Fergusobia colbrani Davies
|
publication ID |
https://doi.org/10.11646/zootaxa.3856.3.2 |
|
publication LSID |
lsid:zoobank.org:pub:060F9D25-F1BF-4DD2-BCF0-379779218E5E |
|
DOI |
https://doi.org/10.5281/zenodo.5618832 |
|
persistent identifier |
https://treatment.plazi.org/id/B37A8794-B47C-356B-FF2C-FA43D5A839FE |
|
treatment provided by |
Plazi |
|
scientific name |
Fergusobia colbrani Davies |
| status |
sp. nov. |
Fergusobia colbrani Davies n. sp.
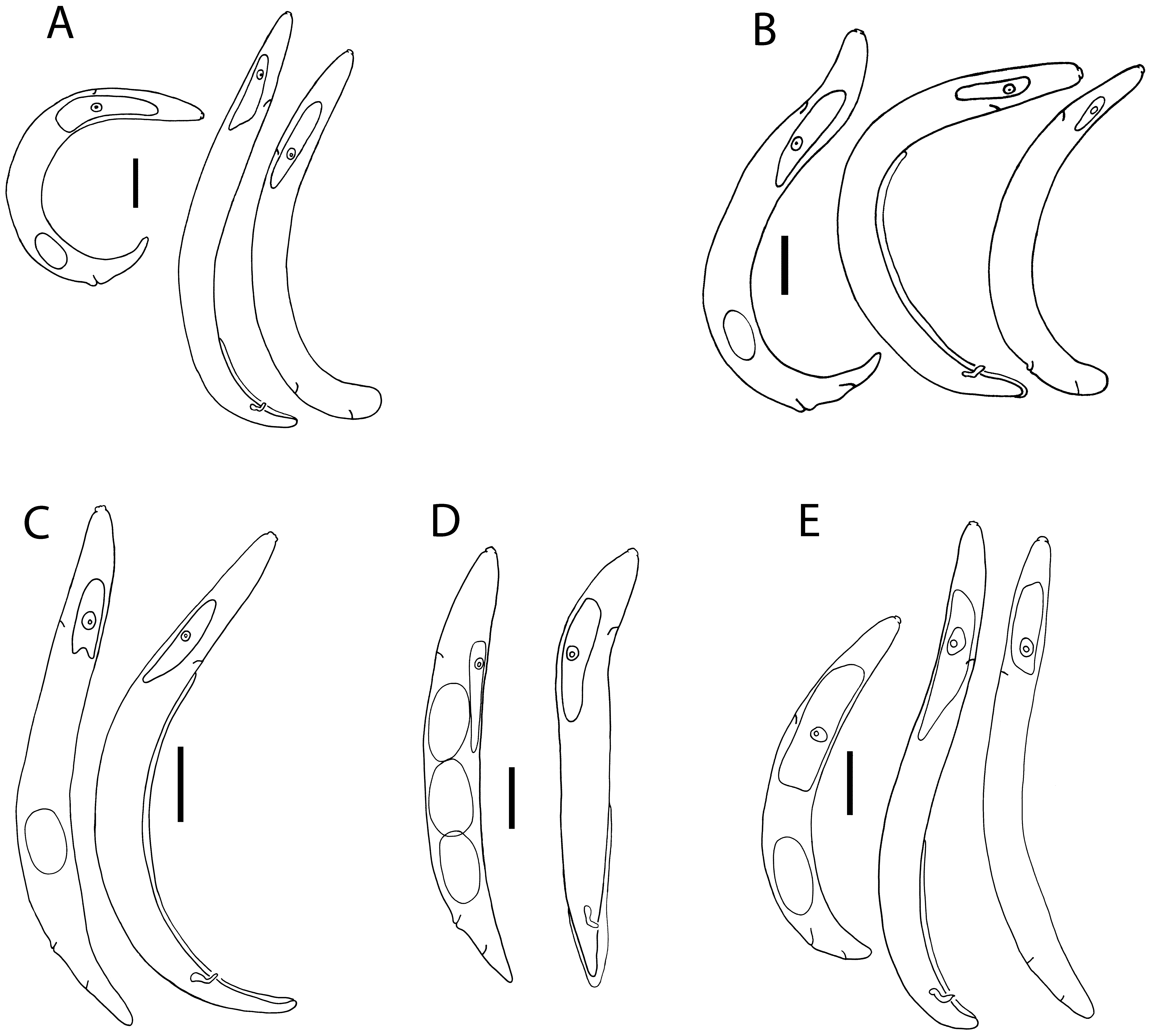
( Figs 1 View FIGURE 1 E, 2E, 3E, 4)
Measurements. Table 3 View TABLE 3 .
Material examined. The description presented here is based on measurements of 26 parthenogenetic ♀s, 2 pre-parasitic infective ♀s, and 26 ♂ s; from a tree of Angophora floribunda (Sm.) Sweet 1830 growing naturally in Paradise Park at Murrurundi, NSW, Australia ( 31º 76’S, 150º 83’ E.); associated with an undescribed species of Fergusonina (MSp 49 in Davies et al. 20011a; WINC nos 0 0 3222, 063857). Extracted from spheroid leaf galls on A. floribunda . Coll. K.A. Davies, 16.iv.2001 and 6.v.2008.
Holotype: a parthenogenetic female, together with an infective female and a male, on a slide deposited in the ANIC, Canberra, ACT, Australia, collected at Murrurundi, NSW, data as above.
Vouchers (collection data as above) deposited at the WINC, The University of Adelaide, SA, Australia, 5 parthenogenetic ♀s, 5 ♂ s; Australian National Museum, Sydney, NSW, Australia, 15 parthenogenetic ♀s, 1 preparasitic infective ♀, 15 ♂ s; and at the USDA Nematode Collection, Beltsville, MD, USA 5 parthenogenetic ♀s and 5 ♂ s.
subvelutina Colbran,1967 Yarraman Description. Parthenogenetic female. From soft spheroid galls on leaves on A. floribunda , containing one or two locules. Body open C-shape to sub-spiral, dorsally curved with ventral side convex; smaller than amphimictic pre-parasitic females and males; body narrows gradually behind vulva to form conoid tail with broadly rounded tip. With light microscope, cuticle appears smooth to weakly annulated, sub-cuticle with longitudinal striae. Lateral fields not seen.
Cephalic region ca 80% diameter of body at anterior end, off-set, ca 2 µm high, unstriated; rounded outline in lateral view, circum-oral area slightly raised. Stylet small with cone 40–50% length, basal knobs rounded, just higher than wide, ca 2 µm wide at base.
Orifice of dorsal pharyngeal gland ca 1 µm posterior to stylet knobs. Anterior fusiform part of digestive tract occupying 51–79% (mean 65%, n = 6) body diameter, 1.9–2.8× diameter; lumen of tract broadens partway along dorsal pharyngeal gland. Pharyngeal glands huge, diameter 35–80% (average 62%, n = 19) of body diameter, extending over intestine, distance from head to end of glands being 45 (37–55) % of total body length.
Secretory/excretory pore opens opposite nucleus of pharyngeal gland; duct obscure, secretory/excretory cell not seen. Hemizonid not seen.
Reproductive tract variable in length, extending to base of pharyngeal gland, part-way along gland or to nerve ring; with 1 to 4 flexures; oviduct with oocytes in pairs for the first few rows, subsequently no apparent pattern of arrangement; quadricolumella not smooth, uterus not extensile, with no eggs in 5 specimens, and 1 in 21 specimens examined; vulva a simple transverse slit with non-protruding rounded lips; no vulval plate. Anus pore-like, in some specimens opens into a small dimple in the cuticle. Tail conoid, short, length 0.5–1.5× anal body diameter; broadly rounded tip.
Infective pre-parasitic female. From spheroid galls on leaves on A. floribunda . Infects mature larval stage of Fergusonina sp. or pupa. Arcuate shape when heat-relaxed; maximum body diameter at mid-body length; body tapers gradually behind vulva. Cuticle obscurely annulated, longitudinal striae of sub-cuticle not apparent with light microscope; lateral fields not seen.
Cephalic region just offset, rounded shape; circum-oral area rounded, flat or slightly raised; stylet slender, with rounded basal knobs higher than wide, ca 1µm in diameter; cone ca 40 – 50% of length.
Orifice of dorsal pharyngeal gland obscure, ca 1µm posterior to stylet knobs. Anterior fusiform part of digestive tract little expanded, occupying 38–63% of body diameter, length 1.6–3.3× daimeter (n = 2). Pharyngeal glands extending over intestine, diameter 55–75% body diameter, distance from head to end of glands is average 27.5 % of body length.
Secretory/excretory pore opens opposite nucleus of pharyngeal gland; duct obscure; secretory/excretory cell not seen. Hemizonid immediately anterior to secretory/excretory pore.
Uterus ca 60% of total gonad length in uninseminated females, packed with sperm in inseminated females; vagina anteriorly directed; reproductive tract extending to level of excretory/secretory pore. Vulva a transverse slit, vulval lips not raised, rounded, no vulval plate present. Anus an obscure pore. Tail sub-cylindroid; relatively short (c = 17–20); length approximates body diameter at anus; tip almost hemispherical.
Male. From spheroid galls on leaves on A. floribunda . Body arcuate to barely J-shaped when heat-relaxed, tail region slightly curved ventrally. Cuticle obscurely annulated, longitudinal striae of sub-cuticle apparent with light microscope; lateral fields not seen.
Cephalic region 6.5–8.0 µm wide, occupying ca 70–80% anterior body diameter, offset, ca 2 µm high; circumoral area not raised, with lightly sclerotised framework; stylet with cone 40–50% of length, round stylet knobs ca 2 µm wide. Diameter of anterior fusiform part of digestive tract 51–86% of body diameter, length 1.4–2.8× diameter. Orifice of dorsal pharyngeal gland ca 1 µm behind knobs. Pharyngeal glands extending over intestine, diameter 41 (29–53) % of body diameter (n = 16), length from head to end of glands 31 (26–47) % of total body length.
Secretory/excretory pore opens opposite or slightly posterior to nucleus of pharyngeal gland; duct obscure, not heavily sclerotised; secretory/excretory cell not seen. Hemizonid immediately anterior to duct, extending over 2 annules.
Testis single, variable in length, usually overlaps dorsal pharyngeal gland and extends to nerve ring but may only reach base of gland; reflexed in a few specimens; cap cell often offset, seminal vesicle and vas deferens not clearly differentiated. Bursa apparently leptoderan, reaching to tail tip but not enveloping it, smooth; may be prominent or obscure; arises 30 – 65% of length of body anterior to tail tip. Spicules paired, angular at about 40% of length, with manubrium and shaft longer than blade; moderately sclerotised; manubrium wider than shaft, not offset; blade having similar diameter to shaft, tip notched, opening terminal. Inconspicuous muscles associated with cloaca. Tail conoid, arcuate, ventrally concave; length 1.5–2.0× diameter at cloaca; bluntly rounded tip.
Diagnosis and relationships. Fergusobia colbrani n. sp. is morphologically characterized by the combination of a small C-shaped parthenogenetic female with a short broadly conoid tail, an arcuate infective female with an almost hemispherical tail tip, and an arcuate to barely J-shaped male with an angular spicule having a notched tip and mid-length leptoderan bursa. Its species status is supported by its development in an unusual spheroid leaf gall found only on host plants of the genus Angophora , and its association with a fergusoninid fly larva having a dorsal shield of the two ‘patches’ form.
Morphologically, Fergusobia colbrani n. sp. is close to F. microcarpae Davies 2013 (in Davies et al. 2013a), F. fisheri Davies & Lloyd 1996 , MSp 11 from E. viminalis , and the Fergusobia spp. from the broad-leaved melaleucas. Molecular sequences are unavailable for phylogenetic analyses.
The parthenogenetic female of F. col br ani n. sp. (C-shape to spiral) differs from F. fasciculosae Davies 2012 (in Davies et al. 2012b) (arcuate); and from F. rileyi Davies 2012 (in Davies et al. 2012a) (almost straight to arcuate). Its length (225–361 um) is shorter than that of F. magna Siddiqi 1986 sensu Davies 2010 (in Davies et al. 2010b) (500–780 µm) and F. i nd i c a ( Jairajpuri 1962) Siddiqi 1986 (525–626 µm). The stylet (7–9 µm) of this parthenogenetic female is longer than in F. curriei Fisher & Nickle 1968 (5–8 µm), F. juliae Davies 2013 (in Davies et al. 2013b) (5–7 µm) and F. microcarpae (9.5–11 µm); and shorter than in F. camaldulensae Davies 2012 (in Davies et al. 2012a) (11–13 µm) and F. pohutukawa Davies 2007 (in Davies et al. 2007) (10 µm). Its stylet is smaller than that of F. tumifaciens Currie 1937 (19 µm). In having a large to enormous pharyngeal gland ( b’ 1.8–2.7), F. colbrani n. sp. is similar to F. quinquenerviae Davies & Giblin-Davis 2004 and F. viridiflorae Davies & Giblin-Davis 2004 , but lacks the extra lobes or flexures. In having a body behind the vulva that narrows gradually, is arcuate and conoid in shape, with a broadly rounded tip, the female of F. col br ani n. sp. differs from F. cosmophyllae Davies 2011 (in Davies et al. 2013b) (narrowly rounded tail tip); F. dealbatae Davies & Giblin- Davis 2004, F. delegatensae Davies 2013 (in Davies et al. 2013b), F. eugenioidae Davies 2012 (in Davies et al. 2012b), F. floribundae Davies 2013 (in Davies et al. 2013b), F. philippinensis Siddiqi 1994 , and F. ptychocarpae Davies 2008 (in Taylor & Davies 2008) (more slender tails); from F. po ro s ae Davies 2013 (in Davies et al. 2013a) (tail with smaller volume); and from F. diversifoliae Davies 2013 (in Davies et al. 2013b), F. morrisae Davies 2012 (in Davies et al. 2012b), and F. pimpamensis Davies 2013 (in Davies et al. 2013b) (bodies narrow rapidly behind the vulva). In having a non-extensile uterus, it is separated from F. brevicauda Siddiqi 1994 , F. jambophila Siddiqi 1986 and F. rileyi , in which the uterus is extensile. It lacks the broad duct of the excretory/secretory system that is present in F. brittenae Davies 2010 (in Taylor & Davies 2010) and F. minimus Lisnawita 2013 (in Davies et al. 2013b). It is similar to F. fisheri , but lacks the wrinkled cuticle on the ventral side. It is also similar to F. nervosae Davies & Giblin-Davis 2004 , but tends to have a shorter tail relative to the anal body diameter (respectively, c ’ 0.6–1.4 vs 1.4–2.0). Morphologically, the parthenogenetic female of F. colbrani n. sp. cannot be separated from that of F. cajuputiae Davies & Giblin-Davis 2004 and F. leucadendrae Davies & Giblin-Davis 2004 , but they do differ biologically, developing on genetically divergent hosts in Angophora and Melaleuca ( Davies et al. 2010a).
The infective female of F. colbrani n. sp. (arcuate) differs in shape from F. brittenae , F. diversifoliae , and F. fasciculosae (open C-shape); from F. floribundae , F. juliae , F. morrisae , F. ptychocarpae and F. viminalisae (strongly curved in posterior region), and from F. porosae and F. rileyi (almost straight). In length (369–405 µm), it is smaller than the infective female of F. curriei (417–489 µm), F. magna (537–633 µm) and F. minimus (419–458 µm); and larger than that of F. c a j u pu t i a e (239–309 µm), F. dealbatae (307–347 µm), F. leucadendrae (270–291 µm), and F. quinquenerviae (259–325 µm). The a ratio (12.7–13.1) is larger than in F. brevicauda (8.9–10.6), F. cosmophyllae (4.0–6.8), and F. microcarpae (10.2–11.8). The infective female of F. c o l b r a ni n. sp. can be separated from that of F. camaldulensae and F. eugenioidae by having a flat, rather than a raised, circum-oral area. Its head capsule is distinctly offset and raised about 2 µm from the rest of the body, separating it from F. delegatensae , in which the head is not so clearly offset or raised. Fergusobia colbrani n. sp. has a broad tail with a sub-hemispherical tip, separating it from F. philippinensis , which has a tip that is truncate in shape. The infective female of F. pimpamensis , with c ’ ratio of 1.5–2.4, has a tail that is longer relative to its diameter than in F. colbrani n. sp. (0.8–1.2). Morphologically, the infective female cannot be separated from that of F. f i s h e r i. While only one infective female of F. nervosae has been examined, it appears to have a longer tail compared to body length than that of F. colbrani n. sp. (respectively, c 12 vs 17.3–20.5, and c ’ 1.6 vs 0.8–1.2). Similarly, F. viridiflorae (n = 1) appears to have a more anterior vulva (V% 79 vs 85–86) than in F. c o l b r a ni n. sp., but more specimens should be examined to confirm this.
In having spicules with a notched tip, the male of F. colbrani n. sp. is separated from all other described fergusobiid males, in which the tip is not notched. The notch is apparently an indent in the cuticle at the point where the channel between the spicules, for passage of the sperm, opens. In addition, the spicule of F. colbrani n. sp. appears to be more heavily sclerotised than in the other species of Fergusobia collected from Angophora .
Form of gall. Warty, spongy, spheroid galls on leaf blades (collected from Murrurundi district, NSW, Australia) ( Fig. 7 View FIGURE 7 F; and see Fig. 3 View FIGURE 3 in Colbran (1964) for a similar gall form), protruding from only one surface of leaf. About 0.5 to 1 cm in diameter. Lack a clear epidermal covering, contain 1 to 3 locules.
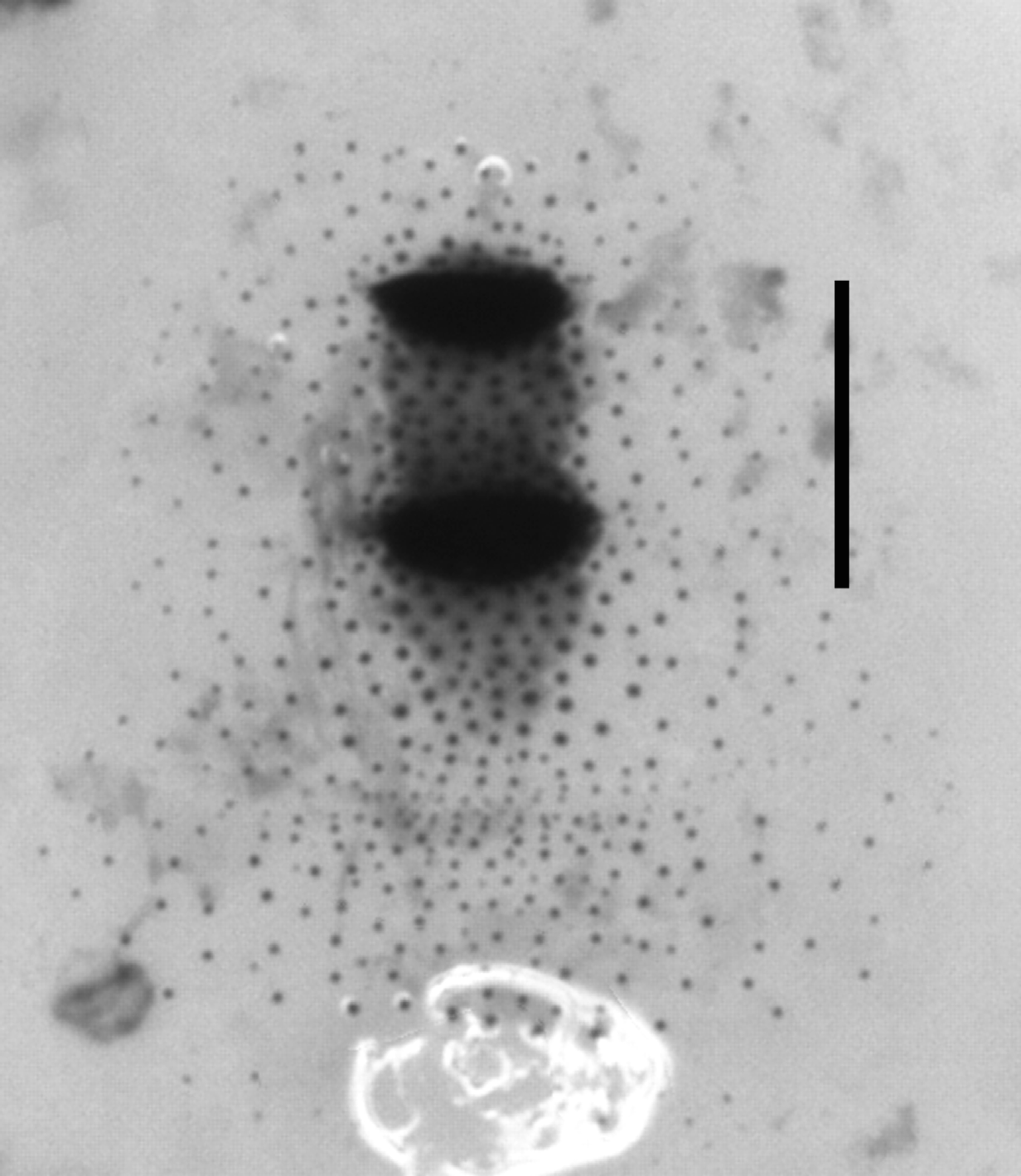
Morphology of dorsal shield. Mature third stage larvae of associated Fergusonina fly with a dorsal shield comprising two separate patches of heavily sclerotised cuticle ( Fig. 8 View FIGURE 8 ). The first is on TS 3, and has a posterior patch of moderately sclerotised cuticle confluent with the second heavily sclerotised patch on AS 1, with a weakly sclerotised patch posterior to it. The whole shield is surrounded by sparse sclerotised raised spicules.
Etymology. Named after the late Dr. Bob Colbran, of Brisbane, Queensland, plant and soil nematologist, who first photographed and recorded spheroid leaf galls on Angophora .
Fergusobia Morphospecies A (WNC no. 2214) ( Figs 1 View FIGURE 1 C; 2C; 3C, 5, Table 2); associated with an undescribed species of Fergusonina (WINC no. 003225).
Form of gall. ‘Leafy’ leaf bud galls (collected from Murrurundi district, NSW, Australia). Similar to, but smaller than, those described from E. viminalis and E. bridgesiana ( Taylor et al. 2005) ; about 0.5 cm in diameter. Galls form within 2 or more fused leaf blades, bounded by developing leaves of the bud which usually grow past the galled tissue. Multilocular.
Morphology of nematodes. Parthenogenetic female of medium size, relatively slender; arcuate to open Cshape; large pharyngeal gland; uterus non-extensile; V about 85%; slender conoid tail. Infective female not collected. Male medium to large; slender, usually C-shape but may be arcuate; medium pharyngeal gland; spicule arcuate to angular with manubrium and shaft longer than blade, manubrium wider than shaft; relatively slender arcuate tail with slightly expanded tip; bursa arises at 70–90% of body length anterior from tail tip, from near excretory/secretory pore to near anterior end of body.
Morphology of dorsal shield. Shield absent.
Fergusobia Morphospecies B (MSp 50 in Davies et al. (2010a), WNC nos 2013, 2077, 2215, 2235) ( Figs 1 View FIGURE 1 D; 2D,F; 3D,F; 6; Table 2); associated with an undescribed species of Fergusonina (WINC no. 003224).
Form of gall. Multilocular axillary vegetative bud ‘stem’ galls (various collections from Breeza, Wiseman’s Ferry and Murrurundi districts, NSW, Australia) ( Fig. 7 View FIGURE 7 B–E). Typically rounded to elliptical, with irregular shape and diameter 0.5–2.0 cm. They are amorphous in form and variable in volume, depending on the number and arrangement of locules, usually <10. There are two types: one is smooth, and the other has small protuberances on the surface which appear to be miniature buds and are often hairy giving the gall a ‘mossy’ appearance. In texture, harden and become ‘woody’ as the gall matures. Loss of the hairs on the ‘moss’ form apparently occurs as these galls mature (Taylor, unpub. data).
Morphology of nematodes. Parthenogenetic female of medium length, stout; almost straight to C-shape when heat-relaxed; cephalic region with raised, peaked circum-oral area; medium pharyngeal gland; extensile uterus; V about 75–85%; short conoid tail with bluntly rounded tip. Infective female not collected. Male of medium length; stout, almost straight to open C-shape; cephalic region with raised circum-oral area; large pharyngeal gland; angular spicule with manubrium and shaft longer than blade, manubrium not wider than shaft; slender tail with bluntly rounded tip; bursa arises near anterior end of nematode.
Morphology of dorsal shield. Shield comprises two patches of heavily sclerotised cuticle; first a patch on the posterior margin of TS 3 confluent with a patch on the anterior edge of AS 1, and second a narrow sclerotised patch on the posterior margin of AS 1 and the anterior margin of AS 2. No raised sclerotised spicules around the patches.
Remarks. The collections from the ‘smooth’ and ‘moss’ forms of the gall may be of different species of Fergusobia —see Discussion.
Fergusobia floribundae Davies 2013 (in Davies et al. 2013b) (WNC 2014, 2350, 2534) ( Figs 1 View FIGURE 1 A, 2A, 3A) associated with an undescribed species of Fergusonina (WINC nos 0 0 3275, 0 0 4314, 0 0 4789, 063866).
Form of gall. Terminal shoot bud galls (collected from Breeza, Mudgee and Hawkesbury districts, NSW, Australia) ( Fig. 7 View FIGURE 7 A). Elliptical to sub-spheroid in shape; mature galls to 2.5 cm in diameter. In some, leaf material grows past the galled tissues. Number of locules>10.
Morphology of nematodes. Parthenogenetic female relatively mid-size, slender; C-shape; large pharyngeal gland; uterus non-extensile, V about 80%; slender arcuate conoid tail. Infective female arcuate shape with a hemispherical tail tip. Male medium to large; slender, arcuate or J-shape; medium pharyngeal gland; angular spicule with manubrium and shaft longer than blade, manubrium wider than shaft but not offset; slender tail; bursa arises at about 25–50% of body length anterior to tail tip.
Morphology of dorsal shield. Shield absent.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |