Glirulus japonicus (Schinz, 1845)
|
publication ID |
https://doi.org/10.5281/zenodo.6604339 |
|
DOI |
https://doi.org/10.5281/zenodo.6604276 |
|
persistent identifier |
https://treatment.plazi.org/id/9B215C43-FFCA-DD0C-C979-FE61FC30FBF7 |
|
treatment provided by |
Carolina |
|
scientific name |
Glirulus japonicus |
| status |
|
16. View On
Japanese Dormouse
Glirulus japonicus View in CoL
French: Loir du Japon / German: Japan-Bilch / Spanish: Liron de Japon
Other common names: Mountain Rat, Yamane
Taxonomy. Myoxus javanicus Schinz, 1845 View in CoL .
Japan.
Schinz’s javanicus was a lapsus for japonicus and was officially conserved as the original spelling by the International Commission on Zoological Nomenclature in 2001. This species is considered to have originated from immigrants that colonized Japan via land bridges formed with adjacent mainland Asia during the Pliocene or early Pleistocene, according to M. Dobson and Y. Kawamura in 1998, and B. D. McKay in 2012. It has traditionally been viewed as monotypic, but recent molecular genetic studies by S. P. Yasuda and colleagues in 2012 that amplified prior results of Yasuda and colleagues in 2009 and 2007 and H. Suzuki and colleagues in 1997 revealed nine distinct genetic lineages that exhibit substantial genetic differentiation; Yasuda and colleagues in 2012 hypothesized that factors such as glacial cycles combined with topographical barriers, or population fluctuations played an important role in shaping present-day population structure. In addition to genetic differences, populations exhibit phenotypic and ecological differences as reported by S. Minato in 1986, Suzuki and colleagues in 1997, K. Funakoshi and colleagues in 2015, and as figured in M. A. Iwasa in 2009; future integrative studies incorporating genetic, morphological, and ecological data will likely show that several lineages are distinct and diagnosable. Monotypic.
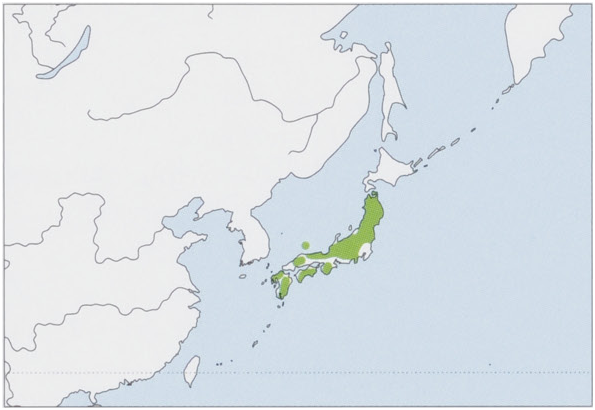
Distribution. Endemic to Japan, recorded from Honshu, Shikoku, Kyushu, and Oki Is (Dogojima). View Figure
Descriptive notes. Head-body 66-93 mm, tail 38-59 mm, ear 6-10, hindfoot 15-18 mm; weight 14-45 g. No sexual dimorphism recorded. Along with the Hazel Dormouse ( Muscardinus avellanarius ), the Japanese Dormouse is one of the smallest species within the family. Dorsal pelage color varies geographically from rich rufous brown to pale rufous beige, with dark brown to black mid-dorsal stripe extending from between ears to base of tail; stripe is widest and most prominent along back. Expression ofstripe is variable. Dorsal pelage is soft, thick, and moderately long, with conspicuous guard hairs. Ventral pelage is gray, lightly suffused with white. Dorsal and ventral pelage colors are not clearly delineated. Head color matches that of dorsal pelage. Eyes are very large, each encircled by prominent dark brown ring; eye mask either appears absent or sometimes as faint darkening beneath vibrissae. Ears are brown, moderately large, and rounded, although they appear small because much of their bases are covered by thick dorsal pelage. No post-auricular patches are present. Cheeks are paler than dorsal pelage, often buffy. Hindfeet are sparsely haired with white fur distally; broad metatarsal streak matches color of dorsal pelage. Tail is moderately short (c.61% of head-body), flattened throughout its length, and covered with hairs extending up to 20 mm. Tail color generally matches dorsal pelage and lacks white tip. Skull is compact, short, and broad. One feature unique to the Japanese Dormouse is lateral wrapping of a projection of basioccipital around posterior margin of each mastoid region and adjoining auditory bulla. Greatest length of skull is 24 mm, zygomatic breadth is 14 mm, and upper tooth row length is 4 mm. Chromosome number is 2n = 46. Females have eight pairs of nipples (2 pectoral + 1 abdominal + 1 inguinal = 8).
Habitat. Subtropical, montane, and subalpine habitats that experience heavy snowfall in regions characterized by primary and secondary mixed broadleaf forest, and mixed coniferous and deciduous forest at elevations of 400-1880 m. Mixed deciduous forests inhabited by Japanese Dormice are dominated by canopy trees such as Japanese chestnut ( Castanea crenata, Fagaceae ), Japanese beech ( Fagus crenata, Fagaceae ), several species of maple ( Acer sp. , Sapindaceae ), Japanese oak ( Quercus crispula, Fagaceae ), Japanese larch ( Larix kaempferi, Pinaceae ), Mongolian oak (Q. mongolica serrata), Japanese white birch ( Betula platyphylla, Betulaceae ), and Japanese red pine ( Pinus densiflora, Pinaceae ) and understory trees and shrubs such as Japanerse bird cherry ( Prunus grayana, Rosaceae ), Chinese mulberry ( Morus australis, Moraceae ), Japanese azalea ( Rhododendron japonicum, Ericaceae ), and Japanese clethra ( Clethra barbinervis, Clethraceae ); mixed deciduous and conifer forests are dominated by conifers such as Japanese cedar ( Cryptomeria japonica), Marie’s fir ( Abies mariesii), northern Japanese hemlock (7 Tsuga diversifolia), and Japanese larch ( Larix kaempferi), but also have Japanese beech, Manchurian walnut ( Juglans mandshurica, Juglandaceae ), and cherry ( Prunus sp. ). In southern Japan, these dormice also inhabit cool temperate mixed deciduous forests at higher elevations, but at lower elevations, they occur in warm subtropical evergreen laurel forest dominated by laurel (Machilus thunbergii), salinica oak ( Quercus salicina),Japanese stone oak ( Lithocarpus edulis, Fagaceae ), with Japanese camellia ( Camellia japonica, Theaceae ) often present in the understory.
Food and Feeding. The Japanese Dormouse is omnivorous. In the wild, it eats insects and their larvae such as bees, moths, and other arthropods; fruits, flowers, and bird eggs are also eaten. Stomach and gut contents with fragments of insects such as cave crickets, earwigs, stink bugs, coleopterans, and centipedes have been recorded. Japanese Dormice have also been said to feed on hardy kiwi ( Actinidia arguta, Actinidiaceae ), fruits of three-leaf chocolate vine ( Akebia trifoliata, Lardizabalaceae ), crimson glory vine ( Vitis coignetiae, Vitaceae ), and fig ( Morus bombycis, Moraceae ); petals, nectar, and fruit of the cherry Prunus jamasakura; and fruit of the giant dogwood ( Cornus controversa, Cornaceae ). In 2004, H. Ida and colleagues studied pre-dispersal nut predation and concluded that Small Japanese Field Mice ( Apodemus argenteus) likely account for a much greater percentage pre-dispersal damage to beechnuts than do Japanese Dormice.
Breeding. Litter sizes ofJapanese Dormice are 3-5 young; as many as seven offspring have been recorded. Females produce 1-2 litters/year: the first in June-July and a second may be produced in October. Gestation is ¢.33 days (range 30-39 days). Parenting behavior exhibited by mothers has been reported to include preventing other dormice from approaching nests, construction and moving of young to new nests, and carrying food to the nest for young; no parental behavior by adult males has been observed.
Activity patterns. The Japanese Dormouse is nocturnal. Hibernation at high elevations in central Honshu lasts ¢.6-7 months from late September to April, ¢.5 months in central Kyushu, and c.4 months on the Kii Peninsula of Honshu. In southern Kyushu, hibernation is either short or consists of bouts interrupted by short periods of activity in mid-winter, and it is hypothesized that some individuals in that region are active year-round during unusually warm winters. Although length of hibernation varies geographically from high elevations that experience extreme snowfall to subtropical lowland forests, this variation may be related more to food availability than solely differences in temperature. Before entering hibernation, Japanese Dormice increase body weights 1-5-2-4 times their mass during non-hibernation periods. Hibernation sites found using telemetry were situated in shallow excavations 5-32 cm underground, under fallen leaves, in decayed branches or trunks, and in tree cavities.
Movements, Home range and Social organization. The Japanese Dormouse is arboreal and solitary. It is a skillful climber, spending most of its life in trees where it nests and feeds. They move quickly through tree canopies and branches, often running upside down, searching for food on undersides of branches and leaves; more than 50% of movements were recorded in an upside down position. A radio-tracking study in April-October in central Honshu revealed that 72% of daily restsites were located in trees vs. 28% in non-arborealsites such as shallow underground sites or in rock crevices near trees. Nest boxes and natural tree cavities constituted most arboreal rest sites. Despite preference for rest sites in trees, Japanese Dormice habitually choose rest sites closer to the ground, at heights of 0-2 m; this may increase predation risk by mammalian predators or snakes. Japanese Dormice sometimes rest in places that offer little cover or protection such as on branches near tree trunks, behind patches of bark, or in a cluster of wild grapes, and some individuals slept with their bodies fully exposed or beneath a few leaves on the ground. They have also occasionally used nesting sites abandoned by the Japanese Squirrel ( Sciurus lis) and the Small Japanese Field Mouse. In 1997, Minato and colleagues reported finding two spherical nests in Japanese azalea bushes 1-1-1-7 m from the ground. Males Japanese Dormice are documented to have larger home range sizes of c¢.2-4 ha than females at c.0-5 ha, although they caution that these values are likely underestimated; males traveled farther than females during a single night, with maximum recorded distances of 336-7 m for males and 173-2 m for females. Home ranges of females did not overlap and males sometimes emit aggressive calls on borders of their home ranges. It has been inferred that the mating system of Japanese Dormice is polygynous or promiscuous. No nesting materials were observed in rest sites used only for one day. Nesting materials used to construct nests in nest boxes include bryophytes, tree bark, lichen, dead leaves, and small sticks; bryophytes represented more than 50% of all nesting materials by weight. Course materials such as bark were used for outer parts of nests and bryophytes for the inner part. Japanese Dormice seemed to select nest sites based on nest material availability because they obtained materials 40-60 cm from their nests; availability of nesting materials in an individual's microhabitat is not thought to be a determining factor for nest box selection, but there is a positive correlation between food resource availability and nest box location. Densities of 1-1-3 ind/ha adult Japanese Dormice have been recorded from central Honshu, contrasting with an annual density of 5-9 ind/ha, including subadults, in a neighboring locality. Japanese Dormice have been recorded to live more than three years in the wild and eight years in captivity.
Status and Conservation. Classified as Least Concern on The IUCNRed List. TheJapanese Dormouse has a large distribution. It is considered to be uncommon, and there are no immediate threats except long-term threat associated with deforestation and degradation of forests through natural calamities or anthropogenic means. The Japanese Dormouse was designated a natural monument ofJapan in 1975 and was listed as “near threatened” in 2002 on the Japanese Red List by the Japanese Ministry of the Environment. Based on mtDNA, nDNA, and yDNA analyses, substantial genetic differentiation among populations has been documented; further study is required to identify evolutionarily significant units to inform conservation action to preserve genetic diversity and evolutionary potential of Japanese Dormice. Presence of Japanese Dormice has been confirmed in areas where they were once feared extirpated or had not previously been recorded; additional use of camera traps in future studies will prove useful in assessing abundance, population trends, and forging conservation strategies. Conservation efforts include construction of arboreal animal bridges connecting forest fragments and allowing safe crossing of potential barriers such as busy highways by arboreal animals, including the Japanese Dormouse. When another similar bridge was constructed in 2011, a Japanese Dormouse used it within seven hours. Such projects that raise public awareness and garner support from the local community members, government agencies, and corporate sponsors positively impact conservation ofJapanese Dormice.
Bibliography. Aoki & Moriya (2009), Dobson & Kawamura (1998), Funakoshi et al. (2015), ICZN (2001), Ida et al. (2004), lijima & Tsuchiya (2015), Ishii (2005), Ishii & Kaneko (2008c), Iwasa (2009), McKay (2012), Minato (1986, 1989, 1994, 1996), Minato & Doei (1995), Minato, Iwabuchi et al. (2012), Minato, Wakabayashi & Hidaka (1997), Ministry of the Environment (2002), Nakamura & Kojo (2011), Nakamura-Kojo et al. (2014), Otsu & Kimura (1993), Rossolimo et al. (2001), Shibata (2000, 2008), Shibata & Kawamichi (2012), Shibata et al. (2004), Suzuki, H. et al. (1997), Suzuki, S. et al. (1975), Takahashi & Takahashi (2013), Tsuchiya (1979), Vogel et al. (2003), Wahlert et al. (1993), Yasuda, M. & Sakata (2011), Yasuda, M. et al. (2015), Yasuda, S.P, Iwabuchi et al. (2012), Yasuda, S.P, Minato et al. (2007), Yasuda, S.P, Nakayama et al. (2009).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.