae or sensory spots, apex slightly produced beyond base of third antennomere, the latter elongate but much smaller than second. Left mandible without incisor teeth between 2–3 apical teeth and mola; epipharynx trapezoidal, with dense, long setae on apical margin and dense, short setae on sides, apex of median area with transverse row of 6 short, blunt pegs and row of 4 circular sensilla proximal to this, epitorma absent. Legs without distal claw, last 3 segments short and broad and densely setose; tibiotarsus reduced to an ovate lobe, length equal to width at base; mesocoxal stridulatory file (pars stridens) with a single line of about 50 quadrate to slightly transverse dense ridges, granulose towards base, and placed in a field of minute granules; metatrochanteral stridulatory file (plectrum) a single line of 45–60 closely placed transverse ridges or granules, increasing in size from base to apex. Anal area of abdomen with two adjacent pear-shaped and minutely but densely setose lobes, without ovate pads, their bases subtending about 90° in ventral view, separated from 10th segment by a dorsal lobe, which is triangular, glabrous and unsculptured; 10th abdominal segment not foreshortened dorsally, ventral apex triangularly excavate, raster confined to apical quarter, consisting of dense, minute, inwardly-directed setae.
Ecology and natural history. The following notes mostly concern the common and widespread species L. aurata ( Fearn 1996, 2015, 2016) and the Lord Howe endemic L. insularis ( Reid 2004) . Lamprima adults are commonly diurnal and are often found on flowers, where they may mate. Adult males snip off the apical shoots of living plant material to provide sap flows. They have been recorded feeding on many genera, both native and exotic, listed by family as follows: Asparagaceae : Lomandra ; Asteraceae : Ozothamnus ; Casuarinaceae : Casuarina ; Fabaceae : Acacia , Virgilia ; Malvaceae : Lavatera ; Myrtaceae : Eucalyptus , Leptospermum , Melaleuca ; Pinaceae : Pinus ; Proteaceae : Banksia ; Rhamnaceae : Alphitonia; Rosaceae : Photinia , Prunus ; Salicaceae : Populus ( Fearn 1996, 2015; Hangay & de Keyzer 2017). Feeding by the males of Lamprima with elongated mandibles (most L. adolphinae and some L. aurata ) has not been described. Female mandibles are apparently non-functional, therefore females fly to the male feeding sites to lap up sap released by males. Feeding sites are used for male-male aggression and for copulation. Fearn (1996) noticed a 3: 1 male to female sex ratio in the field, which is corroborated by the male biased material of most species in collections. Olliff (1889) noted that male L. insularis were much more common than females. In contrast, the material available for L. insularis has a roughly 1:1 sex ratio, but were mostly collected by breaking open logs, so does not represent typical field activity of the species. Adults will also feed on soft fruits (Hangay & de Keyzer 2017). Oviposition is usually underground, the females tunnelling into soil around partly buried decaying wood. There is a great variety of larval host genera, including exotics: Araucariaceae : Araucaria ; Arecaceae : Howea ; Casuarinaceae : Allocasuarina , Casuarina ; Celastraceae : Elaeodendron ; Fabaceae : Acacia ; Lauraceae : Cryptocarya ; Myrtaceae : Eucalyptus , Syzygium ; Oleaceae : Olea ; Salicaceae : Salix ( Fearn 1996; Reid 2004; Hangay & de Keyzer 2017). Lamprima aurata larvae are usually in decaying roots and buried timber in Tasmania ( Fearn 1996) but they prefer standing timber in northern Queensland rainforest ( Wood et al. 1996). Lamprima insularis larvae usually inhabit fallen timber on or above ground level in the subtropical rainforests of Lord Howe Island ( Reid 2004). In wetter areas Lamprima larvae may be better able to survive above ground, or less able to survive below ground, but there may be a trade-off between humidity and temperature, as L. aurata avoids cool temperate rainforests in Tasmania ( Fearn 1996). In logs and stumps the larvae generally bore upwards. Pupation is in a chamber, usually just beneath the wood surface but sometimes in adjacent soil ( Fearn 1996). In Tasmania the entire life cycle is at least three years but it may be 1–2 years in Queensland (Hangay & de Keyzer 2017). In captivity, the life cycle of L. adolphinae is 9–14 months ( Levet 2016).
There are numerous photographs and several videos of Lamprima species on the Internet (for example: Anonymous 2017a), showing: different colour varieties, mating, fighting between males, feeding, rearing methods, larvae and pupae. In copulation and precopulation the protibial spurs of the males have little function. They may scrape lightly over the pronotum of the female as the prothoracic legs are moved backwards and forwards, but this activity seems erratic and brief. In male-to-male combat, each male uses its mandibles to try to embrace the mandibles of the other, so longer mandibles provide a wider net for the embrace. Once one male has enclosed and squeezed together the mandibles of its rival, it shakes the whole animal quickly to one side to unbalance it, then abruptly to the other side, letting go at the end of this second swing. The rival can be flung a few centimetres (see video by Kan 2016). The elongate mandibles of L. adolphinae allow males to grab wayward appendages of rivals rather than gripping the whole head. In fights, the protibial spurs may be used as braces against the substrate and this activity might be their primary function.
The international pet trade is heavily involved in rearing Lamprima species, with goals including production of enlarged mandibles and unusual colour varieties. This may extend the range of variation for each species given here, which is based on field-collected specimens.
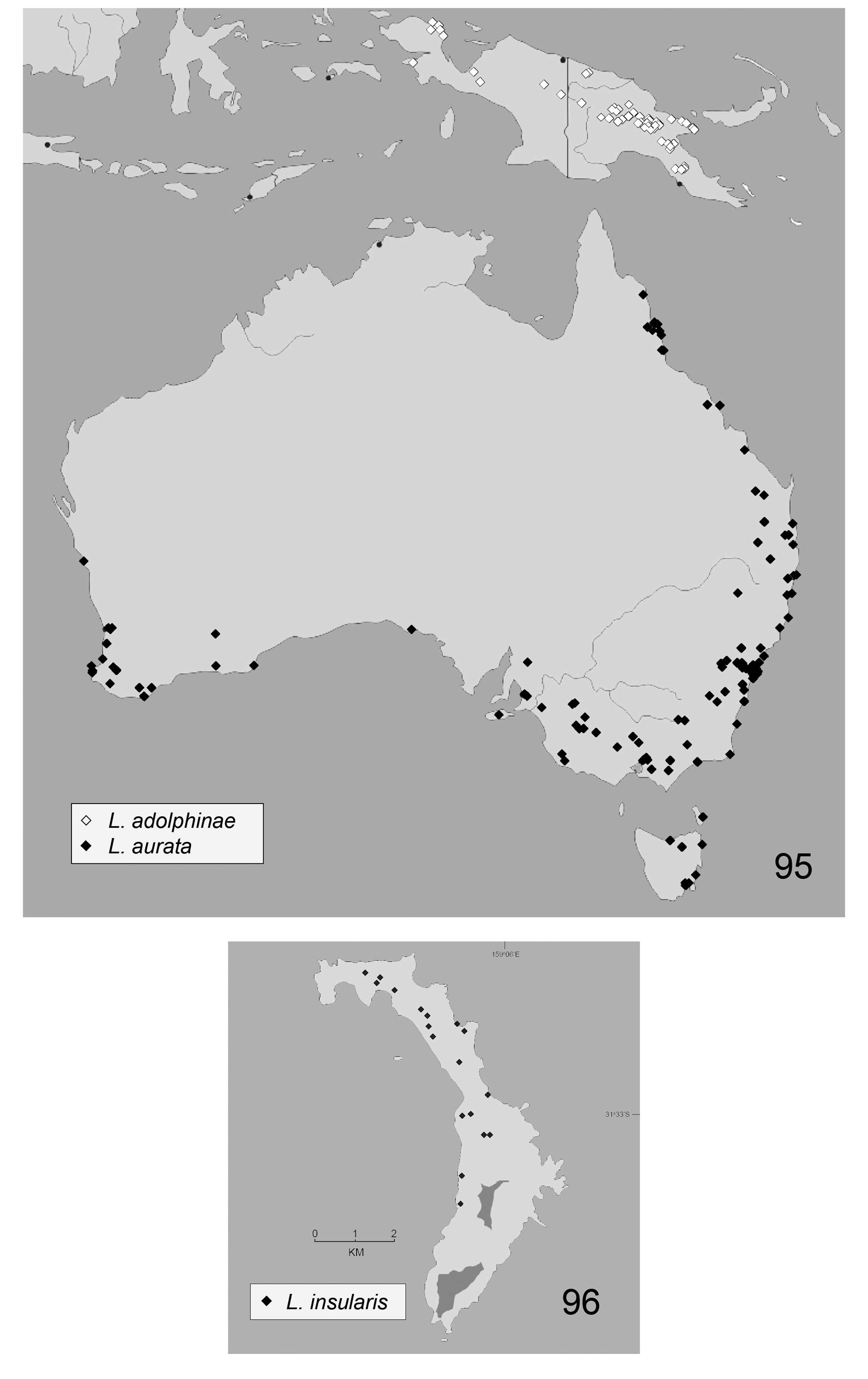
Lamprima is widespread in Australia ( Fig. 95 View FIGURES 95–96. 95 ), occupying almost the entire eastern edge of the continent from Cooktown in northern Queensland to Tasmania and most of the south coast from Mallacoota west to Perth, with an 850 km gap at the Nullarbor Plain. Lamprima occurs up to 400 km inland on the mainland. Lamprima also occurs on two oceanic islands, Norfolk and Lord Howe and a single species is widespread on mainland New Guinea ( Fig. 95 View FIGURES 95–96. 95 ). In New Guinea it occurs up to 2800 m.
Conservation. Conservation status and threats are discussed under each species. In general Lamprima species are extremely popular with stag beetle collectors and most species are being, or have been, reared in commercial quantities in eastern Asia and probably Europe and North America. One species, L. imberbis , is of considerable concern as it has not been collected for 100 years.
Comparison with other genera of Lampriminae . There are four other genera of Lampriminae , all monotypic. The New Zealand genus Dendroblax White, 1846 , is unmistakably different from Lamprima , with a dynastine-shaped body, densely punctate, non-metallic, reddish-brown upper surface, venter with long setae and minimal sexual dimorphism ( Holloway 2007). The Australian endemic Homolamprima W.J. Macleay, 1885 , is relatively easily distinguished from Lamprima by: mesometaventrite junction anteriorly bilobed; apex prosternal process elevated; male protibia with large narrow spines. Homolamprima is also much flatter than any Lamprima species ( Macleay 1885b). The South American genus Streptocerus Fairmaire, 1850 , is similar to Homolamprima but differs from it and all other Lampriminae by the antennal club having four antennomeres ( Paulsen 2010).
Lamprima is morphologically most similar to the northern Australian endemic Phalacrognathus Macleay, 1885 , although this is not supported by an analysis of four gene regions ( Kim & Farrell 2015). Most species of Lamprima can easily be distinguished from Phalacrognathus by male protibia with spur expanded as a flat blade and female protibia without subsidiary teeth between large teeth on outer margin. In Phalacrognathus , the male protibia has a simple spur (as in female) and the female protibia has small subsidiary teeth present between the large teeth on the outer margin. However, Lamprima imberbis , with unknown female, is unusual in Lamprima for its male mandibles lacking internal setae, male protibiae with narrow spurs and without a setal tuft and elytra broadly explanate. It shares these characters with Phalacrognathus , but differs from that genus by: genae prominent anterior to eyes; eyes anteriorly concave; antennomere 7 with flat lateral lobe; temples prominent and notched to accommodate anterior angles of pronotum; male mandible without basal dorsal tooth; anterior of pronotum broadly margined; middle of prosternal process concealed by procoxae; sides of male elytra not crenulate; male protibia without secondary teeth between major teeth; protibial spur on a lobe. All of these characters are common to other male Lamprima and justify placement of L. imberbis in Lamprima .
Included species. The most recent peer-reviewed checklist of Lamprima species ( Moore & Cassis 1992) lists seven in Australia ( Table 1), of which Lamprima insularis is unique to Lord Howe Island and Lamprima aenea is unique to Norfolk Island. One non-Australian species of Lamprima is known, from New Guinea: L. adolphinae , as catalogued by Benesh (1960). The mainland Australian species listed by Moore & Cassis (1992) are L. aurata , L. imberbis , L. latreillii , L. micardi and L. varians . Lamprima micardi is supposedly endemic to Western Australia and L. varians was described from South Australia. The other species, L. aurata , L. imberbis and L. latreillii , were described from the eastern coastal region of Australia, from northern Queensland to Tasmania.
Lamprima Author Date Type Hope in Parry Harold Macleay Boileau Nagel 1930 Benesh Moore & Krajcik This work species rank locality Westwood 1864, 1868 1885a 1913 1960 Cassis 2001 2017
name 1845 1870 1992
L. adolphinae Gestro 1875 View in CoL NG - - - L. - L. L. - L. L. adolphinae View in CoL adolphinae adolphinae adolphinae adolphinae View in CoL
L. aenea Fabricius 1792 View in CoL NI L. aenea L. aenea L. aenea View in CoL L. aenea L. aenea L. aenea L. aenea L. aenea L. aenea L. aenea View in CoL L. aenea Fabricius 1802 View in CoL NI L. L. aenea L. aenea View in CoL - - - - L. aurata View in CoL - L. aenea View in CoL & sensu schreibersi L. aurata View in CoL
Schreibers
L. aenea Fabricius 1805 NI & - L. aurata - L. aurata - - L. aurata L. aurata L. aurata L. aenea & sensu NH L. aurata
Donovan
L. aenea Boisduval 1835 View in CoL NH - L. L. latreillii L. latreillii View in CoL - - L. latreillii View in CoL - L. latreillii View in CoL L. aurata latreillii View in CoL
L. amplicollis Thomson 1862 View in CoL SQ - L. L. latreillii L. latreillii View in CoL - - L. latreillii L. latreillii L. latreillii View in CoL L. aurata latreillii View in CoL
……continued on the next page
Lamprima Author Date Type Hope in Parry Harold Macleay Boileau Nagel 1930 Benesh Moore & Krajcik This work species rank locality Westwood 1864, 1868 1885a 1913 1960 Cassis 2001 2017
name 1845 1870 1992
L. imberbis Carter 1926 View in CoL NSW - - - - - L. imberbis View in CoL L. L. L. L. imberbis View in CoL imberbis imberbis imberbis View in CoL L. insularis Hope 1845 View in CoL WA L. micardi View in CoL - nomen - L. micardi View in CoL - L. micardi View in CoL - L. micardi nomen nudum nudum L. insularis View in CoL W.J. 1885a LHI - - - L. insularis View in CoL - L. insularis L. L. L. L. View in CoL
Macleay insularis insularis insularis insularis View in CoL L. insularis Boileau 1913 View in CoL not - - - - L. micardi View in CoL - - - - L. aurata View in CoL given
L. krefftii View in CoL W.J. 1871 CQ - - - L. krefftii View in CoL - L. latreillii View in CoL ? L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. aurata MacLeay L. latreillii View in CoL W.S. 1819 NSW L. latreillii View in CoL L. L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. aurata View in CoL Macleay latreillii View in CoL L. lulua Kriesche 1940 View in CoL NG - - - - - - adolphinae View in CoL - L. L. adolphinae adolphinae View in CoL L. W.J. 1885a NQ - - - L. - L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. aurata mandibularis Macleay mandibulari s L. mariae Lea 1910 Tas View in CoL - - - - - L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL L. micardi Reiche 1841 View in CoL WA L. micardi View in CoL L. L. micardi View in CoL L. micardi View in CoL L. micardi View in CoL L. micardi View in CoL L. micardi View in CoL L. micardi View in CoL L. micardi View in CoL L. aurata View in CoL micardi View in CoL L. minima View in CoL W.J. 1885a SA - - - L. minima View in CoL - L. varians View in CoL L. varians View in CoL L. varians View in CoL L. micardi View in CoL L. aurata Macleay View in CoL
……continued on the next page
Lamprima View in CoL Author Date Type Hope in Parry Harold Macleay Boileau Nagel 1930 Benesh Moore & Krajcik This work species rank locality Westwood 1864, 1868 1885a 1913 1960 Cassis 2001 2017 name 1845 1870 1992 L. pygmaea View in CoL W.S. 1819 AUS - L. L. latreillii View in CoL L. latreillii View in CoL - - L. latreillii View in CoL L. latreillii View in CoL L. latreillii View in CoL L. aurata MacLeay latreillii View in CoL L. rutilans Erichson 1842 Tas View in CoL - L. aurata View in CoL L. rutilans View in CoL L. rutilans View in CoL - L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL L. aurata View in CoL
L. viridis Erichson 1842 View in CoL not - L. aenea View in CoL L. aenea View in CoL ? - L. aurata L. aurata View in CoL L. aenea L. aenea View in CoL L. aurata View in CoL given
There are good male characters for diagnosing L. aenea View in CoL , L. adolphinae View in CoL , L. imberbis View in CoL and L. insularis View in CoL . There remain the Lamprima View in CoL species, excluding L. imberbis View in CoL , described from mainland Australia and Tasmania. It has already been noted that Matthews (1984) treated L. varians View in CoL and L. aurata View in CoL as one species in South Australia ( L. aurata View in CoL ) and Moore (1984, 1986) suggested that L. aurata View in CoL and L. latreillii View in CoL were variants (“overlapping subspecies”) of one species in southeastern Australia, even though these were originally separated by dorsal punctation and structure of thoracic ventrites ( Macleay 1885a). Lamprima micardi View in CoL in Western Australia and L. varians View in CoL from South Australia were originally distinguished from eastern Australian Lamprima View in CoL by their narrower male protibial spurs ( Reiche 1841; Burmeister 1847) but the presence of intermediates blurs their distinction. We have examined more than 400 specimens of Lamprima View in CoL from throughout southern and eastern Australia and conclude that these represent just one species, L. aurata View in CoL .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Lamprima Latreille, 1804
| Reid, Chris A. M., Smith, Kindi & Beatson, Max 2018 |
Lamprima
| Latreille, 1804a : 150 |
Neolamprima
| Gestro, 1875 : 997 |
| Nagel 1922 : 16 |