Anastatus ( Anastatus ) japonicus Ashmead, 1904
|
publication ID |
https://doi.org/10.11646/zootaxa.4767.3.1 |
|
publication LSID |
urn:lsid:zoobank.org:pub:BAF472F8-CD4E-4518-A279-CCAA12F01737 |
|
DOI |
https://doi.org/10.5281/zenodo.3797147 |
|
persistent identifier |
https://treatment.plazi.org/id/EF69D43A-FFA7-FFD1-FF74-F99EFD47FDFC |
|
treatment provided by |
Plazi |
|
scientific name |
Anastatus ( Anastatus ) japonicus Ashmead, 1904 |
| status |
|
Anastatus ( Anastatus) japonicus Ashmead, 1904 View in CoL
Figs 15–17 View FIGURE 15 View FIGURE 16 View FIGURE 17
Anastatus japonicus Ashmead, 1904: 153 View in CoL . Described: both sexes.
Anastatus japonicus View in CoL ; Kalina, 1981: 17–19, plate 1, fig. 6; Yang et al., 2015b: 163–164, 255, fig. 84.
Anastatus ( Anastatus) japonicus View in CoL ; Narendran, 2009: 82, figs 26, 27.
Anastatus ( Anastatus) japonicus View in CoL ; Chen et al., 2019: 126–128 View Cited Treatment , fig. 5.
Anastatus bifasciatus disparis Ruschka, 1921: 265 View in CoL . Synonymy by Bouček, 1977: 124–124.
Anastatus disparis View in CoL ; Burgess, 1929: 574 (new status).
Anastatus flavipes Sheng and Wang, in Sheng et al., 1997: 6–7 View in CoL (Chinese description). 8 (English abstract), figs 10–13. New synonymy.
Anastatus dendrolimus View in CoL ; Xiao et al., 2001: 204. Misidentification.
Anastatus colemani View in CoL ; Hu et al., 2011: 483, fig. 2. Misidentification.
Anastatus tenuipes View in CoL ; Hu et al., 2011: 483–484. Misidentification.
Anastatus flavipes View in CoL ; Peng et al., 2017: 7–10 View Cited Treatment , figs 8–18.
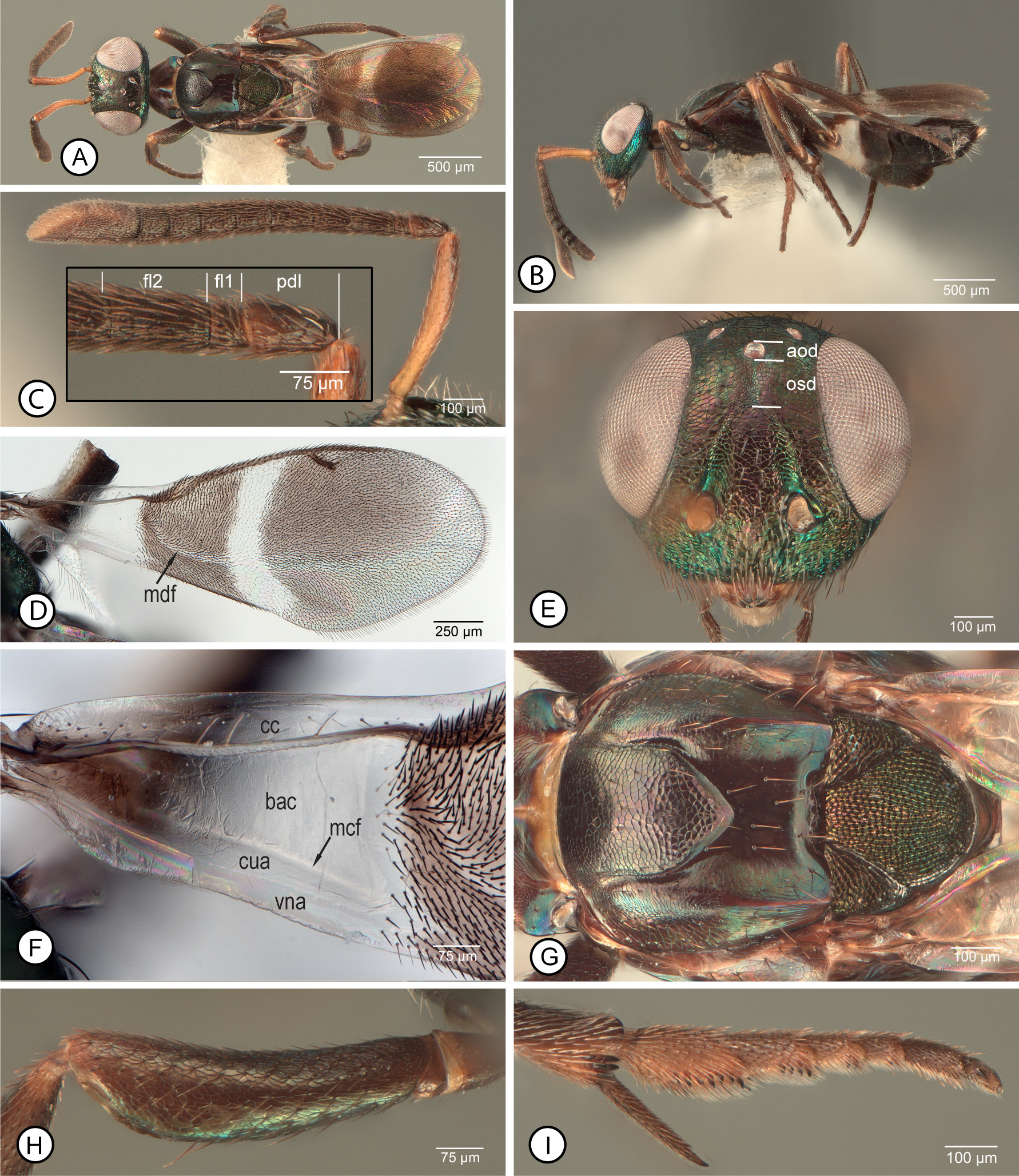
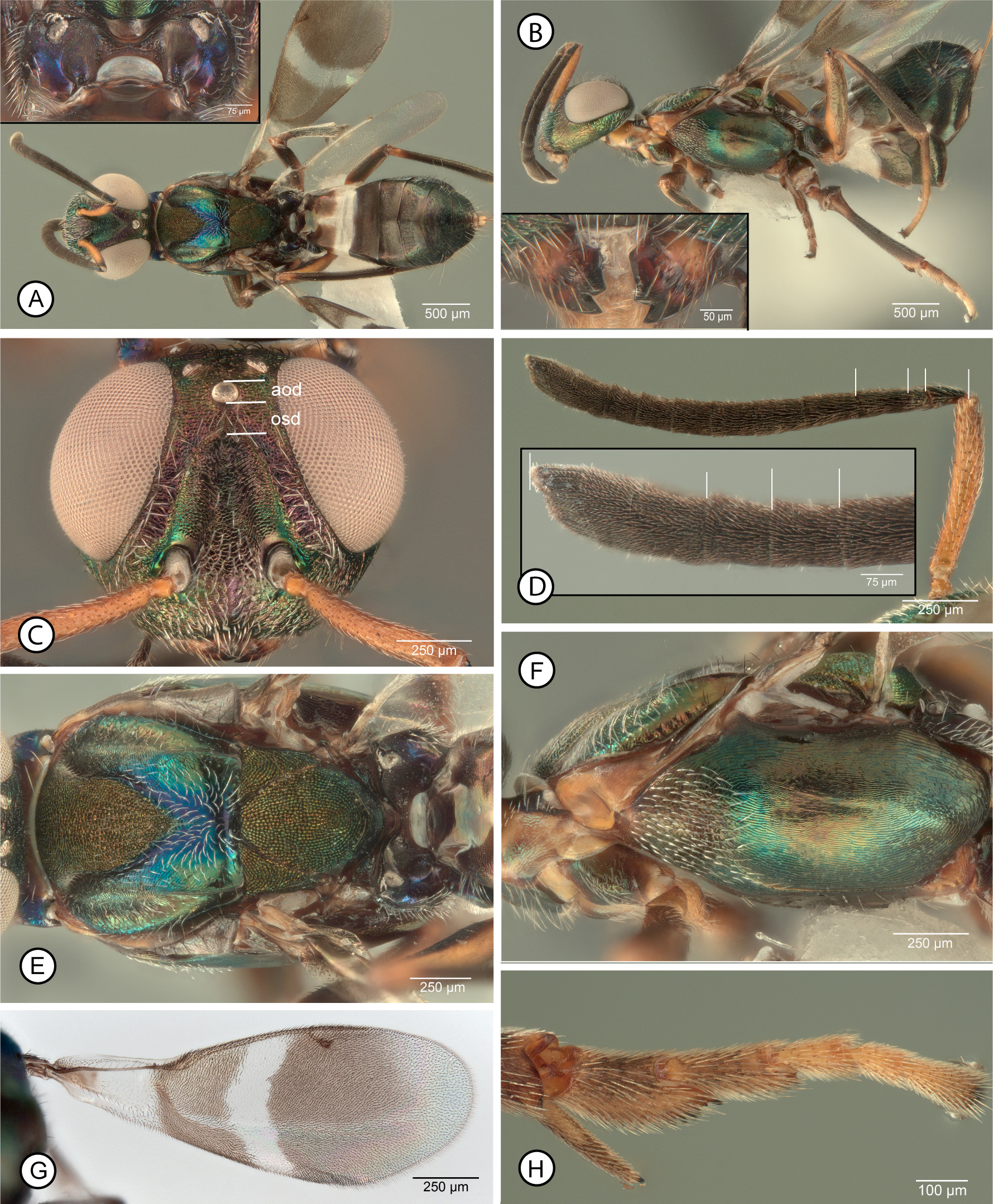
Diagnosis. Female. Macropterous ( Figs 16 View FIGURE 16 A–C, E). Fore wing with hyaline cross band behind marginal vein com- plete and with entirely white setae ( Fig. 15F View FIGURE 15 ); infuscate region basal of hyaline band with uniformly dark setae or sometimes with region of paler, more orangish setae anterior to cubital fold ( Figs 15F View FIGURE 15 , 16F View FIGURE 16 ), but about 2.0–2.5× as wide as cross band ( Fig. 15F View FIGURE 15 ); basal region with basal cell, mediocubital fold and cubital and vanal areas setose, though at least basal cell with comparatively inconspicuous white setae ( Fig. 16F View FIGURE 16 ). Head with scrobal depression separated from anterior ocellus by distance about equal to 1.2–2.0× longitudinal diameter of ocellus ( Figs 15 View FIGURE 15 A–C: lines indicate approximate distances). Antenna ( Fig. 15D View FIGURE 15 ) with fl2 longer than pedicel but not all funiculars longer than wide, with at least apical two funiculars quadrate to slightly transverse ( Fig. 15E View FIGURE 15 ). Mesosoma with mesonotum ( Figs 16G, H View FIGURE 16 ) dark, usually variably distinctly metallic green except concave posterior part of mesoscutum reddishviolaceous to partly blue or purple, but pronotum, procoxa, prepectus, and at least about posterior half of acropleuron contrastingly paler, reddish-brown to yellow ( Figs 16 View FIGURE 16 C–E); mesotibial apical spur pale ( Figs 15G View FIGURE 15 , 16A, C, E View FIGURE 16 ); mesotarsus often with all tarsomeres pale in contrast with dark mesotarsal pegs but sometimes basal two tarsomeres and apical one or two tarsomeres variably distinctly infuscate ( Figs 15G View FIGURE 15 , 16A, C, E View FIGURE 16 ). Mesoscutum ( Figs 16G, H View FIGURE 16 ) with convex anterior part of medial lobe entirely punctate-reticulate, and with posterior concave part of mesoscutum broadly setose with white setae; mesoscutal lateral lobe with bare, minutely mesh-like-coriaceous band anterior of posteromedian carina relative to more oblique, coriaceous-alutaceous sculpture on outer inclined surface ( Figs 16G, H View FIGURE 16 ). Profemur with ventral margin evenly curved, without distinct angulation or tooth apically ( Fig. 1H View FIGURE 1 ).
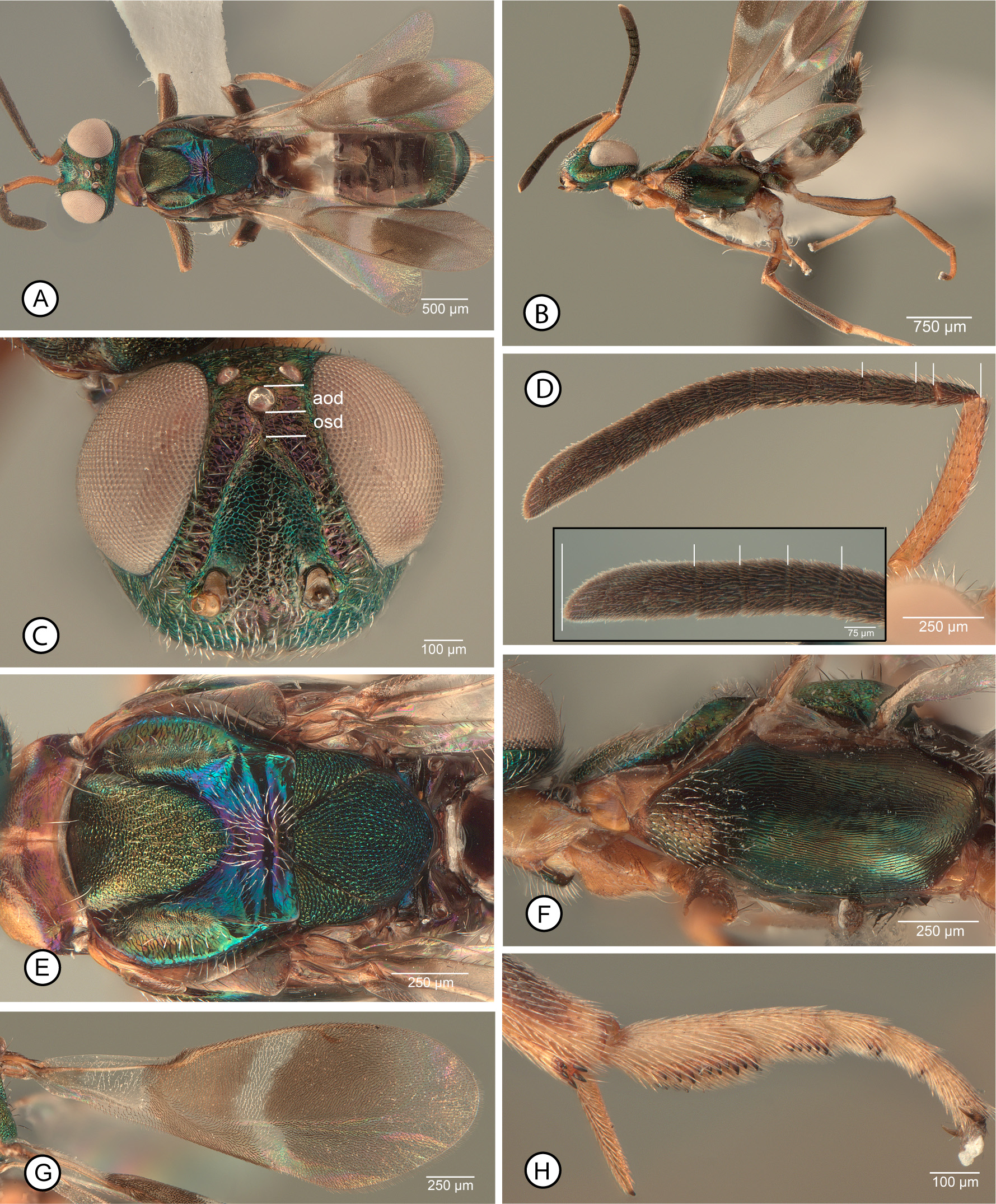
MALE. Antenna ( Figs 17E, F View FIGURE 17 ) with scape entirely dark (examined syntype, Fig. 17F View FIGURE 17 ) to variably extensively yellow ( Fig. 17E View FIGURE 17 ); flagellum uniformly dark such that multiporous plate sensilla not contrasting in colour with surrounding cuticle, and consisting of clava and seven funiculars, the clava variably shorter than ( Fig. 17G View FIGURE 17 ) to longer than (examined syntype, Fig. 17 H View FIGURE 17 ) combined length of fl6–fl8. Head ( Fig. 17F View FIGURE 17 ) with frons mesh-like coriaceous. Mesopleurosternum uniformly dark ( Figs 17 View FIGURE 17 A–C). Legs beyond trochanters at least with all femora and metatibia mostly dark ( Figs 17 View FIGURE 17 A–C), with mesotibia usually entirely pale ( Figs 17B, C View FIGURE 17 ), but type males with about apical half of mesotibia dark ( Figs 17A, J View FIGURE 17 ). Fore wing ( Fig. 17I View FIGURE 17 ) with costal cell dorsally setose along entire leading margin; basal cell entirely, uniformly setose with dark setae; disc with variably large but often comparatively slender, oblique speculum ( Fig. 17I View FIGURE 17 : spc) closed posteriorly by line of dark setae.
Species concept. Our concept of A. japonicus is based on examination of one female labelled “ Japan | Koebele / CoType | No. 7168 | U.S. N.M” and one male labelled “ Japan | Koebele / ♂ Type | No. 7168 | U.S. N.M”, plus online images of a second similarly labelled USNM female available at: www.usnmhymtypes.com/default. asp?Action=Show_Types&Single_Type=True&TypeID=3511). This latter female is labelled as “ ♀ type” and is segregated in the USNM type collection, but all the remaining type specimens of A. japonicus are syntypes because Ashmead (1904) did not originally designate a holotype.
Our concept of A. flavipes is based on examination of the female holotype, male allotype, and remaining 11 female and 5 male paratypes (FAFU) from Jiangxi and Shandong provinces, China as detailed by Peng et al. (2017).
Regional records. Anastatus japonicus was reported previously from China by several authors (see references in Noyes 2019), though at least some of the records associated with parasitism of T. papillosa from mainland China are misidentifications of A. fulloi (see under latter species). The species has not previously been reported from Taiwan, where we confirm it as a parasitoid of T. papillosa .
Regional material examined. Anhui: Bao’er-shan, Quanzhai, 16.VIII.1982, B. Luo ( 1♀ IZCAS). Beijing: Arboretum near Temple of Sleeping Buddha, 5.VII.1992, H.W. Browning, on Juglans regial [ sic] ( 1♀ CNC). Ciyun Temple, 31.VII.1956, D. Zhang ( 1♀ IZCAS). Dashankou, 23.VII.1955, D. Zhang ( 1♀ IZCAS). Haidian, 7.VII.2013 ( 1♀ FAFU), 2013 ( 2♀, 1♂ FAFU), VII.2014 ( 1♀, 2♂ FAFU). Haidian, Baiwang Mountain, VIII2014, J. Zhang and T. Haye, ex. eggs Halyomorpha halys on Robina trees ( 21♀, 3♂ CNC), VII.2015, ex. eggs Cappaea tibialis on Robina trees ( 12♀, 4♂ CNC). Haidian, Baiyushan, VII.2015 ( 2♀, 1♂ FAFU). Haidian, Lengquan village, 2013, J. Zhang and T. Haye, ex. eggs Plautia fimbriata on mulberry tree ( 19♀, 18♂ CNC), VII.2014, ex. eggs Dolycoris baccarum (L.) ( 7♂ CNC), ex. eggs Menida violacea Motschulsky, 1861 ( 10♀, 3♂ CNC). Haidan, Miaofeng Mountain, VIII.2014, J. Zhang and T. Haye, ex. eggs Halyomorpha halys on Robinia trees ( 7♀ CNC). Haidian, Shengshuyuan, 2013, J. Zhang and T. Haye, ex. eggs Plautia crossota on mulberry trees ( 10♀, 13♂ CNC), 18.VI.2013, ex. eggs Halyomorpha halys on Robinia trees ( 3♀, 5♂ CNC). Xishan, VIII.2014 ( 2♀ FAFU). Fujian: V.1992, Lin, reared from Tessaratoma papillosa Drury ( 15♀, 12♂ CNC). Gansu: Kang Country, Longnan City, 23.I.2018, Y. Chen, ex. Caligula japonica Moore egg, laboratory reared on Antherea pernyi (Guérin-Méneville) eggs ( 24♀, 18♂ CNC). Hainan: Nankai, Baisha County, 14.V.2009, T. Hu, light trap, IOZ(E) 1812020 ( 1♀ IZCAS). Hebei: Langfang City, 26.VII.2013 ( 1♀ FAFU). Jiangxi: Bamian Mountain, Huangyangjie, Jinggang Mountains, 29.VII.2014, Q. Liu ( 1♀ FAFU). Jilin: campus of Jilin Agriculture University, Changchun City, 25.VI.2017, Y. Chen ( 1♀ FAFU). Campus of Jilin Agricultural University, Changchun City, 20.VII.2017, Y. Chen, ex. laboratory reared eggs of Antherea pernyi ( 36♀, 21♂ CNC). Liaoning: Tai Shan Shan Forest Farm, Manchu Autonomous County, Benxi City, 23.IV.2017, Y. Chen, ex. Caligula japonica egg, laboratory reared on Antherea pernyi (Guérin-Méneville) eggs ( 29♀, 18♂ CNC). Shaoguoyingzi, Jianping, 23.VIII.1982 ( 1♀ IZCAS). Shaanxi: Haoping, Taibai Mountains, Malaise trap, 26.IX.2014, Z. Yang ( 1♀ FAFU). Shanxi: Lingqiu, 9.VIII.1984, X. Gao ( 2♀ IZCAS). Taiwan: Miaoli, field collect- ed from T. papillosa eggs in 2015 and laboratory reared on T. papillosa eggs, Y.-H. Wu ( 15♀, 12♂ CNC), laboratory reared in National Chung Hsing University, 30.VII.2017 ( 1♀ FAFU). Taipei, 24.037267°N 120.687722°E, V.2018, J.-C. Hsu, laboratory reared (F2) 12.IX.2018, Tessaratoma papillosa ( 2♀ CNC). Taipei, field collected from T. papillosa eggs and laboratory reared on Samia cynthia eggs, 24.VT.2017, Y.-H. Wu ( 7♀, 4♂ CNC). National Taiwan University, Taipei, 28.X.2015, Y.-H. Wu, from Tessaratoma papillosa ( 7♀, 1♂ CNC). Taipei Botanical Garden, Taipei, VIII.2016, L.-J. Wang ( 1♀ CNC). Zhejiang: Tianmu Mountains, 5.VII.2014 ( 4♀ FAFU), 27.IV.2014 ( 1♀, 1♂ FAFU), reared from egg of Hippotiscus dorsalis, H.E. Sunqiang.
Distribution. Regional. ORIENTAL: China ( Fujian, Guangdong, Guangxi, * Hainan, Hong Kong, Jiangsu, *Jiangxi, * Taiwan, *Zhejiang). PALAEARCTIC: China: ( * Anhui, Beijing, * Gansu, * Hebei, * Heilongjiang, Jilin, Liaoning, Shaanxi, Shanxi, Shandong).
Extralimital. Noyes (2019) reported A. japonicus from at least one country from all biogeographic regions except the Neotropics. It is widely distributed throughout the entire Palaearctic Region where it is native, but presence in Canada and United States of America (Nearctic) results from secondary introductions associated with biocontrol of Lymantria dispar (Linnaeus, 1758) ( Lepidoptera : Erebidae ) ( Crossman 1925).
Hosts. Noyes (2019) reported A. japonicus as a parasitoid of over 15 host species in two families of He- miptera ( Alydidae , Pentatomidae ) and five families of Lepidoptera ( Lasiocampidae , Lymantriidae , Notodontidae , Papilionidae , Saturniidae ), sometimes as a hyperparasitoid through Braconidae ( Hymenoptera : Ichneumonoidea) and Encyrtidae ( Hymenoptera : Chalcidoidea) primary parasitoids. Hu et al. (2011), under the misidentification A. colemani , reported Rhynchocoris humeralis as a host, and Chen et al. (2019) newly reported Caligula japonica as a host. New host records reported here are Cappaea tibialis Hsiao and Cheng, 1977 , Dolycoris baccarum (Linnaeus, 1758) , Halyomorpha halys (Stål, 1855) , Menida violacea Motschulsky, 1861 and Plautia crossota (Dallas, 1851) ( Hemiptera : Pentatomidae ). These last records, from specimens originally collected by J. Zhang and T. Haye, represent voucher material from Zhang et al. (2017), in which the parasitoid was identified as Anastatus species. Noyes (2017) also recorded seven plant families as associates of A. japonicus . Here we newly report Juglans regia L. ( Juglandaceae ), and the genera Morus L. ( Moraceae ), and Robinia L. ( Fabaceae ).
As is also discussed under A. formosanus and A. fulloi , A. japonicus apparently is an important parasitoid of T. papillosa in Taiwan, but in southern China it is replaced by A. fulloi as the principal biocontrol agent. Because A. fulloi appears to have been consistently misidentified as A. japonicus in mainland China, the extensive biological literature published under this name in China is brought into question. Li et al. (2014) listed four “challenges” for the implementation of integrated pest management in litchi orchards in China. To this list should also be added a fifth challenge, accurate taxonomy of the biocontrol agents.
Remarks. Females we interpret as A. japonicus are distinguished by a combination of features, being most similar to those of A. fulloi and A. orientalis because of a partly pale mesosoma and absence of a profemoral tooth. However, females we identify as A. fulloi and A. orientalis have a uniformly dark acropleuron with slight metallic luster ( Figs 10F View FIGURE 10 , 20F View FIGURE 20 ) similar in colour to the mesonotum ( Figs 10E View FIGURE 10 , 20E View FIGURE 20 ) whereas females we identify as A. japonicus have the acropleuron variably extensively but noticeably paler than the mesonotum, at least non-metallic brown posteriorly ( Fig. 16C View FIGURE 16 ), and usually conspicuously paler orangish to yellow over at least about its posterior half ( Figs 16D,E View FIGURE 16 ). Females also have the scrobal depression distinctly separated from the anterior ocellus ( Figs 15A, C View FIGURE 15 ), though this distance varies among females we examined. The exact distance between the anterior ocellus and dorsal margin of the scrobal depression can be difficult to measure accurately because the dorsal margin of the depression often is only indistinctly developed. However, the examined female A. japonicus syntype has the scrobal depression separated from the anterior ocellus ( Fig. 15A View FIGURE 15 : osd) by almost twice the longitudinal diameter of the ocellus. Females from western Europe and type females of A. flavipes ( Fig. 15B View FIGURE 15 ) have the scrobal depression separated from the anterior ocellus by about 1.5–2.0× an ocellar diameter, but many eastern Palaearctic females have the scrobal depres- sion separated by as little as 1.2× an ocellar diameter, more similar to that of females of A. fulloi ( Fig. 10C View FIGURE 10 ) and A. orientalis ( Fig. 20C View FIGURE 20 ). There is also variation in mesotarsal colour pattern that appears to form a continuum among females of A. japonicus , A. fulloi , and A. orientalis . Females of A. orientalis have entirely pale mesotarsi ( Fig. 20H View FIGURE 20 ) whereas A. fulloi females often have entirely infuscate mesotarsi, but at least the dorsal and posterior surfaces of the basal two tarsomeres are noticeably darker than the following two tarsomeres ( Fig. 10H View FIGURE 10 ). However, some females we identify as A. japonicus have entirely pale mesotarsi ( Fig. 16D View FIGURE 16 ) similar to A. orientalis females, whereas others have the basal two tarsomeres and sometimes the apical one or two tarsomeres infuscate similar to some A. fulloi , including the syntype of A. japonicus we examined ( Fig. 15G View FIGURE 15 ) and the USNM-imaged syntype. If there are associated males, it is easy to distinguish A. orientalis because males of this species have entirely yellowish legs ( Fig. 21B View FIGURE 21 ) or at most the metafemur variably dark ventrally ( Fig. 21E View FIGURE 21 ) as well as basally pale antennae ( Fig. 21G View FIGURE 21 ). However, we cannot presently distinguish males of A. japonicus from those of A. fulloi reliably, and because of observed variation among females of the two species confident identification of the species sometimes is difficult. Even our present concept of A. japonicus requires further study. Ashmead (1904: 153) originally described males as having the legs “aeneous-black…an annulus at base of the tibiae…white”, and when Crawford (1913: 249) described A. formosanus he stated that the species resembled A. japonicus but in males of the latter species “the middle tibiae are dark”. The male syntype of A. japonicus we examined ( Fig. 17A View FIGURE 17 ) has about the apical half of the mesotibia dark ( Fig. 17J View FIGURE 17 ). There are three other male syntypes of A. japonicus in the USNM, only one of which has the mesotibiae remaining, but this also has the mesotibiae darkened apically, whereas other USNM males from China, Iran, Japan, Spain, and the United States of America have entirely pale mesotibiae (Michael Gates, personal communication) as do all the males we record from China as well as males identified as A. japonicus from Canada and the United States of America in the CNC. Further, the examined male syntype has the clava quite distinctly shorter than the combined length of the apical three funiculars ( Fig. 17H View FIGURE 17 ), whereas other males we identify as A. japonicus have the clava at least as long as the apical three funiculars ( Fig. 17G View FIGURE 17 ). Apparent variation in relative length of the clava was also noted for A. fulloi (see further under this species) and it remains questionable whether this indicates intraspecific variation in the species or possibly unrecognised cryptic species. However, the presence of apically dark mesotibiae for type series males but entirely pale mesotibiae for all subsequently identified A. japonicus males, even those from Japan ( e.g., Tachikawa (1959) under the name A. bifasciatus ), is certainly anomalous. Ashmead (1904: 153) stated that A. japonicus was described “from several specimens received from Mr. A. Koebele”. The implication is that the association of the sexes was based on their being reared together, though there is no explicit statement of this in the publication or labelling. Three possibilities could explain the apparent anomalous leg colour pattern—1) males of A. japonicus are conspicuously variable in mesotibial colour pattern, which seems unlikely based on the diversity of subsequently reared specimens examined; 2) the type material of A. japonicus was not reared and is a mixed series with females and males belonging to different species; or 3) A. japonicus has been incorrectly interpreted subse- quent to the original description and all literature records, including our own, actually represent some other very similar but much more widely distributed species. If the latter, the correct name would be the current oldest junior synonym Anastatus disparis because Ruschka (1921: 266) described the male as having the “legs brown, femora green, front and middle tibiae and the tarsi except the last segment yellow”. We also newly synonymise A. flavipes under A. japonicus . Females of the type series of A. flavipes are somewhat unusual in having the acropleuron entirely pale ( Peng et al. 2017, fig. 9) or almost so ( Fig. 16D View FIGURE 16 ), but otherwise are typical of females we recognise as A. japonicus , including having the scrobal depression and anterior ocellus comparatively widely separated ( Fig. 15B View FIGURE 15 ), as noted above. Type males of A. flavipes are similar in leg colour pattern and antennal structure ( Peng et al. 2017, figs 15–18) to other non-type males we recognise as A. japonicus . Because of the unusually wide world distribution of A. japonicus , even if sometimes the result of intentional introductions, as well as diverse host records and morphological diversity of both sexes, molecular analyses are required to investigate whether our current concept of A. japonicus includes one or more cryptic species.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Anastatus ( Anastatus ) japonicus Ashmead, 1904
| Peng, Lingfei, Gibson, Gary A. P., Tang, Lu & Xiang, Jiawei 2020 |
Anastatus ( Anastatus ) japonicus
| Chen, Y. M. & Gibson, G. A. P. & Peng, L. F. & Iqbal, A. & Zang, L. S. 2019: 126 |
Anastatus colemani
| Hu, T. Y. & Hu, H. Y. & Xiao, H. 2011: 483 |
Anastatus tenuipes
| Hu, T. Y. & Hu, H. Y. & Xiao, H. 2011: 483 |
Anastatus ( Anastatus ) japonicus
| Narendran, T. C. 2009: 82 |
Anastatus dendrolimus
| Xiao, H. & Huang, D. W. & Zhang, G. Y. 2001: 204 |
Anastatus flavipes Sheng and Wang, in Sheng et al ., 1997: 6–7
| Sheng, J. K. & Wang, G. H. & Yu, Y. X. & Yu, J. C. 1997: 7 |
Anastatus japonicus
| Yang, Z. Q. & Yao, Y. X. & Cao, L. M. 2015: 163 |
| Kalina, V. 1981: 17 |
Anastatus disparis
| Burgess, A. F. 1929: 574 |
Anastatus bifasciatus disparis
| Boucek, Z. 1977: 124 |
| Ruschka, F. 1921: 265 |
Anastatus japonicus
| Ashmead, W. H. 1904: 153 |