Hirtuleiodes, Hennemann & Conle, 2024
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5444.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:5DE4A9DD-99F7-4E23-AD50-58DC491BB75E |
|
persistent identifier |
https://treatment.plazi.org/id/03FD87D9-FE84-D957-FF55-F6F42BF5E3F6 |
|
treatment provided by |
Plazi |
|
scientific name |
Hirtuleiodes |
| status |
|
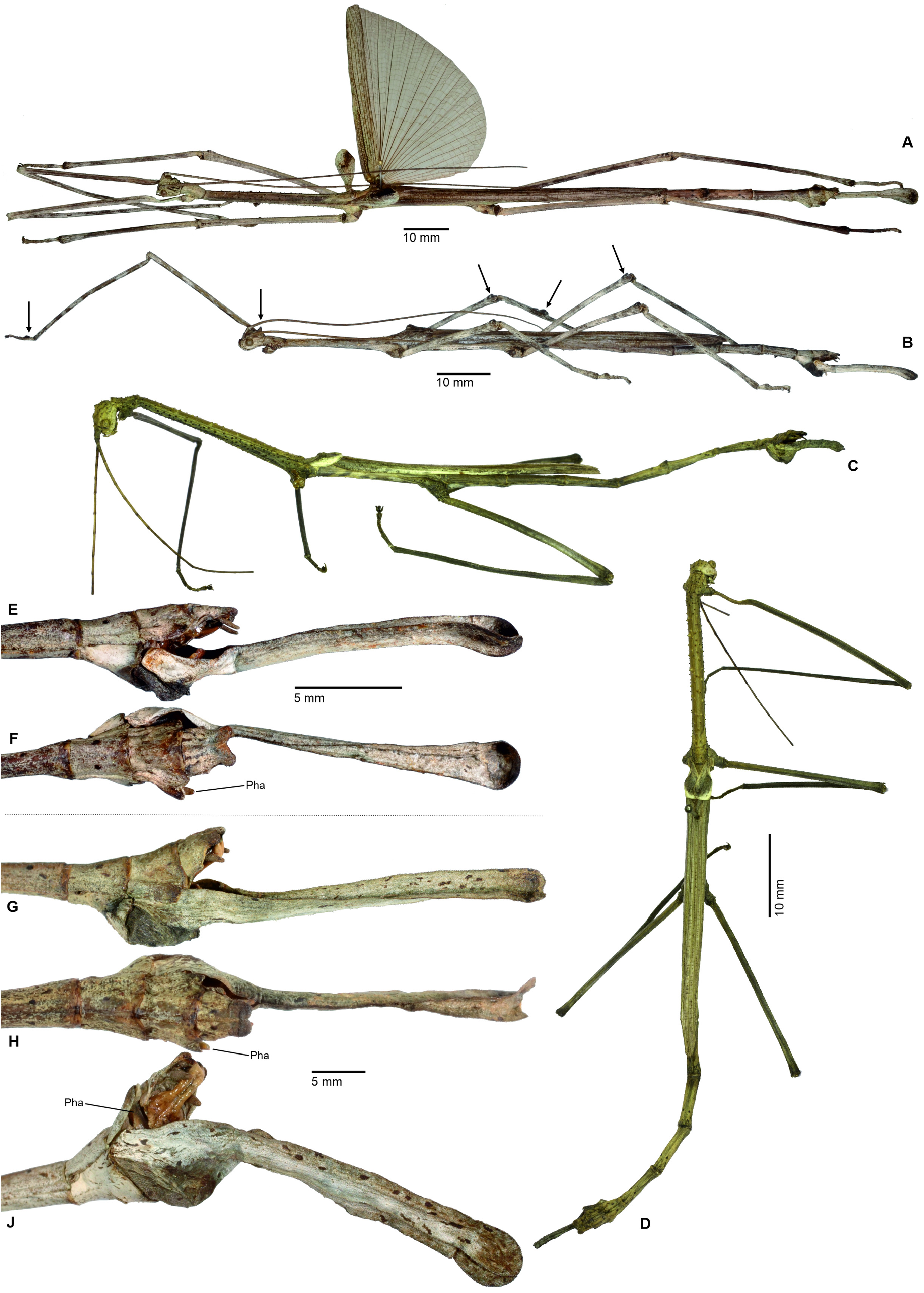
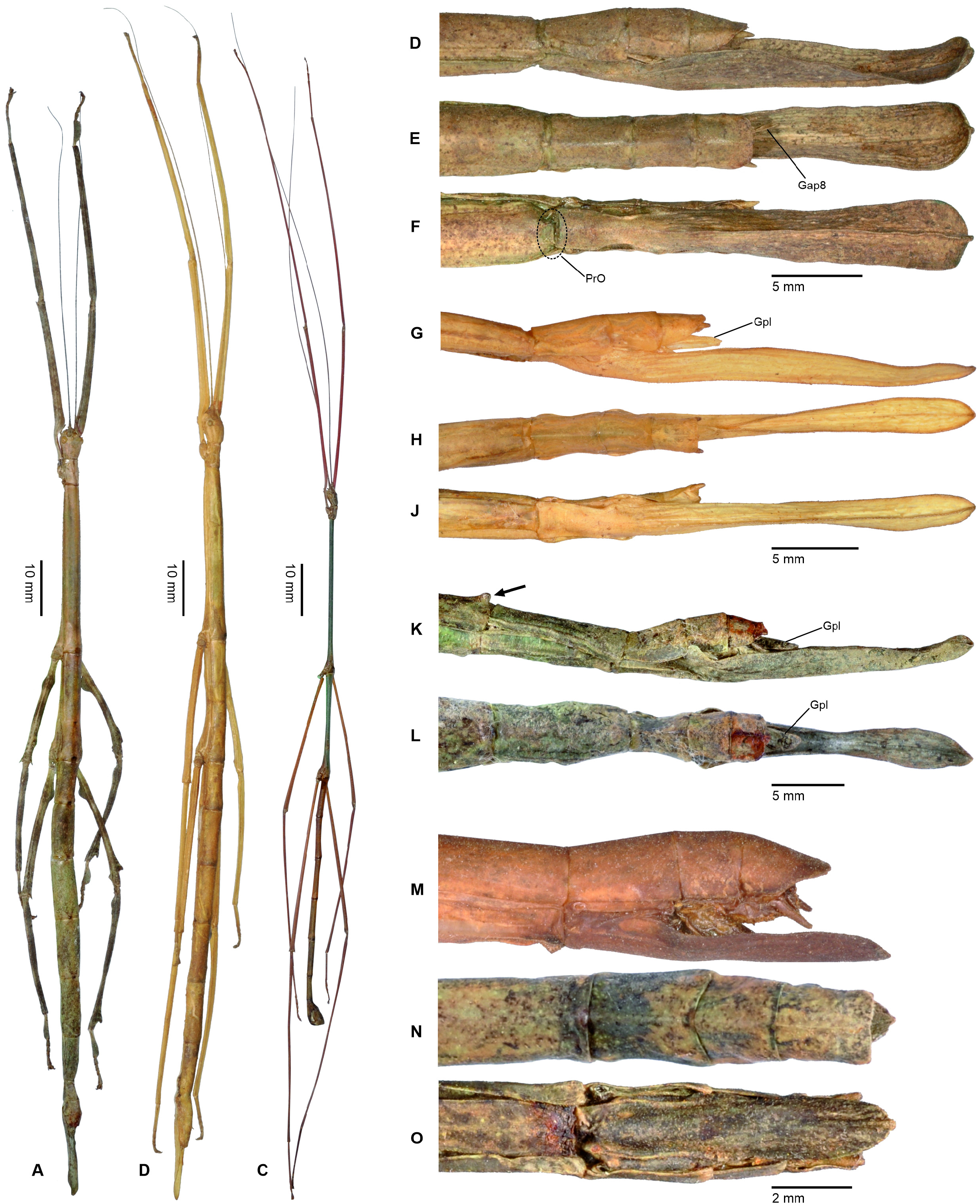
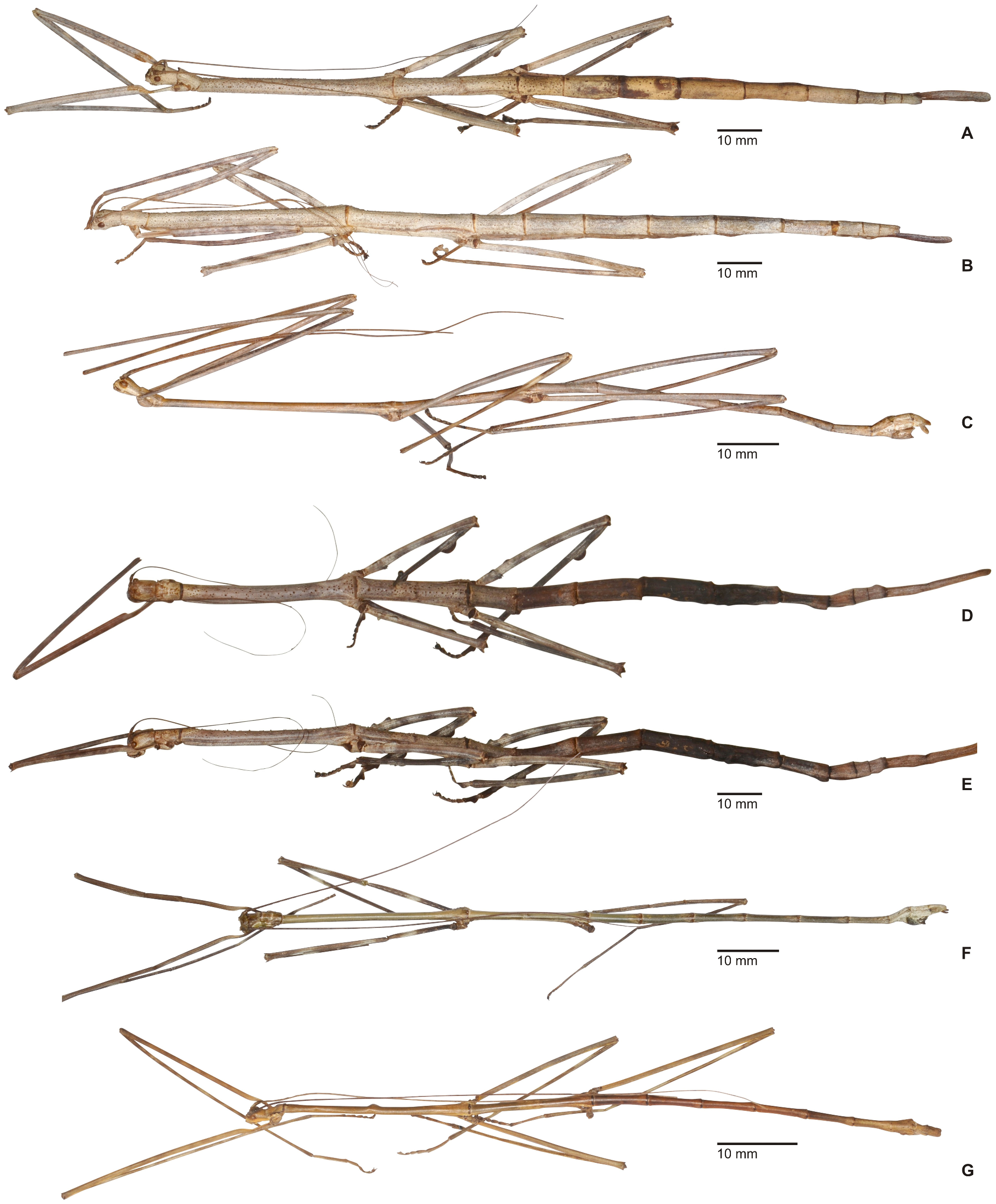
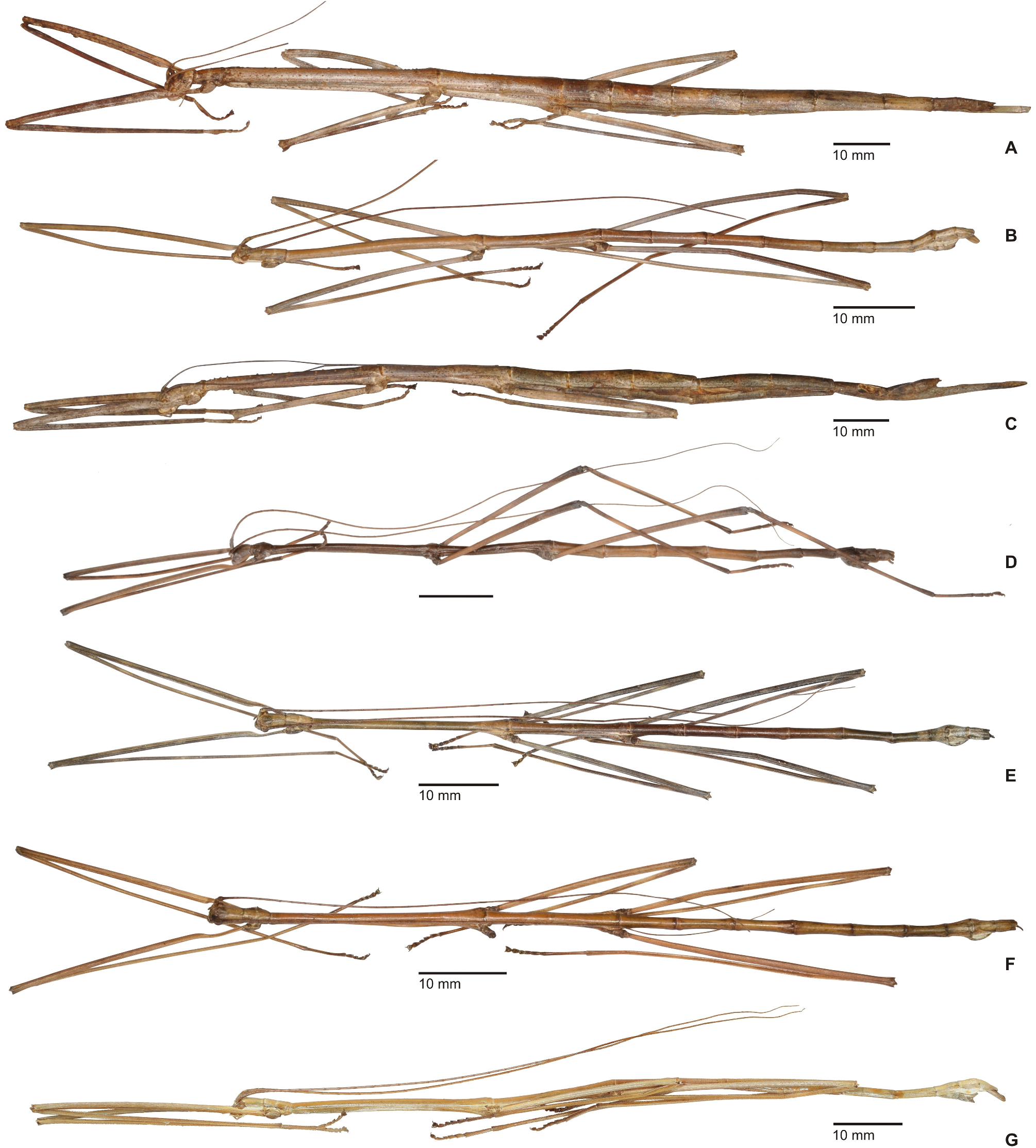
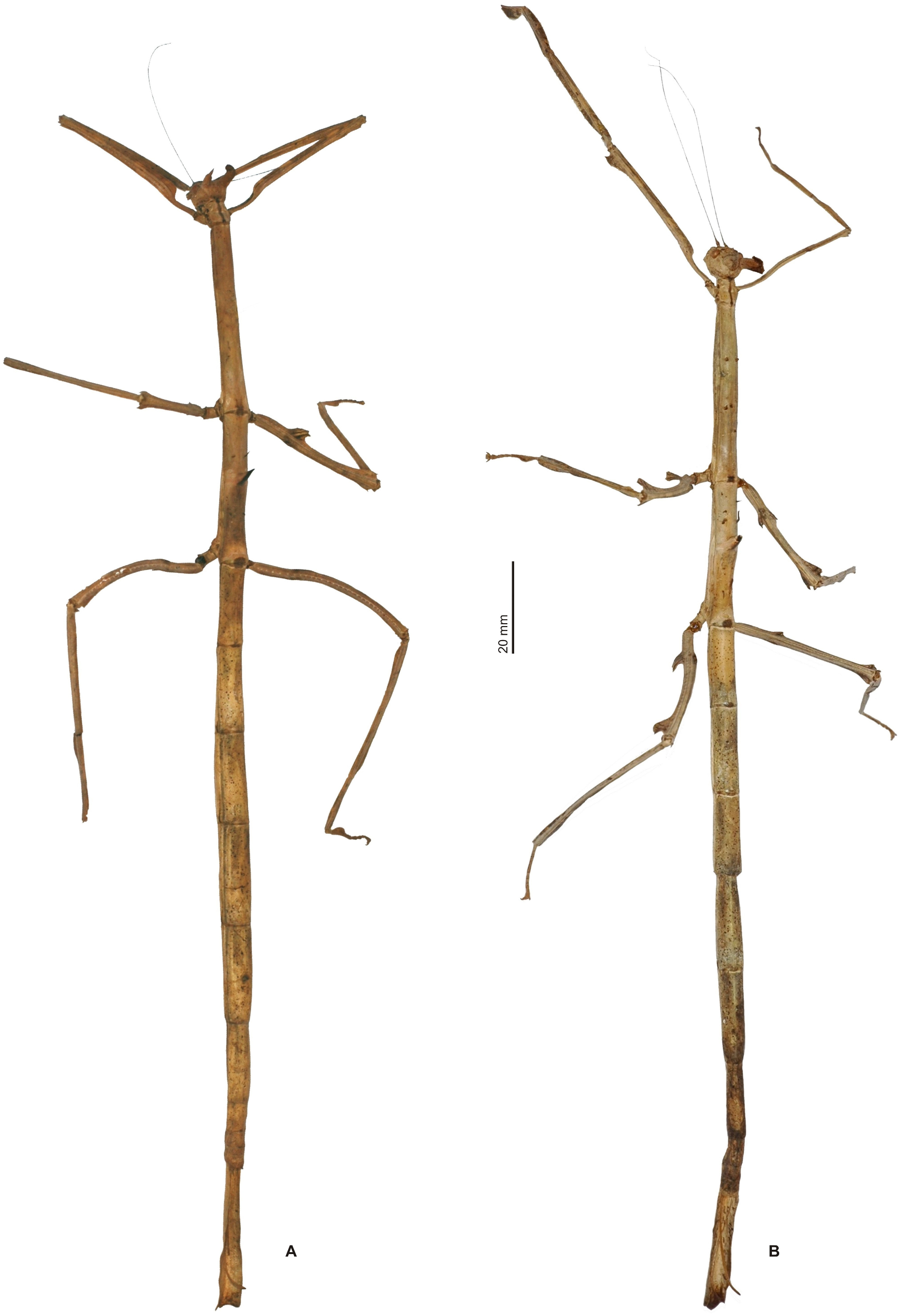
1. Xylodus ceratocephalus ( Gray, 1835: 15) . HT, ♀: Bras.; Phibalosoma ceratocephalus Gray, Westw. pl. 36, fig. 2 [OUMNH, No. 613]. comb. n. ( Figs. 6D View FIGURE 6 , 81–82 View FIGURE 81 View FIGURE 82 , 83H–J View FIGURE 83 , 94B View FIGURE 94 , 104 View FIGURE 104 )
= Xylodus adumbratus Saussure, 1859: 62 View in CoL . HT, ♀ (penultimate instar nymph): ♀ 189/53, Portorico, Brésil, Mr. T. F. Gidét [MHNG]. (Synonymised by Redtenbacher, 1908: 427)
= Phibalosoma michaelis Redtenbacher, 1908: 427 . HT, ♀: Mus. Caes. Vind., Bahia View in CoL , ex coll. Sommer; det. Redtenb. Phibalosoma michaelis ; 7643; Phibalosoma michaelis Redt. , Bahia View in CoL ! Type! [NHMW, No. 831]. syn. n.
Remarks: Examination of the holotype of Xylodus adumbratus Saussure, 1859 in MHNG ( Fig. 81D View FIGURE 81 ) has shown this to be a penultimate instar ♀ nymph of C. ceratocephalus Gray, 1853 , hence the synonymy established by Redtenbacher (1908: 247) is here confirmed. The holotype of Redtenbacher’s Phibalosoma michaelis in NHMW ( Figs. 81A–B View FIGURE 81 ) is undoubtedly conspecific with X. ceratocephalus and hence synonymised (syn. n.). The ♂ ( Figs. 82A–B View FIGURE 82 ) was described based on a specimen from Espírito Santo in ZMUH by Redtenbacher (1908: 427). This peculiar species appears to be very rare, because so far only a total four specimens have become known.
Distribution: Brazil [OUMNH]; E-Brazil, Est. Bahía [NHMW]; E-Brazil, Est. Espírito Santo [ZMUH]; NE-Brazil, Est. Paraná, Rio Paraná, Porto Rico [MHNG].
6. Biogeography
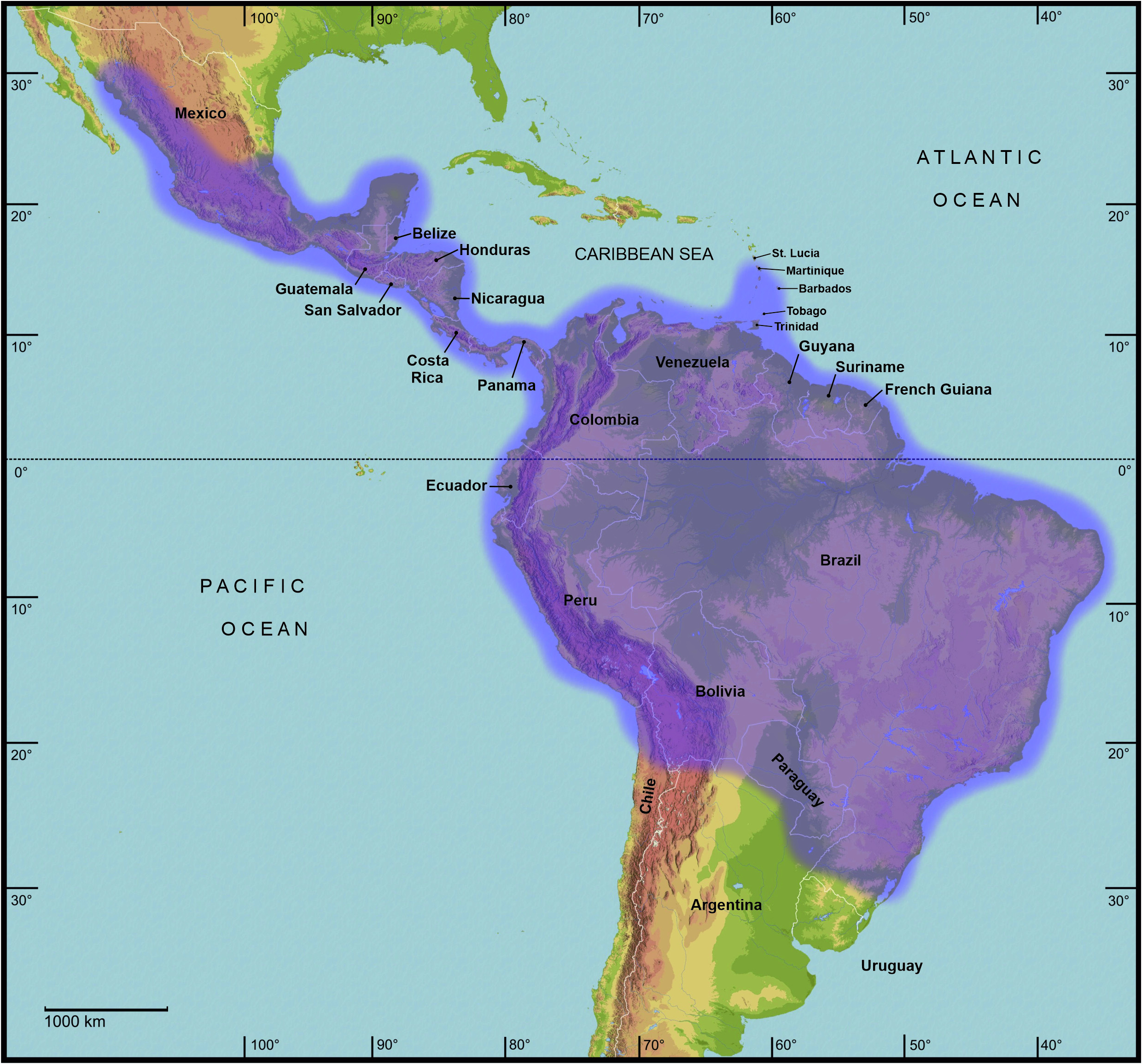
Biogeography is the discipline that describes distributional patterns of specific and supra-specific groups of organisms and aims to explain how they have come about by hypothesising historical and ecological processes ( Morrone, 2006). Based on pan-biogeographic and cladistic analyses of the entomofauna Latin America and the Caribbean are biogeographically divided into a hierarchically arranged system of regions, subregions, dominions and provinces. Between the boundaries of biogeographic regions are transition zones, which are areas of biotic overlap and harbour a mixture of different biotic elements. The northern portions of Central America, including great parts of northern and north-eastern Mexico, belong to the Nearctic region. The two central Mexican cordilleras basically form the Mexican transition zone. The remainder of Central America, the entire Caribbean and the bulk of South America east of the Andes and as far south as north-eastern Argentina represent the Neotropical region. This region basically comprises the tropics of the New World. The southern portion of South America, comprising southern and western Argentina as well as Chile is regarded as the Andean region. Between the Neotropical and Andean regions lies the South American transition zone. It extends along the highlands of the Andes between western Venezuela, northern Chile and west-central Argentina ( Morrone, 2006) . In the extended composition here presented, the Cladomorformia tax. n. clade has a very wide distributional pattern, which covers almost the entity of Latin America and the Caribbean with the only exceptions being the Greater Antilles and the highly mountainous Andean regions. The highest diversity of taxa is found throughout the Neotropical region and northern portion of the South American transition zone between Venezuela and Bolivia. Only two genera are found in the very northern part of Latin America that belongs to the Nearctic region (i. e. most of Mexico) and merely four known species are distributed on the southern Lesser Antilles as far north as the island of Dominica.
Cladomorformia represents the majority of the large to very large Phasmatodea of the New World and comprises the longest of all known insects in this part of the world, with ♀♀ of a considerable number of species exceeding body lengths of 20 cm. Not only does this clade have a very wide distributional range ( Fig. 2 View FIGURE 2 ) but members also occur in wide range of different habitats including primary forest, secondary forest montane and lowland forest, semi-arid to dry scrub-land, savannas, agricultural or even urban areas such as public parks, private gardens or small areas of secondary forest or scrub-land towards the outskirts of cities. The principal factor that determines the presence of certain taxa of Phasmatodea in certain localities is the vegetation, since phasmatodeans are dependent on their preferred host plants ( Berger, 2004: 24). In a group of insects like the Phasmatodea however, which are so well known for their masquerade crypsis, whereby they morphologically and in behaviour resemble parts of their environment and host plants, the evolutionary process of adaption inevitably goes along with the type of vegetation. A second but perhaps less important factor is represented by the climate and microclimate as well as the presence of predators.
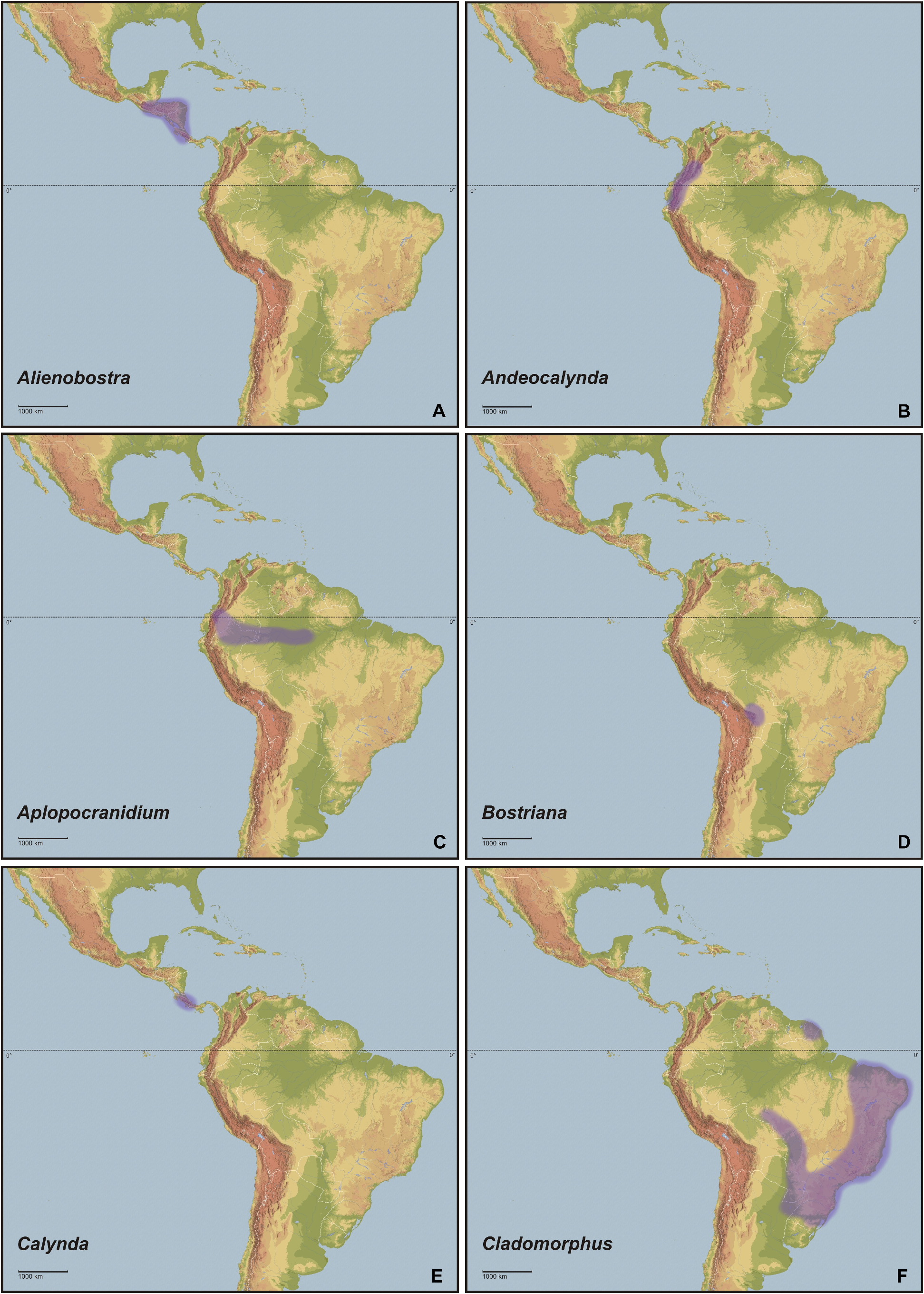
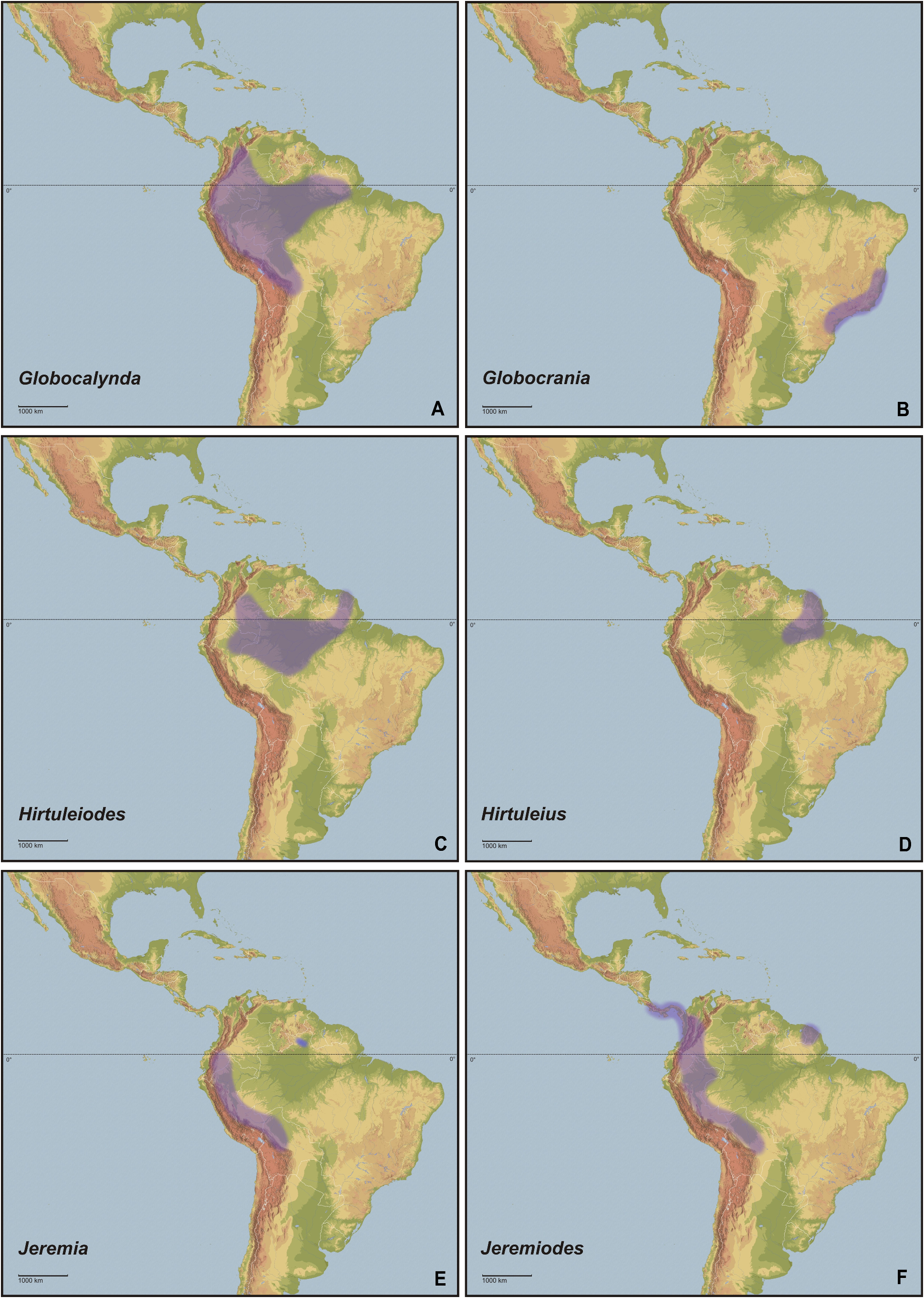
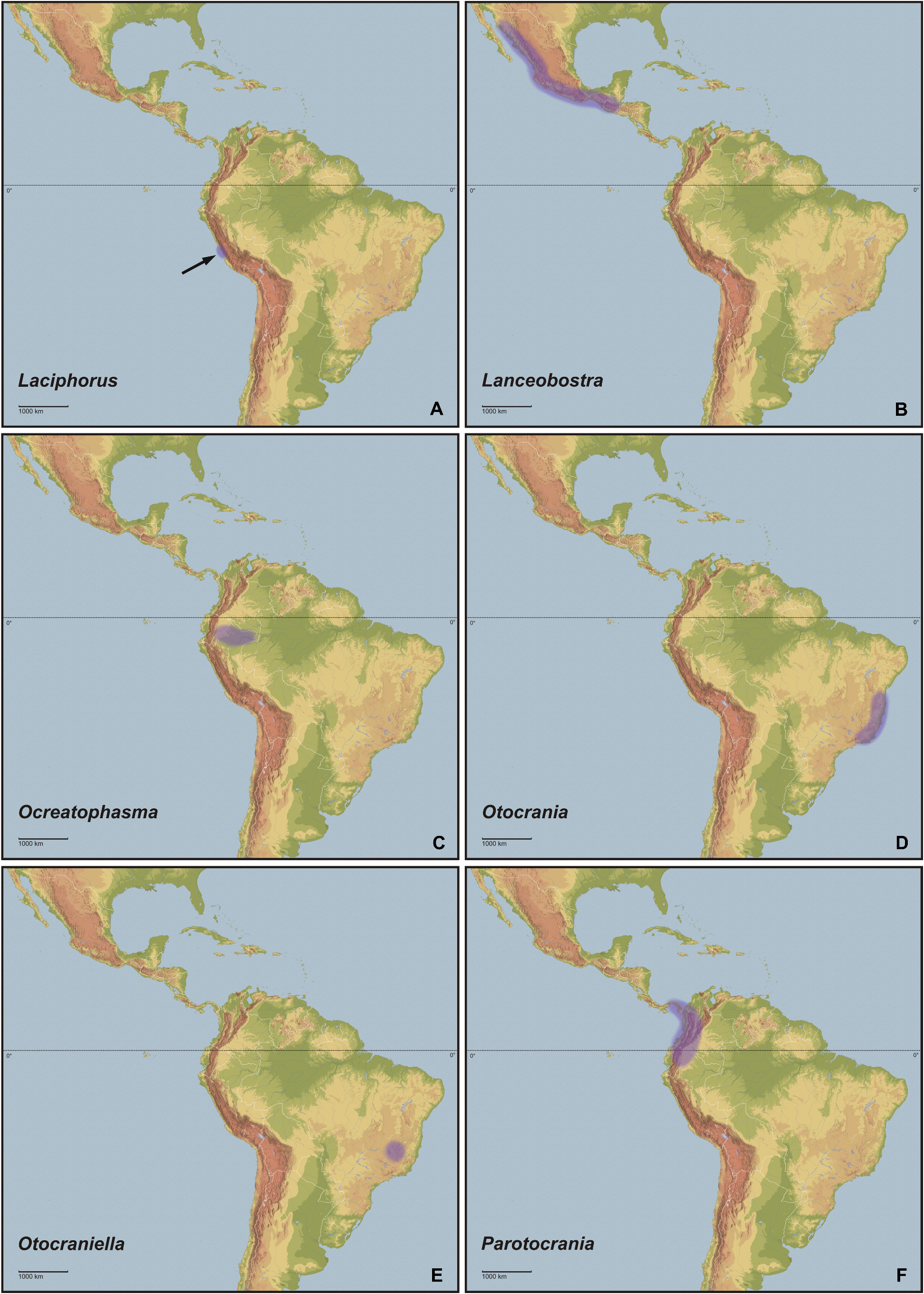
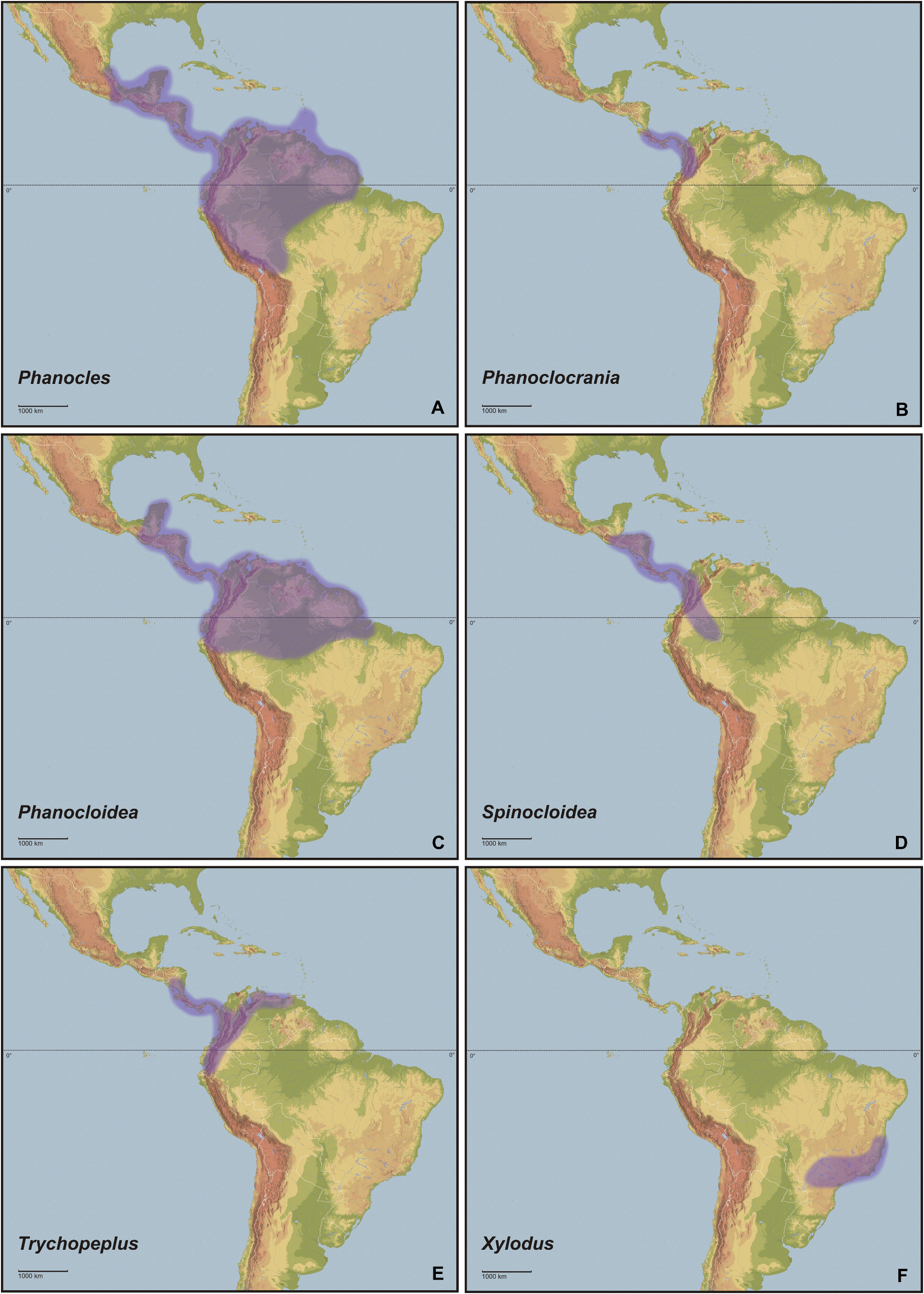
Concerning habitats, the vast number of taxa of Cladomorformia are found in humid biotopes with a clear preference to tropical lowland and montane forests ( Figs. 130B–F View FIGURE 130 , 131B–D View FIGURE 131 ) and cloud forests ( Figs. 130E–F View FIGURE 130 , 132C–D View FIGURE 132 ). One monotypic genus however, Laciphorus Redtenbacher, 1908 , is peculiar to the “Coastal Lomas” of western Peru, hilly areas of fog-watered vegetation that biogeographically lie within the dry Coastal Peruvian Desert (Hennemann & Conle, 2020: 5). The evaluations of recorded localities range from sea-level to about 3500 metres in the Andes of Colombia, Ecuador and Peru. While for most genera the greater number of species occurs in regions below 1500 metres, certain genera appear to be restricted to mountainous dwarf forest habitats throughout the Andean regions of Colombia and Ecuador (e. g. Andeocalynda Hennemann & Conle, 2020 ; Fig. 131A View FIGURE 131 ). The majority of genera, including the two speciose and widely distributed Phanocles Stål, 1875 and Phanocloidea Zompro, 2001 , have species not only in lowland but also in montane regions up to almost 2500 metres. Only few genera like Hirtuleiodes gen. n., Hirtuleius Stål, 1875 or Jeremia Redtenbacher, 1908 seem to be entirely restricted to lowland forests throughout Central and South America east of the Andes ( Figs. 102 View FIGURE 102 , C, D, E). Lanceobostra gen. n. ( Fig. 103B View FIGURE 103 ) is geographically restricted to Mexico with only two species found in northern Guatemala ( L. freygessneri and L. guatemalensis ) and almost all members of this genus are peculiar to semi-arid and dry forests, scrub-land or savannas ( Fig. 130A View FIGURE 130 ).
The genus with the by far widest distributional range is the speciose Phanocles (35 known species), which occurs from as far north as southern Mexico ( Ph. berezini , Ph. burkartii , Ph. chiapasense, Ph. horni , Ph. mexicanus and Ph. saussurei ) to as far south as Bolivia, and even has three species ( Ph. barbadosense , Ph. keratosqueleton and Ph. mutica ) on the southern Lesser Antilles ( Fig. 104A View FIGURE 104 ). Although widely spread throughout most of the northern half of the Neotropical region east of the Andes, known species diversity decreases notably towards the east and there are no records from south-east Brazil, the Chacoan and Paraná subregions or the Atlantic Forest. The highest species diversity is found in southern Central America ( Costa Rica and Panama) and the hilly to mountainous regions of Colombia, Ecuador and Peru. A similar distributional range is seen in the closely related Phanocloidea (26 species), which however is absent in Mexico with the northern-most records being in Belize and Guatemala ( Ph. incolumis , Ph. magistralis and Ph. magnifica ) and with only one species ( Ph. tobagoense ) on the island of Tobago ( Fig. 104C View FIGURE 104 ). Several genera are geographically restricted to southern Central America and north-western South America and share a very similar distributional range, i. e. Parotocrania gen. n. ( Fig. 103F View FIGURE 103 ), Phanoclocrania gen. n. ( Fig. 104B View FIGURE 104 ), Spinocloidea gen. n. ( Fig. 104D View FIGURE 104 ) and Trychopeplus ( Fig. 104E View FIGURE 104 ). The only two genera that occur exclusively in Central America are Alienobostra ( Fig. 101A View FIGURE 101 ) and Calynda ( Fig. 101E View FIGURE 101 ), both of which share a long and lanceolate subgenital plate in ♀♀ and distinctively elongated, incurved cerci in ♂♂ that are used to grasp the ♀ abdomen during the copulation. Lanceobostra gen. n. is the northern-most distributed genus (19 species) and the only genus of Cladomorformia that is found in the Nearctic region of Latin America, i.e. throughout the western Mexican states of Oaxaca, Michoacan, Colima, Jalisco, Nayarit Sinaloa and Sonora ( Fig. 103B View FIGURE 103 ). Globocrania gen. n. ( Fig. 102B View FIGURE 102 ) as well as the two striking and monotypic genera Otocrania ( Fig. 103D View FIGURE 103 ) and Xylodus ( Fig. 104F View FIGURE 104 ) are endemics of the Brazilian Atlantic Forest (Portuguese: Mata Atlântica) and the very distinctive monotypic Otocraniella ( Fig. 103E View FIGURE 103 ) appears to be endemic to some high elevation plains in the state of Minas Gerais, East Brazil. Finally, the type-genus Cladomorphus (6 species) has a moderately large distributional range and settles most of eastern South America from French Guiana in the north to central Argentina in the south and Paraguay in the west ( Fig. 101F View FIGURE 101 ).
Without any doubt analysing the distribution patterns of the genera of Cladomorformia and bringing them in correlation with morphological and molecular data may serve to better understand the still poorly known intergeneric relationships within this clade and presenting hypotheses on the geographic origin of the Cladomorformia .
7. Biology
As explained above Cladomorformia tax. n. occurs in a wide array of habitats and biotopes. Much of the information presented below is based on unpublished personal observations that the authors were able to achieve during field trips in various types of habitats in Brazil, Costa Rica, Ecuador, French Guiana, Panama and Peru (→ 3.). In general, and not surprising in aspect of their relatively large size, members of Cladomorphini mostly perch in heights between 1–5 metres off the ground, usually in undergrowth of forests, along forest edges or on solitary lowgrowing shrubs and small trees throughout scrub-land habitats. Spatially phasmids possess higher species diversity and density in forest edge habitats compared to the forest understory ( Berger, 2004: 24, table 2–1), which in several members of Cladomorformia correlates with the dietary preferences to fast growing pioneer trees species and lianas of high nutritious quality ( Coley, 1983; Coley et al., 1985) and refusal of persistent late successional tree species ( Berger, 2004). Apparently, among taxa of Cladomorformia there seems to be no preference to the canopy layer of forests, which is indicated by the lack of any confirmed records from these higher levels of the forest vegetation. Winged ♂♂ of certain species that are capable of active flight may occasionally be attracted by light traps but there is no available information on where the insects actually perched. The fact that the authors have frequently found such winged ♂♂ in lower vegetation however suggests that also these taxa do not show any specialisation to the canopy layer. Principally, like the majority of Phasmatodea of tropical regions members of Cladomorformia are not particularly abundant in neither of their natural habitats ( Novotný & Basset, 2000; Berger, 2004) and no species has so far been mentioned to occur in large or even pest-like numbers, which renders these insects generally unimportant for agriculture. Actually, most species are rarely found and occur rather solitary or may be aggregated on individual host plants. Only few species can be found in frequent numbers in certain localities, which include Bostriana boliviana in the Sara Province of eastern Central Bolivia and Lanceobostra aetolus at Venvidio in the state of Sinaloa in north-west Mexico (seen by a large series of specimens from the same locality and period in the collection of ANSP) or Phanocloidea muricata in French Guiana (personal observation in September 2001) and the state of Pará in north-east Brazil. Little is known about the natural diets of members of Cladomorformia and host plants have as yet only been cited for a very few species, some of which may be summarised here. Laciphorus capitatus is peculiar to Ophryosporus pubescens ( Asteraceae ) in the Lomas de Pachacamac east of Lima, Peru ( Aguilar, 1970: 4; Hennemann & Conle, 2020a). It is the only species that is known to be a specialised feeder, which however may be reasoned by the low diversity of vegetation in these particular habitats. Other species are known to be moderately polyphagous in their natural habitats and have been found on a variety of different plants. In the Pacific lowlands of Guanacaste Province, Costa Rica Alienobostra remiformis is known to feed on swollen thorn acacias ( Acacia collinisi and A. cornigera , Fabaceae ) during the dry season and on Mimosa pigra ( Fabaceae ), Indigofera sp. ( Fabaceae ) and Guazuma ulmifolia ( Malvaceae ) and possible other plants during the wet season ( Windsor et al., 1996: 355). Eilmus (2008: 4) provided information on the host plants of Phanocles saussurei near Puerto Escondido, Oaxaca in southern Mexico and reported the species was found on an unidentified liana of the family Combretaceae , Sida acuata ( Malvaceae ), Malvaviscus arboreus ( Malvaceae ) and an unidentified Acacia- species ( Fabaceae ) of which Sida acuata was accepted as food in captivity in Mexico. Berger (2004: 18) recorded Phanocles ploiaria from Barro Colorado Island, Panama and provided information on its dietary habits. This author stated that 34% of individuals were found on plants of the family Rhamnaceae (e. g. Gouania lupuloides ) and exclusively along forest edges. The observation that in captivity also representatives of the plant families Bignoniaceae , Melastomaceae , Sapindaceae ( Paullinia spp. and Serjana spp.), Malvaceae and Tiliaceae were accepted ( Berger, 2004: 22), suggest Ph. ploiaria to be rather polyphagous. This is supported by the wide range of alternative food plants that are accepted by P. ploiaria in breeding conditions in Europe (peronal observations), which include various Rosaceae ( Rubus spp. , Rosa spp. ), Fagaceae ( Quercus spp. , Fagus sylvatica ), hazel ( Corylus avellana , Betulaceae ) or Gaultheria shallon ( Ericaceae ). Berger (2004) stated Parotocrania panamae sp. n. (erroneously referred to as “ Otocrania sp. ”) was exclusively found on Sapindaceae on Barro Colorado Island, which can be supported by personal observations at Valle de Antón and Altos de Campana, Panama, where the type specimens were also exclusively found on members of this plant-family. Moreover, Berger (2004) stated that Parotocrania panamae sp. n. and Phanocles ploiraia were both exclusively found on pioneer tree species and lianas on Barro Colorado Island, an observation on the dietary preferences that is largely in accordance to personal observations on various Phanocles -species and related taxa throughout localities in Costa Rica, Panama, Ecuador, French Guiana and Brazil. The distinctive Cranidium gibbosum was reported to feed on pseudo-ficus or the American Cambodge tree Vismia guineenis (in fact possible referring to the South American V. baccifera , Hypericaceae ) in French Guiana ( Roubaud & Lelong, 1993: 23). On the Lesser Antilles Phanocles keratosqueleton has been reported to be rather polyphagous and natural food plants to include Acacia spp. ( Fabaceae ), Calliandra slaneae ( Fabaceae ), Canavalia campylocarpa ( Fabaceae ), Centrosema sp. ( Fabaceae ), Chamaecrista glandulosa ( Fabaceae : Caesalpinioideae), Haematoxylon campechianum ( Fabaceae ), Leucaena leucocephala ( Fabaceae ), Mimosa camporum ( Fabaceae ), Pithecellobium unguis-cati ( Fabaceae ) and Teramnus labialis ( Fabaceae ), which shows a clear preference to plants of the Fabaceae family ( Langlois et al., 2000; Lelong et al., 2003; Langlois et al., 2006). On the islands of Tobago and Trinidad ( Langlois & Bellanger, 2012: 109; Bellanger et al., 2012) Phanocles muticus has been reported to feed on Chamaecrista nictitans ( Fabaceae ) and Cecropia sp. ( Urticaceae ) and Phanocloidea tobagoensis seems to be peculiar to Lantana sp. ( Verbenaceae ) on Tobago. In Monteverde, Costa Rica Trychopeplus laciniatus is known to feed exclusively on orchids like Prosthecha campilostelix and Oncidium spp. ( Orchidaceae , pers. communication Kenji Nishida).
Alternative food plants often accepted in captivity in Europe include guava ( Psidium guayava , Myrtaceae ), oak ( Quercus spp. , Fagaceae ), beech ( Fagus sylvaticus , Fagaceae ), hazel ( Corylus avellana , Betulaceae ), roses ( Rosa spp. , Rosaceae ), bramble and raspberry ( Rubus spp. , Roasaceae) as well as salal ( Gaultheria shallon , Ericaceae ). It is not unusual that individual species feed on several of these plants in captivity, which alludes to the supposed polyphagy of these species in their natural environment.
As for most phasmatodeans, members of Cladomorformia are nocturnal in their natural habitats and rest almost motionless on their host plants during the day. Thereby the insects profit from their often remarkable camouflage in mostly resembling twigs and branches of their environment or their host plants. Among Cladomorformia this twig mimic is most distinctly developed in Ocreatophasma gen. n., a genus so far only known from the ♀♀. The thoracic sterna and limbs of this particular genus show striking specialisations that are unique not only within Cladomorformia but the whole of Neotropical Occidophasmata. The very slender and laterally compressed mid legs are tightly laid alongside the mesosternum, which has an acute medio-longitudinal keel and lateral surfaces that are slightly concave longitudinally to hold the legs. The protibiae have the dorsal carina deflexed to form a blade-like lamella. In that all legs are possibly held in an axis to the body when the insects are in their resting position (catalepsy), this provides a stunning twig mimic that remarkable resembles behaviour seen in Oriental genera of the Lonchodidae : Lonchodinae (e. g. Hermagoras Stål, 1875 or Leprocaulinus Uvarov, 1940 ). These taxa are known to often just hang headlong by their posterior tarsi to appear like a straight hanging down filament or stick, a behaviour which may also be assumed for Ocreatophasma . Members of Trychopeplus are very peculiar for showing impressive morphological crypsis mimicking moss in their natural habitats. As in practically all phasmids feeding, moulting and copulating almost exclusively take place at night. Defensive reactions include remaining motionless, dropping to the ground and quickly walking away. While the wingless ♀♀ often show very hectic movements and curl on the forest or cage floor, ♂♂ that are capable of active flight may fly off if disturbed.
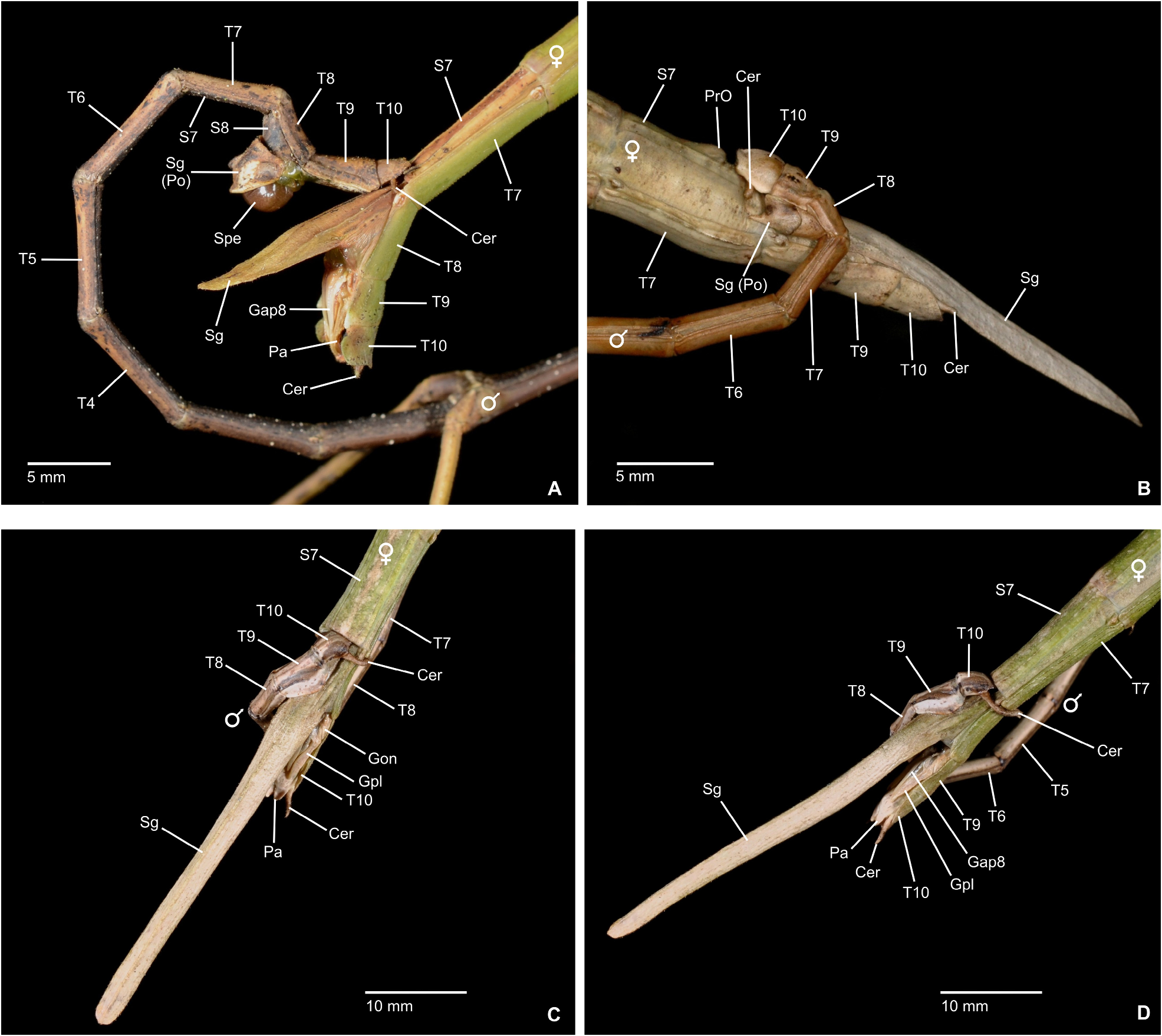
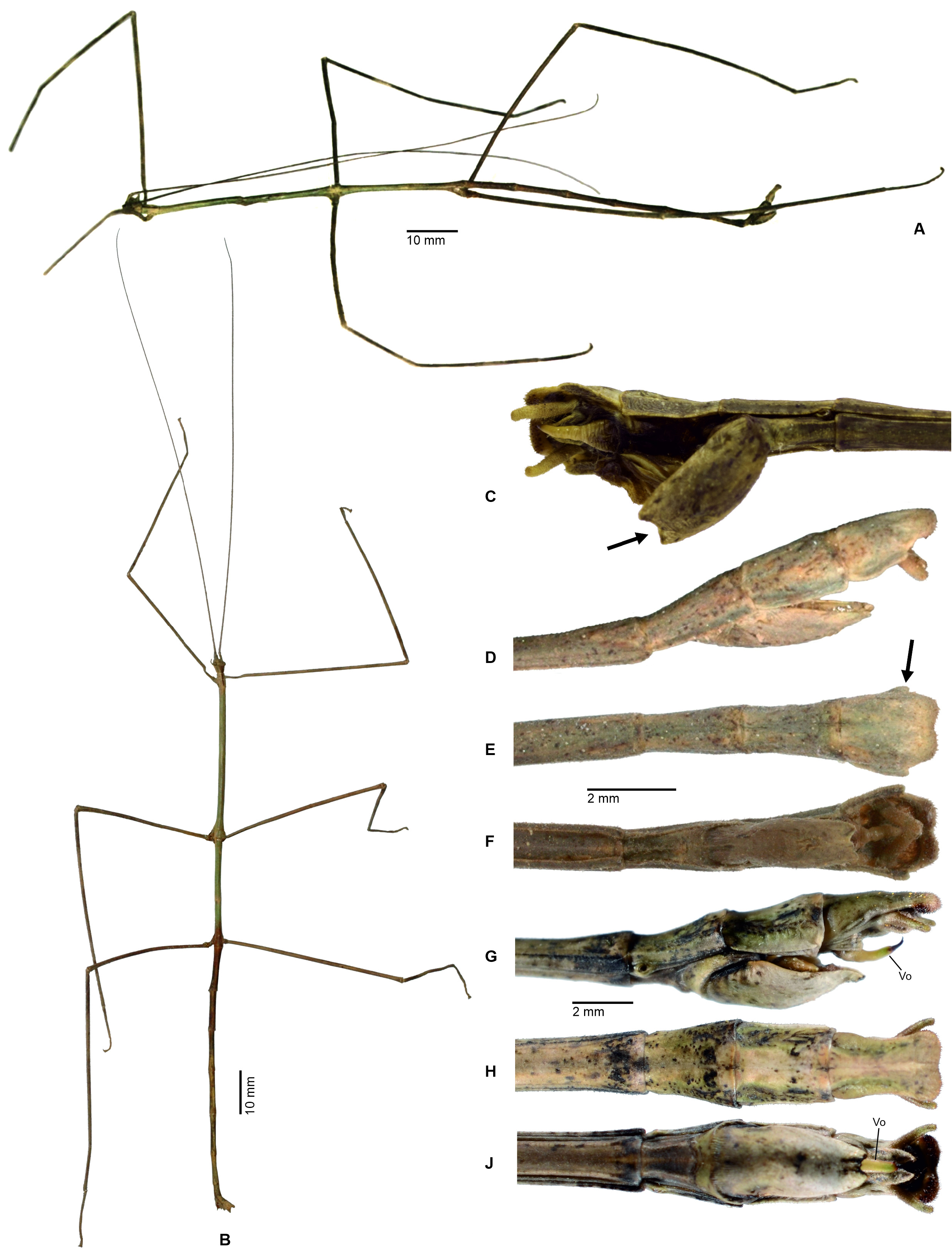
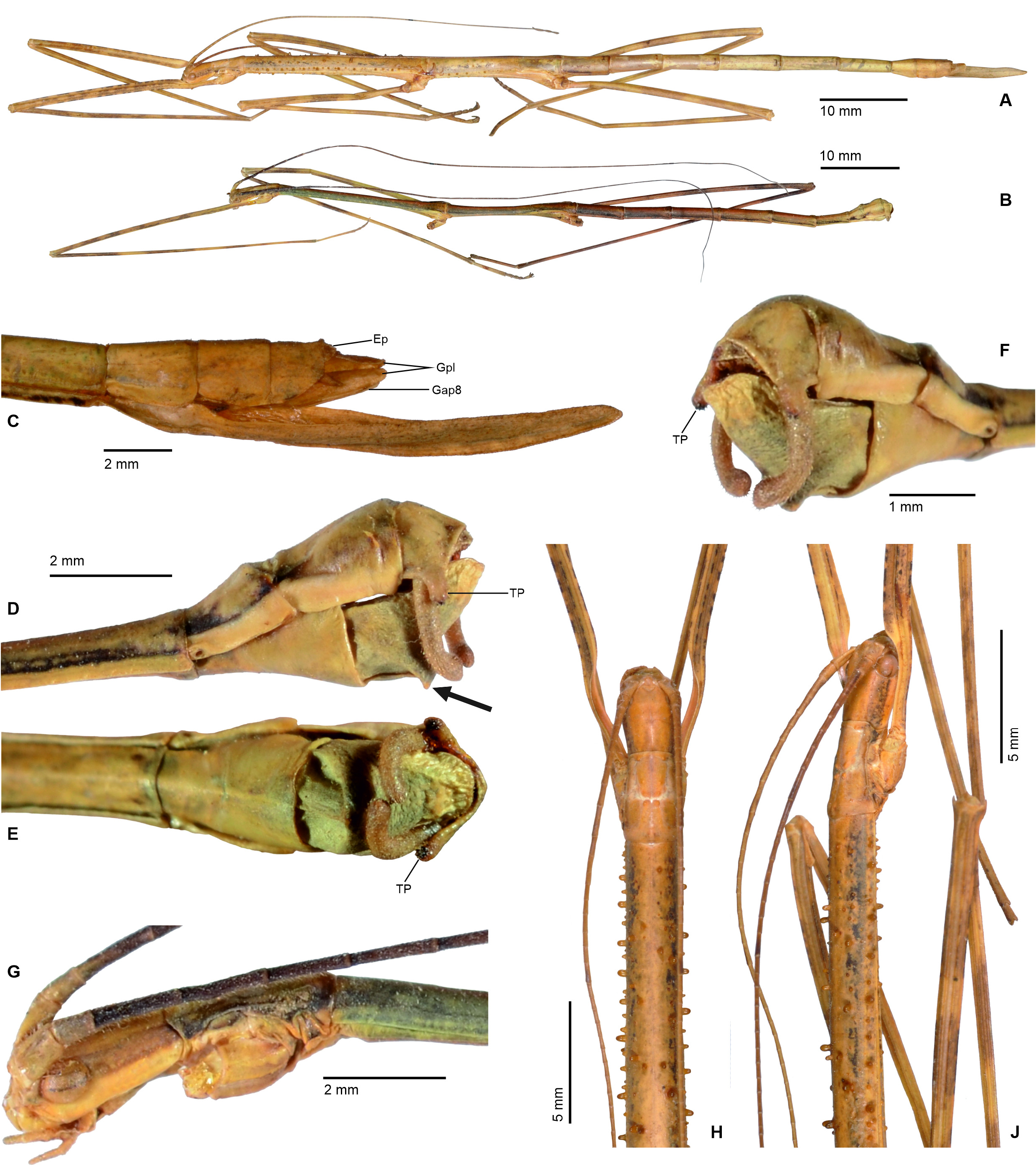
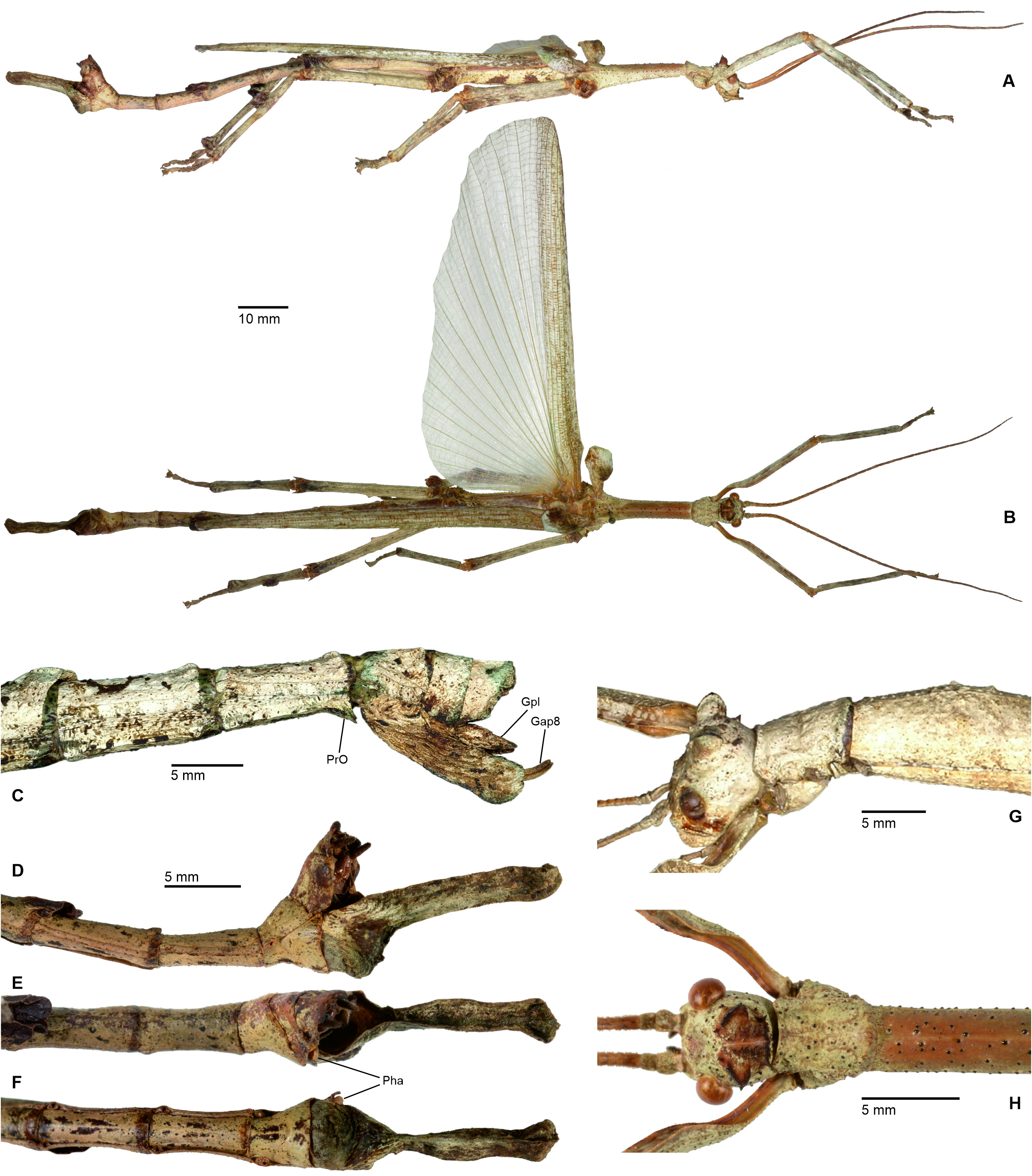
Mating mostly takes place during the night, when the insects become active and search for food or mates. While ♀♀ are rather immobile and restrict their movements to oviposition and feeding, ♂♂ are notably more mobile and actively search for mates. In some species, e. g. the Mexican Phanocles horni ( Redtenbacher, 1908) , ♂♂ are frequently seen to struggle for mates and the first author has observed a single ♀ in captivity to carry three competing ♂♂ on its back all of which tempted to anchor their terminalia to its abdomen. This behaviour was seen even though there was no imbalance of numbers of ♂♂ and ♀♀ in the breeding cage. Males of P. horni will usually remain on the back of the same ♀ and anchored to its abdomen for the entire lifetime and even if the ♂♂ dies before the ♀, the dehydrated remains of its terminalia may remain attached to the ♀ abdomen. In captivity ♂♂ of this species have even been observed trying to mate with branches or the fingers of the human breeder when handling the insects. The terminalia of ♂♂ of Cladomorformia show a notable array of morphological specialisations, which include the shape and structure of the poculum, vomer, anal segment and cerci in particular. All taxa have a more or less well-developed vomer that serves as an anchorage on the praeopercular organ of the ♀, which is variably shaped structure near the posterior margin of abdominal sternum VII. An exception is represented by the ♂♂ of Alienobostra , which have the vomer secondarily reduced and functionally supported by strongly elongated sickle-shaped cerci that serve as claspers that are closed around the abdomen of the ♀ during the copulation ( Figs. 6C–D View FIGURE 6 ). This is also the case in Calynda and Spinocloidea gen. n. but in these two genera the cerci are notably shorter than compared to Alienobostra and cannot be closed around the ♀ abdomen, only being pressed against the lateral surfaces of abdominal tergum VII of the ♀ ( Fig. 106D View FIGURE 106 ). Moreover, these two genera have a well-developed vomer that certainly is the main anchor during copulation. Cerci that are similarly specialised to those of Alienobostra , but less distinctly elongated, are also seen in Aplopocranidium , Jeremia and Jeremiodes . These are assumed to have the same function but observations of the mating habits of these genera are not available. In Aplopocranidium , Jeremia and Jeremiodes the vomer is increasingly reduced. With the exception of the three genera of the Jeremia group (see below) the ♂♂ of all genera of Cladomorformia possess a pair of thorn-pads on the lower outer angles of the anal segment that under muscular control assist in grasping the ♀ praeopercular organ. While copulating, the ♂ rests on the back of the ♀ and the abdomen is bent downward with the ventral genitalia of the anal segment covering the sternum VII of the ♀. Fertilization is brought about by transfer of single spermatophores, a gel-like, spherical and often whitish capsule that contains an amount of male sperm ( Brock, 1992; Brock & Büscher, 2023) or sperm is transmitted directly ( Schulten, 1995).
No specialised egg laying techniques are known to occur among members of Cladomorformia . Eggs are simply dropped or flipped to the ground singularly with the ♀♀ frequently catapulting the eggs away by an abrupt movement of the abdomen to ensure optimum dispersal. This is generally regarded as the ancestral technique of oviposition ( Goldberg et al., 2015; Robertson et al., 2018; Brock & Büscher, 2023). Although the catapulting of eggs a priori appears as an effective way to disperse eggs, the limited mobility of ♀♀, which perch on an individual host plant as long as there is foliage that serves as nutrition, the oviposition of eggs will only be concentrated around the bases of the host plants ( Windsor et al., 1996: 365) why phasmids are generally regarded as having limited dispersal ability ( Compton & Ware, 1991). Mostly the eggs resemble plant seeds and remain on the ground during the entire embryonic development. A more effective mechanism of egg dispersal that is caused by an impressive convergence between the animal and plant kingdom involves ants, a signal adaption for zoochory and co-evolution with plants ( Stanton et al., 2015; Brock & Büscher, 2023: 132). For Cladomorformia this has been reported for Alienobostra remiformis in the Pacific lowlands of Guanacaste, Costa Rica by Windsor et al. (1996), who erroneously referred to the species as “ Calynda bicuspis ”. This study has shown that the spongy internal matrix of the capitula of the eggs, that to a variable degree is present throughout all genera of Cladomorformia , attracts ectatomine ants ( Ectatomma ruidum (Roger, 1869) , Ectatomminae). The reason for this is that the ants nutritively profit from the elaisomes contained in the proteinaceous egg capitula. While some ants immediately transport eggs away and discard them, others carried eggs into their underground nest where the capitulum was removed. In the latter case however, ants were commonly observed to return eggs to the surface why this mechanism must be regarded an aboveground dispersal ( Windsor et al., 1996). In Trychopeplus the egg capsule is all over covered with hair-like fringes that expand after contact with water or humidity and contribute to making the eggs adhesive to moss and various other plant parts in the natural environment of these insects to ensure safe embryonic development. This suggests that the majority of eggs do not reach the forest floor but remain in the understory vegetation of the humid mossy forests in which species of Trychopeplus live (personal observation). Females are prolific egg producers with some species, e. g. Phanocles ploiaria , possessing oviposition rates of 5– 6 eggs per day (personal observation) and ♀, which makes a total of more than 1000 eggs in an individual female’s lifetime. Windsor et al. (1996) reported an average of 4– 8 eggs per day and ♀ for solitary Alienobostra remiformis and observed a notable increase of the oviposition directly after the cessation of mating with up to five eggs produced per hour. Breeding of various species in captivity has shown the incubation times for eggs to vary considerably from species to species, but to be rather similar in closely related species. It mostly ranges between four and eight months and also depends on the average temperatures, the incubation time generally decreasing with raising temperatures.
8. Summary
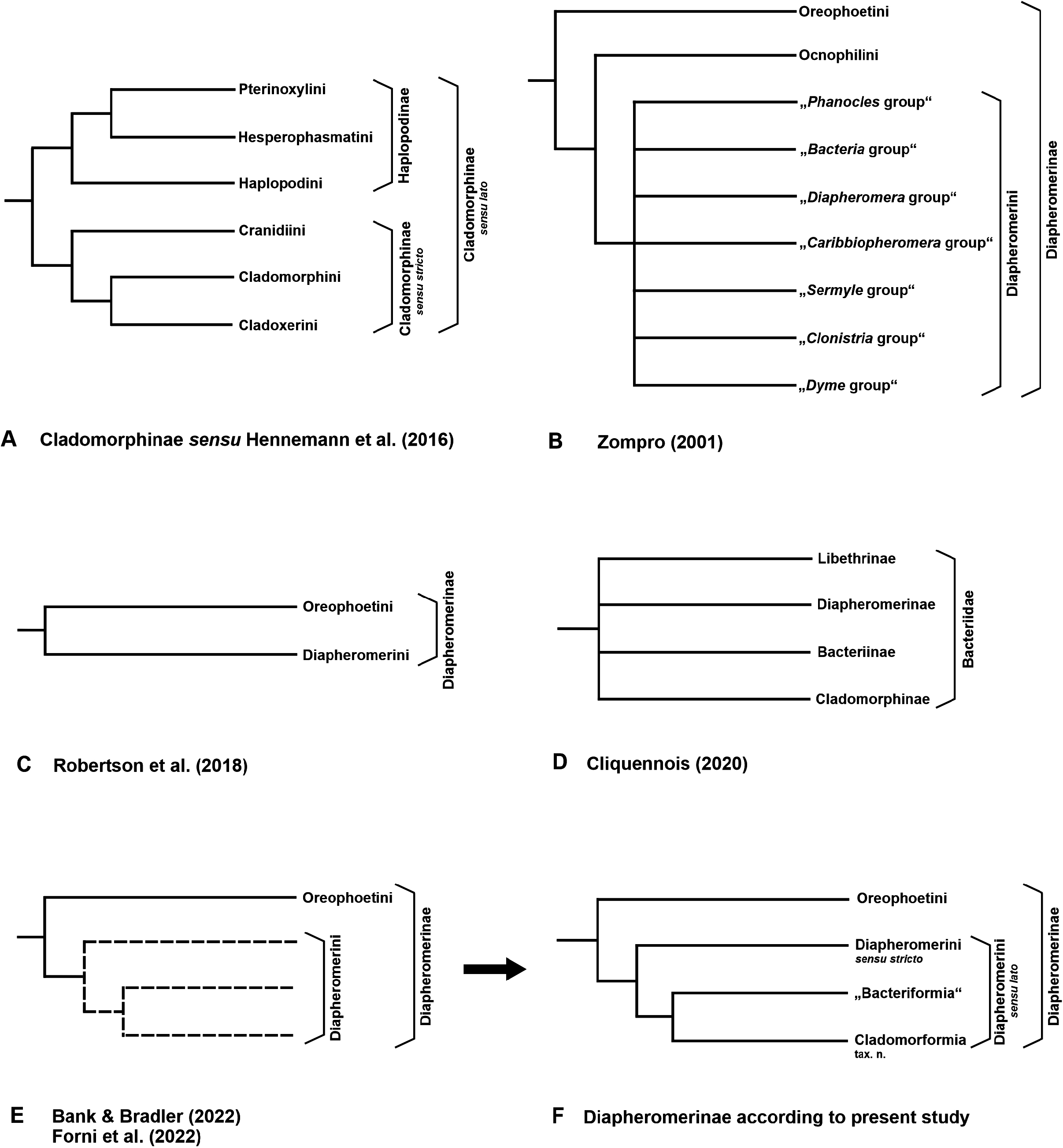
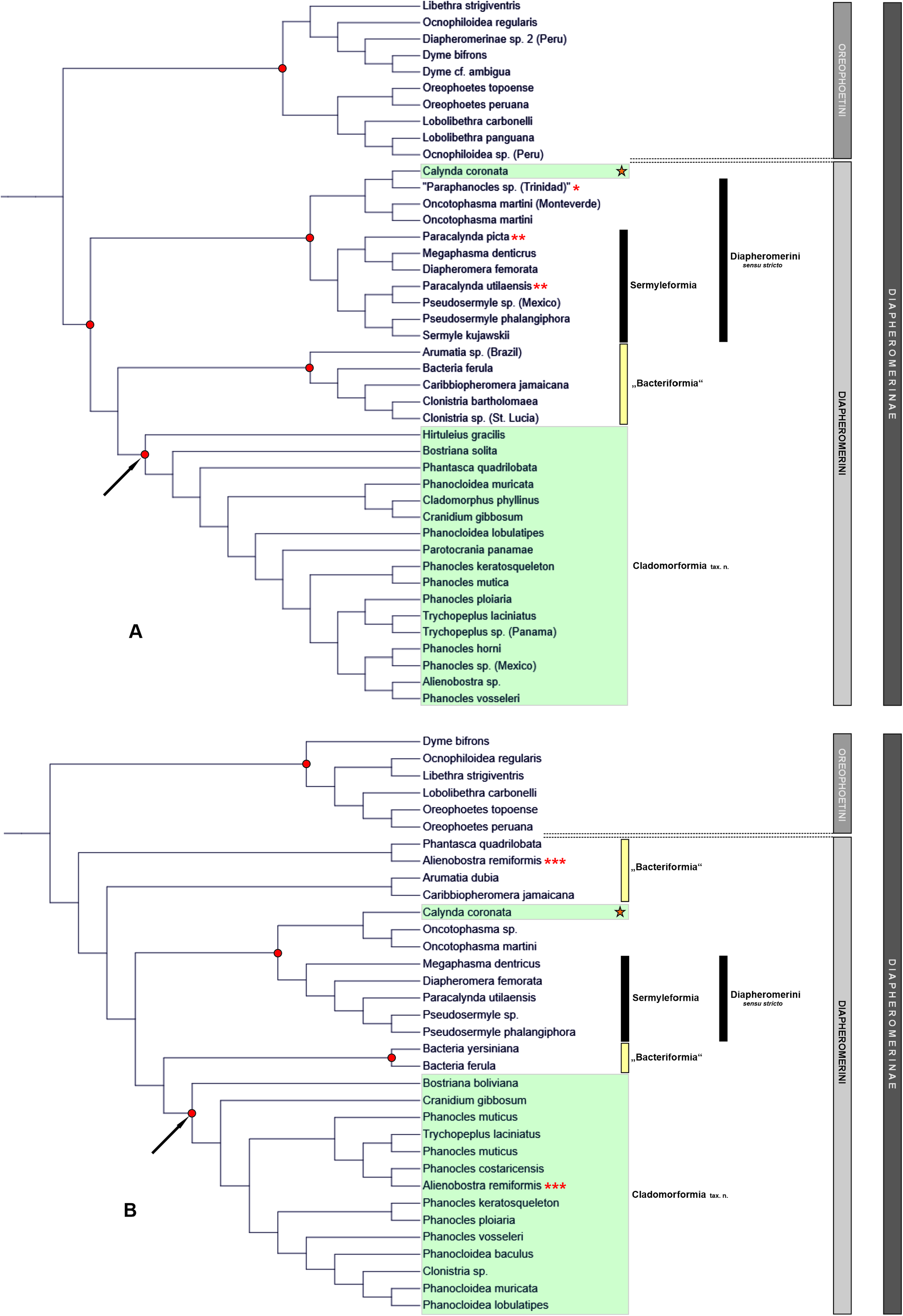
The new rank-free Cladomorformia tax. n. has been introduced as a possibly monophyletic sub-ordinate lineage of Diapheromernae: Diapheromerini . The monophyly of the lineage is suggested by previous phylogenetic studies based on molecular data ( Bank & Bradler, 2022; Forni et al., 2022) with the inclusion of the genera previously classified as Cladomorphinae : Cladomorphini sensu stricto ( Hennemann & Conle, 2016) and the genus Cranidium , all of which were here formally transferred to Diapheromerinae . An exception is represented by the genus Calynda , which has resulted as belonging to Diapheromerini s. str. (see Figs. 133A–B View FIGURE 133 ) in the abovementioned analyses but morphological traits provide no support for this placement and assign Calynda to Cladomorformia instead. Cladomorformia . The complicated history of the classification of Diapheromerinae has been outlined and previous competing arrangements of the Diapheromerinae have been briefly surveyed and illustrated ( Fig. 1 View FIGURE 1 ). The two tribes currently contained in Diapheromerimae ( Robertson et al., 2018), namely Diapheromerini and Oreophoetini , have been re-diagnosed and anatomical characters have been summarised to support the future delimitation of these two clades and identification of autapomorphies. Some discrepancies between the morphological traits achieved herein and previous results based on molecular data were subjected to a critical discussion. According to the present study Cadomorformia comprises 25 valid genera and 163 known species (→ 8.3.). All genera have been re-diagnosed and differentiated from morphologically similar and presumably closely related genera and complete lists of species were presented for each genus, which include all synonyms and provide detailed data of type specimens as well as information on the distribution of each species. Seven of the genera and 41 species are newly described herein (→ 8.1.). Careful examination of all necessary type material has revealed 44 new synonymies and 64 species had to be transferred to other genera, which has resulted in the need of 132 taxonomic changes (→ 8.4.). For justification of the new synonymies here introduced and ensuring stability of the affected names 25 lectotype designations were established (→ 8.2.). With all synonymic species included a total of 233 specific names have been covered and clarified in this morphology-based taxonomic study of the Cladomorformia .
The array of new genera and species here described within Cladomorformia and very numerous necessary taxonomic changes made in the present survey emphasize on our still very fractional knowledge of this lineage of New World Phasmatodea . This is even more stunning in aspect of Cladomorformia comprising the bulk of the New World so-called “giant stick insects” with ♀♀ of a reasonable number of species exceeding body lengths of 20 cm. The lineage also contains the longest of all known extant insects of this part of the world. Females of the newly described Panamanian Phanocles maximus Hennemann, Conle & Cambra sp. n. are the longest insects of the Americas and the whole of Occidophasmata. They reach a maximum recorded body length of 285.0 mm and overall lengths with the legs stretched out of more than 400.0 mm. This size is only excelled by a number of Old-World species of the Oriophasmata (e. g. Hennemann & Conle, 2008). Among the Occidophasmata ♀♀ of the eye-catching Brazilian Otocrania aurita reach a maximum total body length of 270.0 mm, thus are the second longest insect of the New World. The third longest species is the Panamanian Phanocles subvolans with the longest recorded specimen measuring a total body length of 240.0 mm. However, while ♀♀ of Phanocles subvolans have a very short subgenital plates that hardly exceeds the tip of the abdomen, Otocrania aurita only achieves the 270.0 mm thanks to having the subgenital plate enormously elongated and greatly projecting beyond the abdomen. Thus, if the body length sensu stricto is regarded, then both Otocrania aurita and Phanocles subvolans together must be regarded the second longest New World insect, because both have a maximum recorded body length of 232.0 mm when the subgenital plate is disregarded. The stunning fact that the longest extant insect of the New World has just recently been discovered and is described herein is a perfect example for demonstrating our still fractional knowledge of the phasmid fauna of the Americas and that as yet there is good potential that still impressive unknown species can be found.
The intergeneric relationships within Cladomorformia are still poorly understood and much broader sampling in future phylogenetic approaches is necessary for any more in-depth discussion and achieving a reliable classification of the clade. The comprehensive morphological data made available by the present study will hopefully serve for forthcoming morphology-based phylogenetic studies. Besides, they allow a number of conclusion that shall be summarised below. However, since no phylogenetic approach has been conducted herein the following observations can only be hypothetical. The now available data indicate five groups of morphologically similar and supposedly closely related genera, that are outlined below, though these generic groups are not reflected in the determination keys presented above (→ 5.) and are rather meant to contribute to a better understanding of the distribution of morphological traits within Cladomorformia .
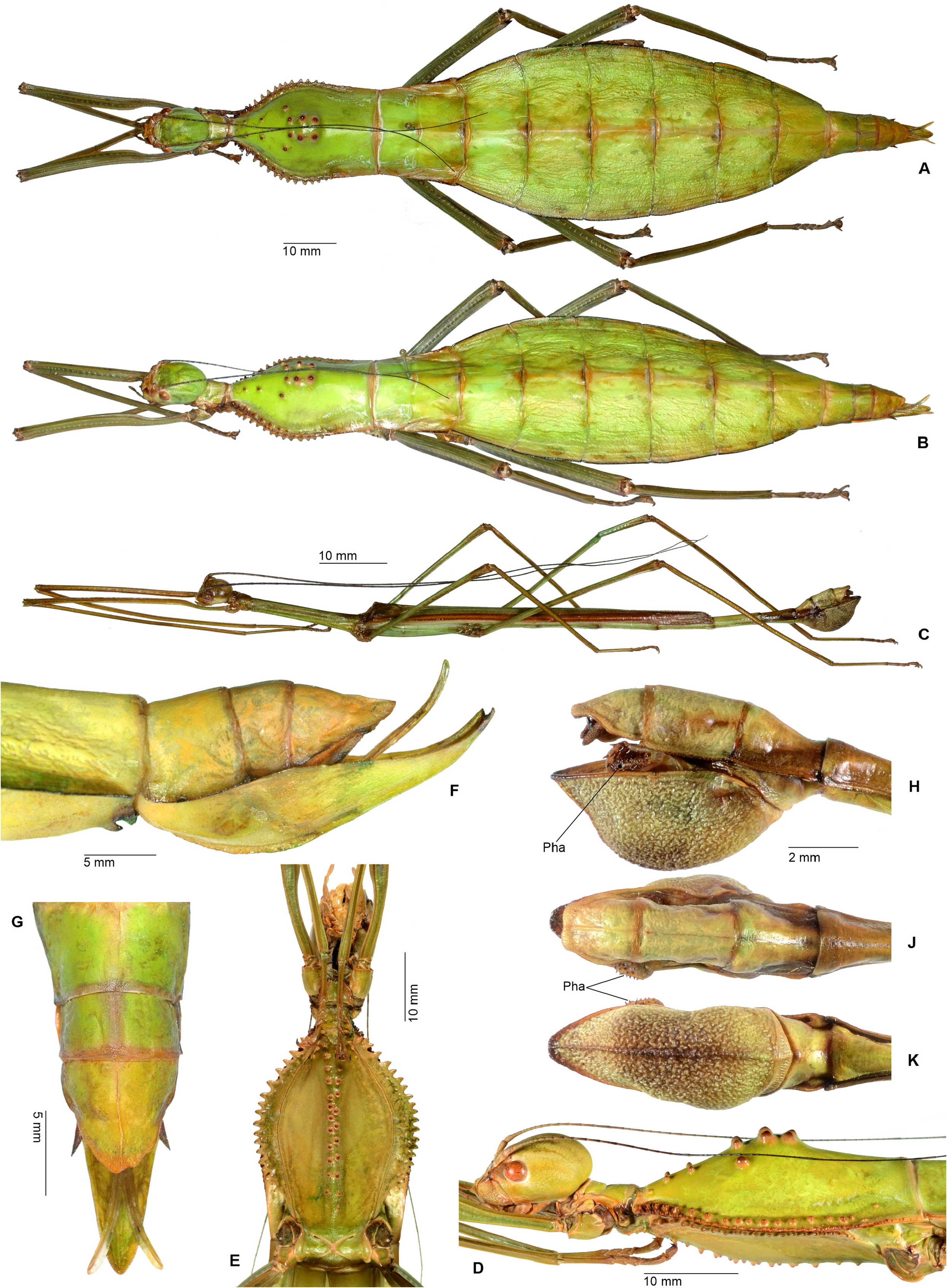
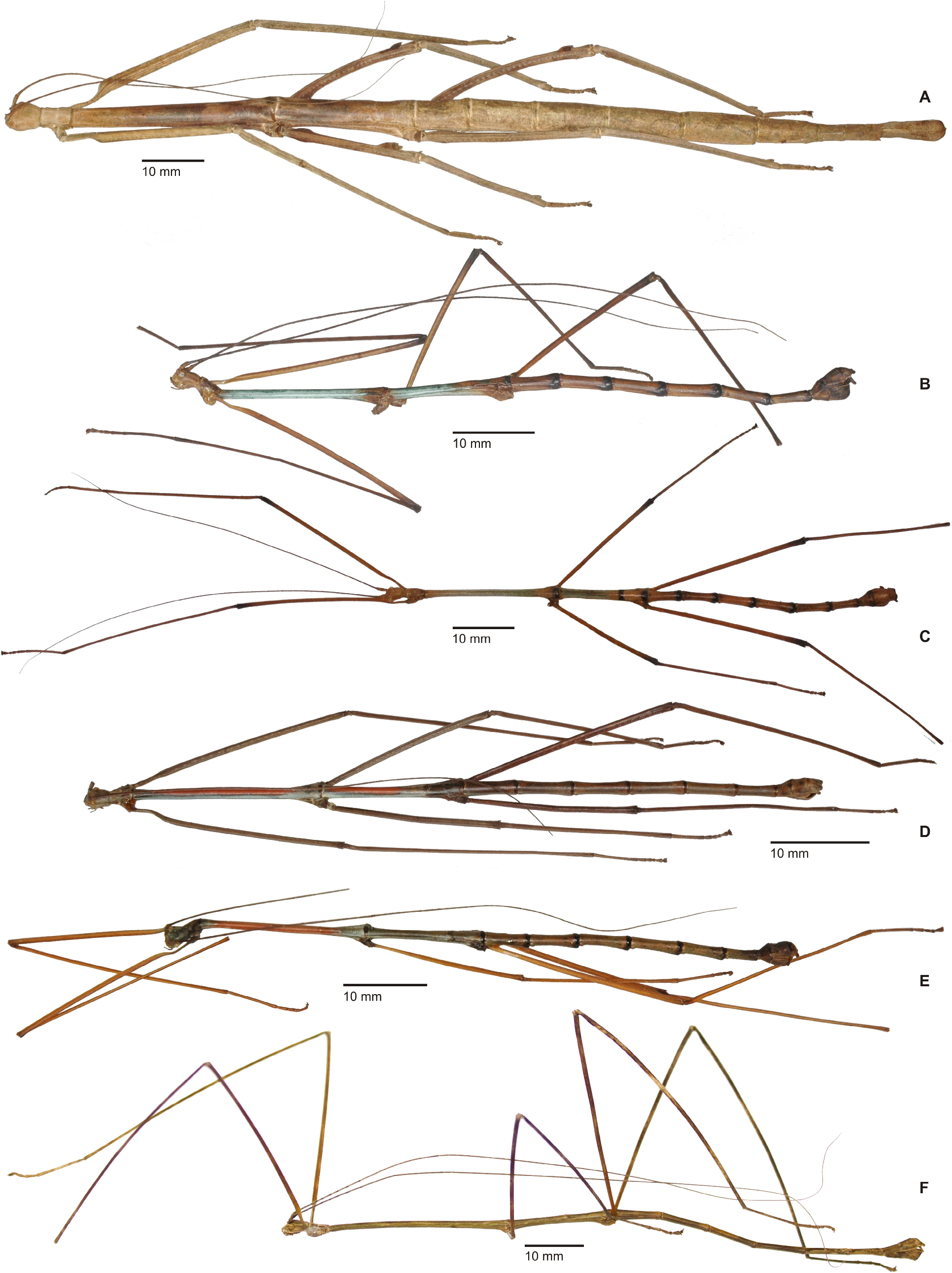
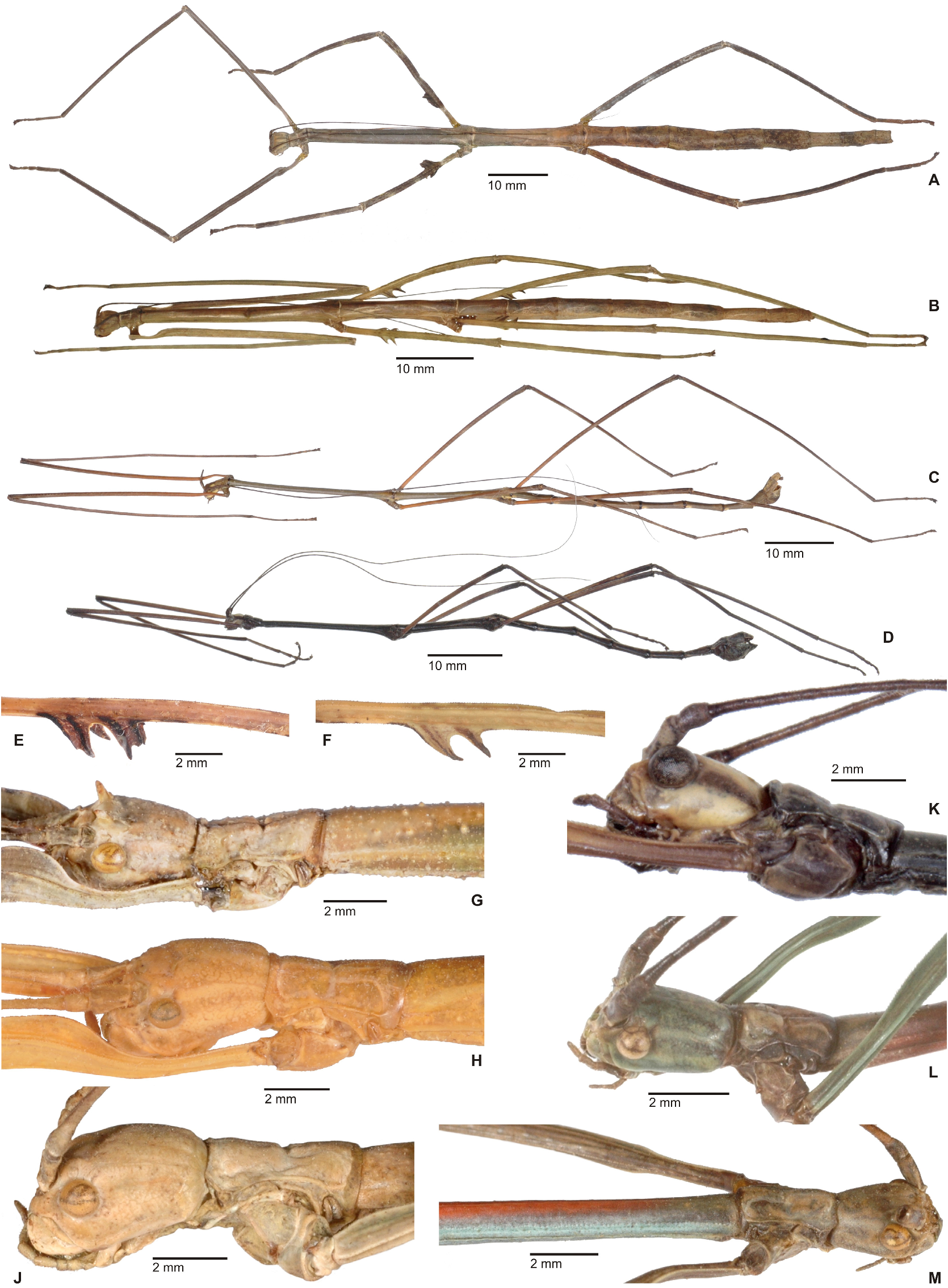
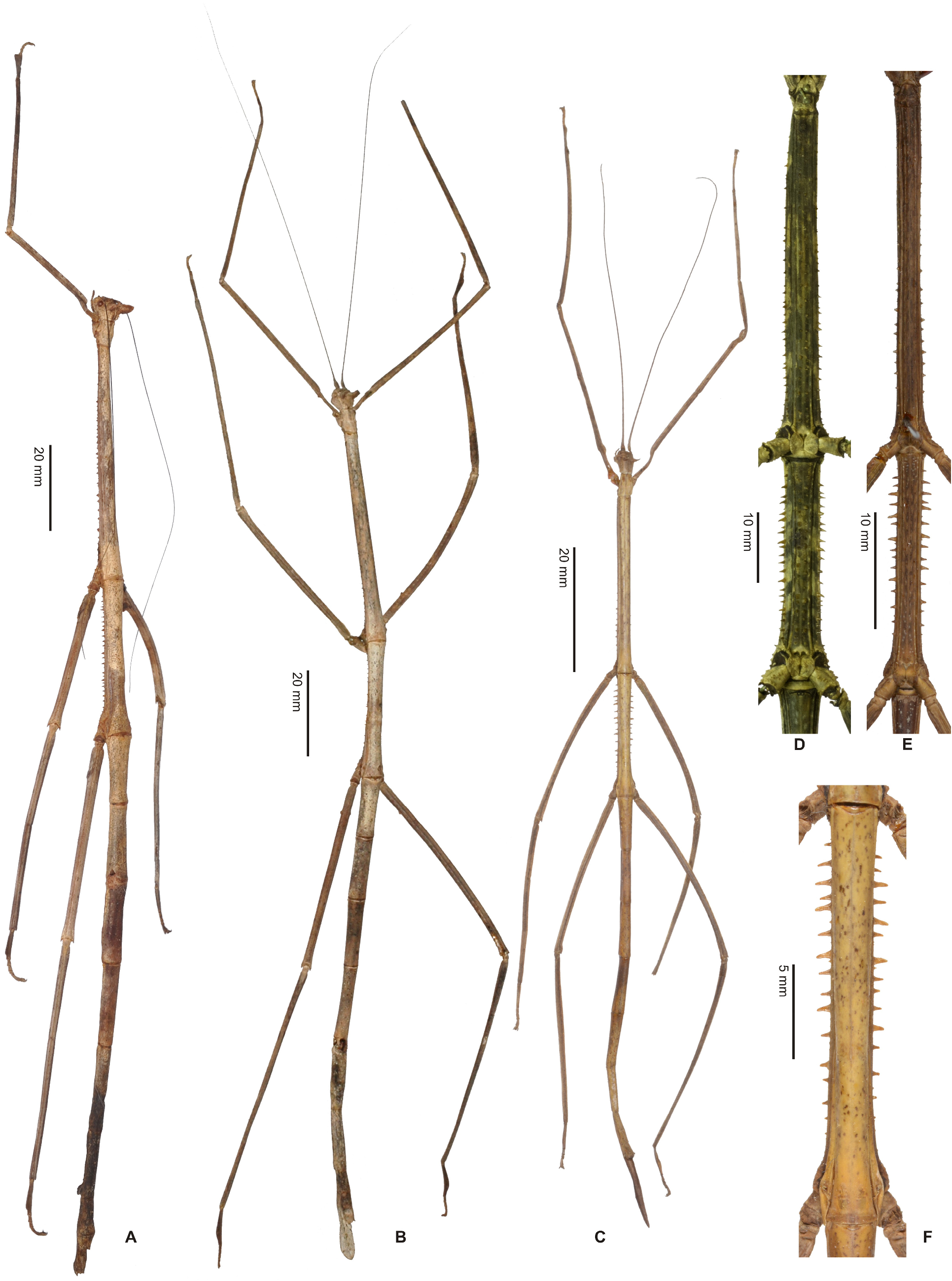
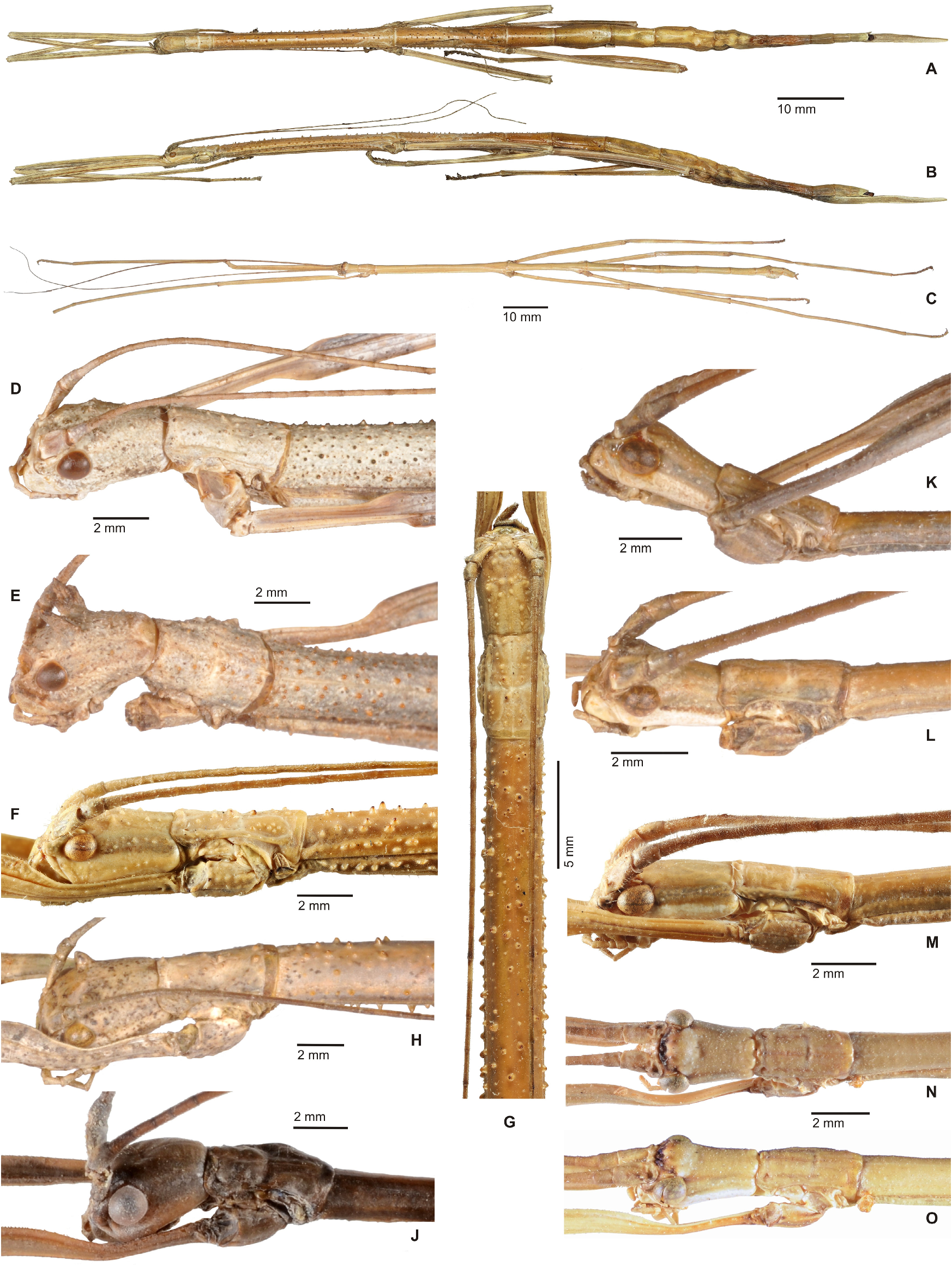
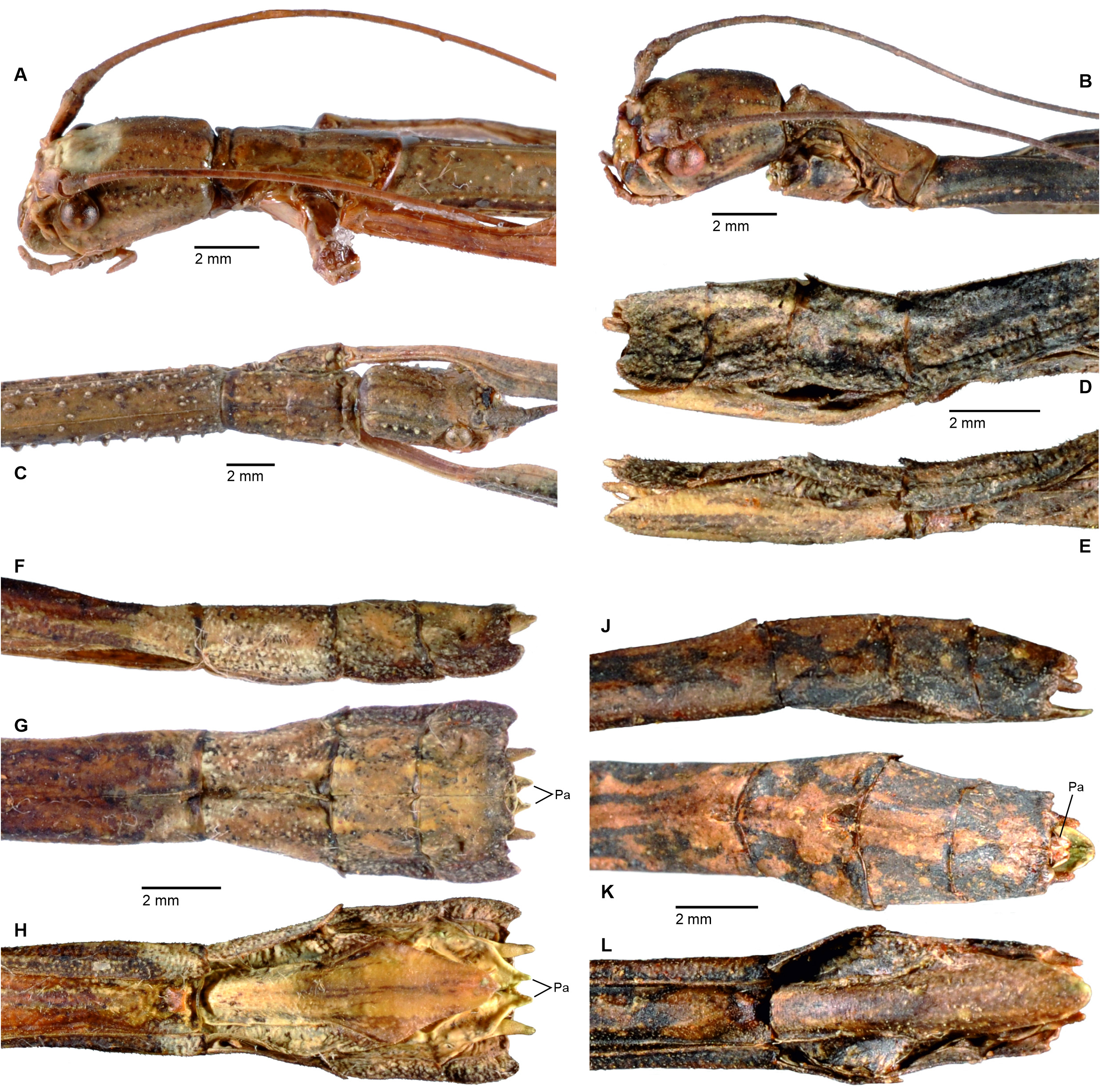
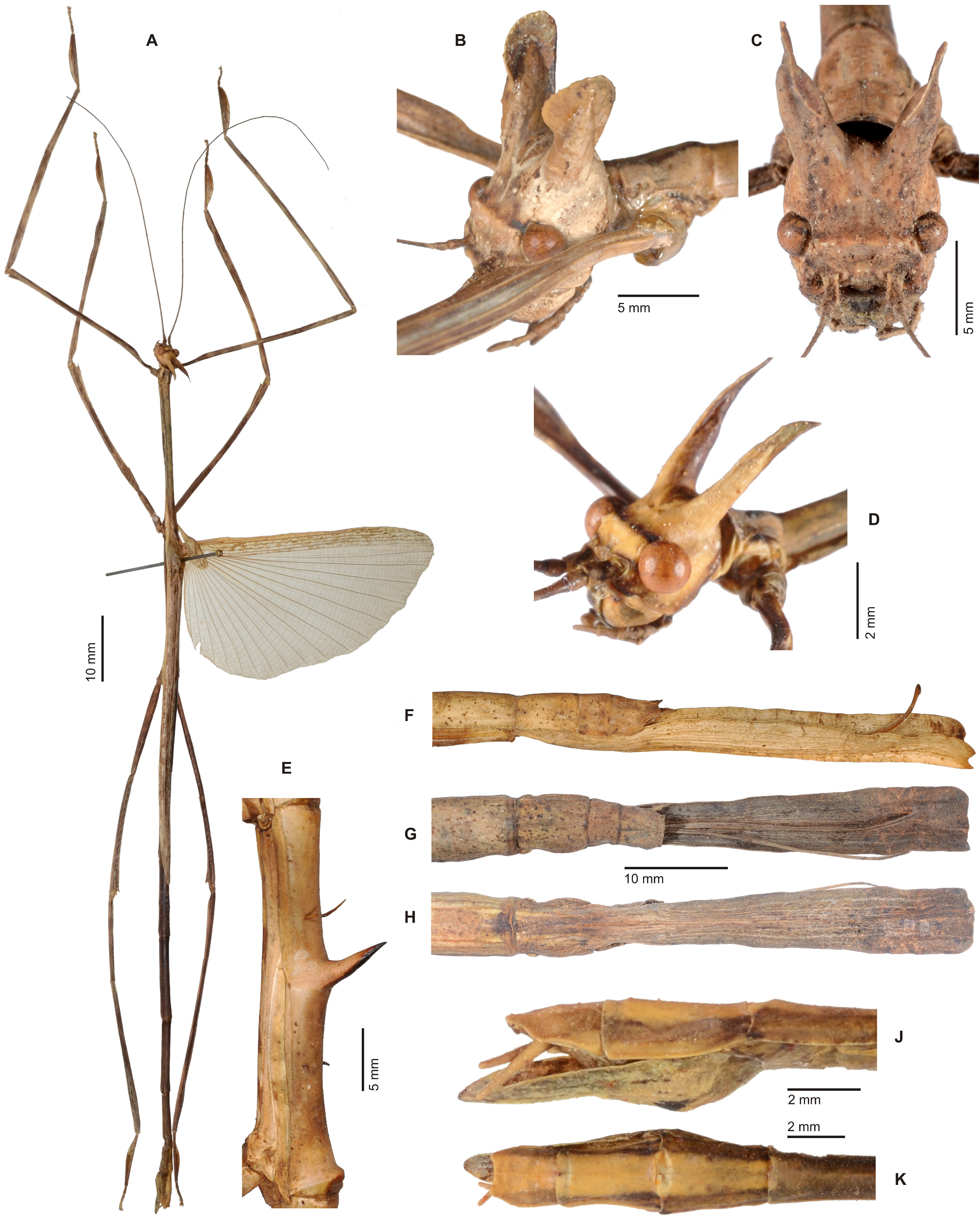
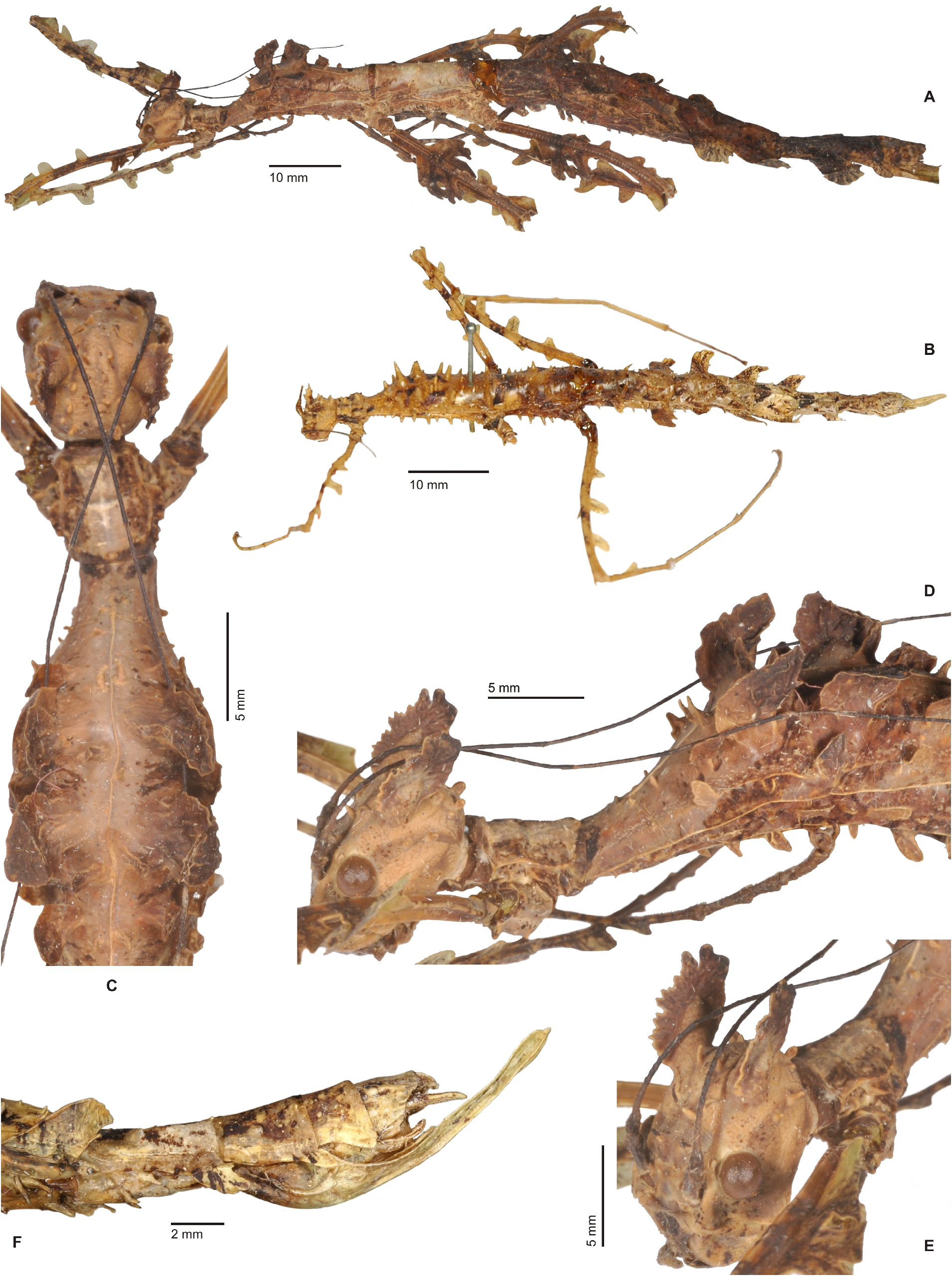
Cladomorphus group. A first rather distinctive generic group is formed by the five genera Cladomorphus , Hirtuleiodes gen. n., Hirtuleius , Otocraniella and Xylodus . All are mostly distributed throughout the eastern Amazon basin as far south as northern Argentina and Paraguay and with only one known species in the eastern Colombian lowlands ( Hirtuleiodes granuliceps ). The genera of this group are conspicuous for the various specialisations of the terminalia, which comprises the subgenital plate, gonoplacs, gonapophyses and praeopercular organ of ♀♀ as well as the anal segment, phallus, vomer and poculum of ♂♂.
This group contains some of the largest Phasmatodea of the New World and with the exception of Otocraniella all are rather stocky, rough bodied insects often with variously armed and furnished limbs. Males are almost exclusively alate with the only exception represented by Otocraniella . Both sexes have profemora that are distinctly triangular in cross-section, with the dorsal carinae strongly approaching each other and the anterodorsal carina prominently raised (more pronounced in ♀♀). The medioventral carina is very distinct, ± lamellate and strongly displaced towards the anteroventral carina to form an almost plain ventral surface of the femur. The body surface of both sexes is strongly sculptured (thorax in particular), and ranges from scabrous over densely granulose and tubercular to spinulose. In both sexes abdominal tergum VI (sometimes also V) bears a pair of ± distinct crest-like posterior swellings or excrescences. The mesosternum is simple and does not bear a medio-longitudinal carina or keel like in the Phanocles group. The metanotum of ♀♀ usually bears a ± distinct tubercle or rounded swelling posteromedially, which resembles a rudimentary version of the prominent metanotal spine seen in Otocrania ( Phanocles group). All genera have well-developed paired ventral thorn pads at the posterior margin of the anal segment and a well-developed variously specialised vomer ( Figs. 94A–E View FIGURE 94 ). It is either basically triangular in shape and bears a single terminal hook ( Hirtuleiodes Fig. 94E View FIGURE 94 and Otocraniella Fig. 94D View FIGURE 94 ) or is angled in outline, ± enlarged and bears two posterolateral hooks ( Cladomorphus Fig. 94A View FIGURE 94 , Hirtuleius Fig. 94C View FIGURE 94 and Xylodus Fig.- 94B). The phallus is ± enlarged and exposed as a digitiform sinistral appendix that laterally projects over the poculum and sometimes even the lateral margin of abdominal tergum IX (most pronounced in Cladomorphus Figs. 4A View FIGURE 4 , 94A View FIGURE 94 and Xylodus Figs. 4D View FIGURE 4 , 94B View FIGURE 94 ). The slightly deflexed and sometimes triangular lateral margins of abdominal tergum IX seen in Cladomorphus and Xylodus resemble the genus Phanocloidea ( Phanocles group). Cladomorphus , Otocraniella and Xylodus share the very distinctive and long, spatulate or tube-like apical appendix of the poculum that projects considerably over the apex of the abdomen and resembles an elongated ♀ subgenital plate ( Figs. 4A, D View FIGURE 4 , 17E–J View FIGURE 17 , 82B–D View FIGURE 82 ). This specialisation is unique within the Occidophasmata, readily distinguishes these genera from all other members of Cladomorformia and certainly represents a synapomorphy of these three genera. The function of this specialisation is not known. The conspicuously enlarged phallus is a possible autapomorphy of the Cladomorphus group, whereas bifid vomers are also found within the Phanocles group. The genital morphology of ♀♀ widely corresponds to that of the Phanocles group, common features being the often enormously elongated and filiform gonapophyses VIII, which greatly extend beyond the apex of the abdomen, and prominent praeopercular organ on abdominal sternum VII ( Figs. 90A–E View FIGURE 90 ). The praeopercular shows a wide range of different shapes. In contrast to the Phanocles group however the subgenital plate is never lanceolate but always ± broadened, spatulate and obtuse angled, and often has the upper margins ± undulate and lobed (almost always entire in the Phanocles group). The gonoplacs are prominent but usually no longer than the gonapophyses IX. In Xylodus however they are noticeably enlarged and extend over the apex of the anal segment, which may be an autapomorphy of this genus ( Fig. 82A View FIGURE 82 ). In Cladomorphus the paraprocts are noticeably enlarged and tri-carinate in cross-section ( Fig. 3D View FIGURE 3 ), a feature also seen in certain members of the Phanocles group.
Only the eggs of Cladomorphus ( Figs. 98H–J View FIGURE 98 ) and Otocraniella are known. They are characteristic for the ovoid capsule with a rounded polar area, almost smooth but dull capsule surface and roundly convex opercular protuberance (matrix capitulum), which is a hollow and open net-like structure.
Jeremia group. Morphology suggests the three genera Aplopocranidium , Jeremia and Jeremiodes are closely related, which was already suggested by Hennemann & Conle, (2010), who provided a table that compared and distinguished the three genera in detail. Together they form a fairly distinctive generic group that is well characterised by the morphology of the terminalia of ♂♂. All three genera are distributed throughout the Amazone basin ranging from northern Colombia and French Guiana to as far south as Bolivia with Jeremiodes also having one species in the lowland forests of the Osa Peninsular in south-west Costa Rica. Males of all four genera have well-developed alae and a median segment that is much longer than the metanotum. In ♀♀ the median segment is slightly longer than the metanotum. Females have a notably elongated subgenital plate that projects beyond the apex of the abdomen by at least the length of the anal segment and has the apex either pointed ( Aplopocranidium Figs. 11J–L View FIGURE 11 and msot species of Jeremiodes Figs. 34A–C, J–L View FIGURE 34 ) or broadened and obtusely rounded ( Jeremia Figs. 31C–G View FIGURE 31 ). The gonapophyses VIII are strongly elongated, filiform and project considerably over the apex of the abdomen and the praeopercular organ is weakly developed (two small carinae in Jeremia Fig. 89G View FIGURE 89 ) to almost fully reduced ( Aplopocranidium Fig. 89F View FIGURE 89 and Jeremiodes Figs. 89H–J View FIGURE 89 ). In accordance to the obscure praeopercular organ of ♀♀, which would serve as a contact point for the ♂♂ genitalia during the copulation, ♂♂ of all three genera share conspicuously enlarged and specialised cerci that act as claspers to hold fast to the abdomen of ♀♀ while mating. The cerci are compressed dorsoventrally with the apex rounded or sickle-shaped in Jeremia ( Figs. 31 View FIGURE 31 H-K) laterally compressed and angled inward in Aplopocranidium ( Figs. 31F–H View FIGURE 31 ) and club-shaped with the apex thickened and ± osteoid to hook-like in Jeremiodes ( Figs. 34M–R View FIGURE 34 ). Moreover, the paired posteroventral thorn pads of the anal segment are fully reduced secondarily in all three genera and abdominal sternum IX is just vaguely to not transversely divided into a poculum and a smaller basal (anterior) portion. The poculum is large, bulgy and roundly tub-shaped with the posterior margin either entire ( Jeremia Fig. 31K View FIGURE 31 ) or bidentate ( Aplopocranidium Fig. 11H View FIGURE 11 and Jeremiodes Figs. 34M–R View FIGURE 34 ). A vomer is present in all three genera ( Figs. 94F–M View FIGURE 94 ), but whereas it is well-developed, elongate and triangular in shape with an acutely pointed terminal hook in Aplopocranidium and Jeremiodes , it is noticeably reduced secondarily and merely represented as a small, spatulate appendix in Jeremia . The reduction of the thorn pads in ♂♂, which are functionally replaced by the enlarged and specialised cerci, is likely to represent a synapomorphy of Aplopocranidium + Jeremia + Jeremiodes and the secondarily reduced vomer can with great certainty be interpreted as an autapomorphy of Jeremia . Another character that most certainly can be regarded as an autapomorphy of Jeremia is the prominently dentate medioventral carina of the meso- and metatibiae in both sexes, which is unique within Cladomorphini and readily separates the genus from all other members of the tribe ( Fig. 31M View FIGURE 31 ). Apparent from morphological aspects, Aplopocranidium and Jeremiodes are closely related and may with quite some certainty represent adelphotaxa, which has already been suggested by Hennemann & Conle (2010: 105). For instance, ♂♂ share the entirely unarmed legs and bidentate posterior margin of the poculum and ♀♀ have the lanceolate, mostly apically pointed subgenital plate in common. Jeremia is likely to be sister to Aplopocranidium + Jeremiodes and readily distinguishable from both genera by a good number of characters, some of which are summarised above.
Some morphological characteristics of Jeremia such as the morphology of the profemora, which are almost triangular in cross-section with the anterodorsal carina conspicuously raised and the ± lamellate medioventral carina strongly displaced towards the anteroventral carina, crest-like posterior pair of protuberances on abdominal tergum VI of both sexes and broadened, apically obtuse subgenital plate of ♀♀ suggest affinity to members of the Cladomorphus group. The ovoid and unarmed head, mostly unarmed limbs, smooth abdomen of both sexes, more or less glossy body surface and lanceolate subgenital plate of ♀♀, as well as the large, tub-shaped poculum of ♂♂ of Aplopocranidium and Jeremiodes strikingly resemble the genus Cranidium Burmeister, 1838 and suggest close relation.
So far, only the egg of Jeremia has become known ( Fig. 98K View FIGURE 98 ). From the known eggs of Cladomorformia it most closely resembles that of Cladomorphus in having a very long, slender and almost parallel-sided micropylar plate and only a small, irregularly convex and spongy opercular protuberance. However, the capsule is more laterally flattened than that of Cladomorphus has an obtuse longitudinal bulge along the dorsal and ventral surfaces, the polar area is distinctly indented and the surface is much more severely pitted.
Cranidium group. The striking Cranidium is anatomically very well recognised among Cladomorformia and differs from all other members by the wholly unarmed extremities and indistinct medioventral carina of the profemora of both sexes, which is roughly central on the ventral surface of the profemur, strongly flattened and laterally dilated, leaf-like body of ♀♀, which is rhomboidal in cross-section, as well as the spinulose phallus of ♂♂ ( Figs. 19H–K View FIGURE 19 ) and eggs that have a narrowing of the posteromedian notch of the micropylar plate before it widens into the gap and a distinctly separated median line ( Hennemann et al., 2016: 18, Fig. 49 View FIGURE 49 ). All these distinctive characters are unique within Cladomorformia and are most likely autapomorphies of Cranidium , the leaf-like body ( Figs. 19A–B View FIGURE 19 ) as well as the tumescent mesonotum ( Fig. 19D View FIGURE 19 ) and longitudinally tectate mesosternum of ♀♀ ( Fig. 19E View FIGURE 19 ) being even unique within the whole of Occidophasmata. The mostly smooth and shiny body surface of both sexes suggests close affinity to the Jeremia group, with which several specialisations of the terminalia of ♂♂ are shared although the cerci are small and not enlarged ( Fig. 19H View FIGURE 19 ). Close relation to Aplopocranidium in particular is suggested by the reduced armature of the limbs of both sexes, tubercular mesonotum and mesopleurae of ♀♀ and enormously enlarged tub-shaped poculum of ♂♂. Moreover, the bright green general colour of ♀♀ is exclusively shared with Aplopocranidium .
Phanocles group. This group contains the bulk of diversity of Cladomorformia and comprises the genera Alienobostra , Lanceobostra , Globocrania , Otocrania , Parotocrania , Phanocles , Phanoclocrania , Phanocloidea and Trychopeplus . These cover almost the whole known distributional range of the clade with the exception of north-east Argentina and Paraguay. As the northernmost genus Lanceobostra is restricted to Mexico and northern Guatemala ( Fig. 102B View FIGURE 102 ), Alienobostra is endemic in northern Central America ( Fig. 101A View FIGURE 101 ) and as the southernmost representatives Globocrania and Otocrania are endemic to the Atlantic Forest of eastern Brazil ( Figs. 102B View FIGURE 102 , 103D View FIGURE 103 ). All known eggs of members of this group (only those of Otocrania are not known) have ± angular capsules with a flattened or indented polar area and a large, hollow crest-like opercular excrescence that is formed by membranous, lamellate extensions of the outer margin, which extending by at least one-third of the capsule length.
Although Trychopeplus ( Figs. 79–80 View FIGURE 79 View FIGURE 80 ) at first glance appears anatomically very different from all other genera of this group and the whole of Cladomorformia , the stunning crypsis is merely an extreme adaption to its natural environment where the numerous appendages, lobes and teeth of the body and legs provide an impressive mimicry of mosses. With the cryptic appendages disregarded the genus clearly keys out as a member of Cladomorformia , e. g. by having a distinct praeopercular organ, an elongated subgenital plate and conspicuously elongated and filiform gonapophyses VIII in ♀♀ and the typical morphology of the terminalia in ♂♂. The molecular studies of Forni et al. (2022) and Bank & Bradler (2022) have shown Trychopeplus as nested within Phanocles ( Figs. 133A–B View FIGURE 133 ), but there is no clue for this position based on morphological traits. Although there is one Ecuadorian species in Phanocles ( Ph. decorus Fig 54C View FIGURE 54 ) that has a spinose thorax and prominently lobed legs, these are developed to a much lesser extent. Moreover, characters such as the strongly globose to roundly conical vertex, head that is higher than long, perlamorph antennae that are hardly longer than the profemora as well as the strongly arched and medially swollen mesothorax of ♀♀, armed mesothorax of ♂♂, laterally lobed abdominal terga of both sexes and strongly hairy eggs readily separate Trychopeplus not only from Phanocles but from all other member of Cladomorformia . Thus, the validity of the genus is strongly supported from morphological aspects with all of the mentioned adaptions certainly representing autapomorphies of Trychopeplus .
Among the genera of this group Alienobostra ( Fig. 8 View FIGURE 8 ) takes on a somewhat isolated position because it shows some distinctive specialisations of the ♂♂ terminalia that readily separate it from all other genera ( Fig. 4C View FIGURE 4 ). The strongly elongated and incurved cerci and secondarily reduced vomer have even prompted Bradler (2009: 201) to assume Alienobostra as the possible adelphotaxon of the Sermyleformia and this author furthermore interpreted the reduced vomer as a possible intermediate step towards an entire loss of this organ in the Sermyleformia. Although these characters certainly represent autapomorphies of Alienobostra , there is no clue for them to indicate close affinity within Diapheromerini s. str. or even the Sermyleformia. The vomer is notably reduced and rather spatulate in shape with two blunt apices but is well sclerotized ( Fig. 95H View FIGURE 95 ) and not functionally replaced but merely supported by the long, incurved cerci that serve as claspers that are closed around the abdomen of the ♀ during the copulation.A similarly reduced vomer in combination with enlarged cerci can be observed in Jeremia ( Jeremia group) and distinctly enlarged cerci alone but combined with a well-developed vomer are seen in Aplopocranidium , and Jeremiodes ( Jeremia group) as well as Calynda and Spinocloidea ( Calynda group). In all these taxa and in accordance to the long cerci that serve as claspers, ♀♀ merely have a very indistinct praeopercular. Other characters that shut out close affinity within Diapheromerini s. str. but prove the position of Alienobostra within Cladomorformia are: the long median segment that is about two-thirds the length of the metanotum; distinct and noticeably displaced medioventral carina of the profemora; medio-longitudinally keeled mesosternum ( Fig. 85E View FIGURE 85 ); very long and lanceolate subgenital plate of ♀♀; developed thorn-pads of the anal segment, large poculum und ventrally open abdominal tergum IX in ♂♂ as well as the strongly sculptured surface of the angular and posteriorly flattened egg-capsule and large, hollow fibrous excrescence of the operculum ( Figs. 98L–M View FIGURE 98 ). These characters place Alienobostra in the Phanocles group and the flattened, sub-cylindrical head and glossy body of ♂♂, as well as the very similar general appearance of ♀♀ and distribution in northern Central America support close affinity to Lanceobostra .
The striking Brazilian Otocrania ( Figs. 43–44 View FIGURE 43 View FIGURE 44 ) is endemic to the Atlantic Forest of eastern Brazil ( Fig. 103D View FIGURE 103 ) and is another genus that takes on a fairly isolated position within the Phanocles group, being readily characterised by two in all likelihood apomorphic characters, i.e. the huge posterior spine of the metanotum in ♀♀ ( Fig. 44E View FIGURE 44 ) and the apically elongated, spatulate poculum of ♂♂ that projects notably over the tip of the abdomen ( Figs. 44J–K View FIGURE 44 ). While both these characters indicate affinity to members of the Cladomorphus group, the remaining morphology of Otocrania obviously places the genus in closer relation to Globocrania and Phanocles . Females of most genera of the Cladomorphus group have a posteromedian swelling or hump on the metanotum which might be interpreted as a rudimentary version of the spine seen in Otocrania . Males of certain genera of the Cladomorphus group, i. e. Cladomorphus , Otocraniella and Xylodus , possess a long apical appendix of the poculum that is much longer than that seen in Otocrania . Whereas the globose head, which bears a pair of huge spiniform (♂♂, Fig. 44D View FIGURE 44 ) or auriform (♀♀, Figs. 44B–C View FIGURE 44 ) lobes, and the distinct medio-longitudinal keel of the mesosternum ( Fig. 86C View FIGURE 86 ) are also found in some species of Globocrania , Parotocrania, Phanocle s and Phanoclocrania , the apically broadened, angular and tridentate apex of the subgenital plate of ♀♀ ( Figs. 44 View FIGURE 44 F-H) and presence of a prominent rounded dorsal lobe on the remarkably elongated basitarsi of ♂♂ ( Fig. 44A View FIGURE 44 ) readily separate Otocrania from other members of this group.
Globocrania gen. n. ( Figs. 24–26 View FIGURE 24 View FIGURE 25 View FIGURE 26 ) is geographically restricted to the Atlantic Forest of eastern Brazil ( Fig. 102B View FIGURE 102 ) and as the distribution suggests, several morphological traits indicate relation to Otocrania but some traits also imply affinity to Phanoclocrania gen. n. which has two species in southern Central America and Colombia.All three genera share a ± distinct medio-longitudinal carina or keel on the ventral surface of the thorax, a globose and often prominently armed head, a distinctive black medio-longitudinal line or stripe on the pronotum and dorsally raised or lobed basitarsi in both sexes. Both sexes have a median segment that is slightly shorter than the metanotum. The exclusively apterous and very slender ♂♂ of Globocrania however are characteristic for the long anal segment that is strongly widened in the posterior portion and the widest of all abdominal segments ( Figs. 25C–J View FIGURE 25 ), the very slender and digitiform vomer ( Figs. 95N–O View FIGURE 95 ) and the fairly small, flat and scoop-shaped apically bidentate or bilobate poculum ( Figs. 25C–J View FIGURE 25 ). Females are very long and slender insects, which usually exceed 18 cm in body length. The subgenital plate projects beyond the tip of the abdomen by more than the length of the two terminal abdominal terga taken together and is variable in shape, ranging from slender and lanceolate over ovate and scoop-shaped in dorsal aspect to angular and spatulate ( Figs. 26C–E, H–J View FIGURE 26 ) and there is a very prominent praeopercular organ on abdominal sternum VII. They however differ from the ♀♀ of the two aforementioned genera by the distinctly dentate or spinose meso- and metapleurae as well as the two longitudinal, parallel rows of tubercles on the meso- and metasternum. With high probability the shape of the anal segment and poculum of ♂♂ as well as the two closely spaced medio-longitudinal rows of tubercles on the meso- and metasternum may represent autapomorphies of Globocrania . The armed meso- and metapleurae closely resemble certain species of Phanocloidea (e. g. Ph. turgida, Ph. submutica or Ph. vacca ) and are also seen in one Costa Rican species of Phanocles ( Ph. pleurospinosus sp. n.).
Intergeneric relationships among the remaining genera of this group are difficult to detect and deserve further phylogenetic analyses. All share a ± distinct medio-longitudinal carina or keel on the ventral surface of the thorax and profemora that are triangular in cross-section with the anterodorsal carina notably raised and the distinct medioventral carina in both sexes conspicuously displaced towards the anteroventral carina, dorsally crested basitarsi (more developed in ♀♀) and a pronounced praeopercular organ in ♀♀. Males are either apterous, brachypterous or fully alate with the latter taxa capable of active flight. While ♂♂ of Lanceobostra gen. n. and Phanoclocrania gen. n. are exclusively apterous, all other genera comprise taxa that have apterous and alate ♂♂. The median segment is variable in length, being somewhat shorter than the metanotum in taxa with apterous ♂♂ and equal in length or longer than the metanotum in ♀♀, whose corresponding ♂♂ are brachypterous or alate. In alate ♂♂ the median segment is usually much longer than the metanotum, the only exception being Phanocloidea muricata and Phanocloidea venezuelica sp. n. whose ♂♂ possess a very long median segment but only have tiny vestigial alae ( Figs. 73J–K View FIGURE 73 ). The principally Mexican Lanceobostra (19 known species) is a morphologically rather uniform genus ( Figs. 36 View FIGURE 36 , 37 View FIGURE 37 , 38A–C View FIGURE 38 ) in which the different species strongly resemble each other, ♀♀ always having a long and lanceolate subgenital plate, a distinct bifid praeopercular organ ( Figs. 91A–C View FIGURE 91 ) and and a rather flattened head that bears a pair of variably sized cephalic horns ( Figs. 38D–H View FIGURE 38 ), and ♂♂ having a sub-cylindrical, exclusively unarmed head with a white or light cream lower portion of the genae ( Figs. 38J–O View FIGURE 38 ) and a ± distinct, spiniform central protuberance of the poculum ( Figs. 39O–V View FIGURE 39 , 40 View FIGURE 40 ). While ♀♀ often possess single teeth or lobes on the mid and hind legs, the limbs of ♂♂ are exclusively unarmed.Autapomorphies of Lanceobostra are difficult to determine at this point. The eight known species of Parotocrania ( Figs. 46–48 View FIGURE 46 View FIGURE 47 View FIGURE 48 ) are distributed in Panamá, Colombia, Ecuador and northeast Peru ( Fig. 103F View FIGURE 103 ). The genus is typical for having ♂♂ with a granulose, nodose or tubercular head and thorax, which might well be an autapomorphy. Females have the medio-longitudinal carina of the mesosternum only obscure and have a rather flattened, just weakly keeled subgenital plate that at best slightly projects beyond the apex of the abdomen and has the apex ± downcurved ( Figs. 46F–N View FIGURE 46 ). The prominent cephalic horns seen in three species (♀♀ of P. panamae sp. n. and ♂♂ of P. cassicephala and P. segmentaria ) resemble Phanoclocrania and provide clue for close affinity to this genus, which is supported by the distribution. Phanoclocrania ( Fig. 68 View FIGURE 68 ) has only two very distinctive species that are distributed in Panama and Colombia ( Fig. 104B View FIGURE 104 ). Males are characteristic for the huge spiniform and backward-directed appendage of the poculum ( Fig. 68J View FIGURE 68 ), which possibly is an autapomorphy. Whereas the Colombian P. speciosa has a small triangular vomer with a single terminal hook, the vomer is rather angular in outline with three terminal points in the type-species P. dorsuaria ( Fig. 95K View FIGURE 95 ) The latter character is unique within the entire Cladomorformia and apparently an autapomorphy of that species. The ♂ of P. speciosa at the other hand has strongly elongated, gently arched and downward-directed cerci and an anal segment in which the posterolateral angles are protruded into an obtusely rounded process. Both species have the dorsal carina of the basitarsi of both sexes rounded and possess an acute medio-longitudinal keel on the meso- and metasternum ( Figs. 86K–L View FIGURE 86 ). The two most speciose, polymorphic and most widely distributed genera of this group and the whole of Cladomorformia are the closely related Phanocles (35 known species) and Phanocloidea (26 known species). Both genera comprise species with apterous and pterous ♂♂, species with or without cephalic armature in one or both sexes and various head shapes throughout both sexes (see further comments below), ♀♀ with short or enormously elongated and variously shaped subgenital plates as well as variously shaped praeopercular organs and species with smooth to strongly sculptured body surfaces. Cephalic armature in ♂♂ however only occurs in Phanocles , whereas the head is always unarmed in Phanocloidea . Moreover, a wide range of different armature and furniture of the extremities is seen among ♀♀ of Phanocles in particular. Females of Phanocles almost always have the lateral margins of abdominal tergum VII deflexed or dilated into a ± distinct lobe (the following species have this lobe just very weakly developed although: P. aequatorialis , P. berezini sp. n., P. horni P. maximus sp. n. and Ph. significans ), but while ♀♀ of Phanocloidea mostly have the lateral margins of abdominal tergum VII straight, one species has lobes that are similar to those frequently seen in Phanocles (i. e. Phanocloidea pallidenotata ). Phanocloidea contains two closely related northwest South American species that have conspicuously shortened and small subgenital plates, which do not reach to the tip of the abdomen ( Ph. gracilis and Ph. ibaguena ), a character that within the whole of Cladomorformia is elsewhere only found in the genus Bostriana . The spinose meso- and metapleurae seen in certain ♀♀ of Phanocloidea (e. g. Ph. incolumis , Ph. magistralis, Ph. turgida, Ph. submutica , Ph. tobagoense or Ph. vacca ) resemble the Brazilian Globocrania . Whereas the morphology of the terminalia is fairly uniform and only shows variability in the shape of the anal segment, vomer and poculum in Phanocles , notable polymorphy and specialisations are observed in ♂♂ of Phanocloidea . In this latter genus abdominal tergum IX may have the lateral margins almost straight to prominently dilated to form a semi-circular or ± large triangular lobe and the poculum shows various specialisations including posterolateral impressions and/or excavations of the lateral margins and/or different shapes of the posterior margin ( Figs. 96A–K View FIGURE 96 ). While ♂♂ of Phanocles are mostly various shades of brown or grey and rarely show greenish or bluish tones on the meso- and metathorax, ♂♂ of Phanocloidea are often fairly colourful insects that show green, blue or red colours on the thoracic segments or almost always have reddish bases of the profemora. In contrast to the insect, the eggs of both genera can be frequently and readily distinguished. Whereas those of Phanocles have distinctly pitted capsules ( Figs. 99 View FIGURE 99 , 100A–B View FIGURE 100 ), those of Phanocloidea are never pitted but show various other sculpturing including rugulae, ridges, scale-like structures or even fimbriate appendages ( Figs. 100G–P View FIGURE 100 ).
Calynda group. A last group of genera, that share several common characters and therefore appear to be closely related, is formed by the Central American Calynda and Spinocloidea gen. n., the Andean Andeocalynda , Globocalynda that is distributed in the eastern slopes of the Andes from Colombia to Bolivia in the south and the monotypic Laciphorus , which is peculiar to the “Coastal Lomas” of Peru. The distribution is restricted to southern Central America and the South American transition zone (see Morrone, 2006). Males of all genera are exclusively apterous and both sexes have a median segment that is always shorter than the metanotum but often strongly shortened. The mesosternum is simple and lacks a medio-longitudinal carina. Females all exhibit a considerably elongated subgenital plate that projects notably beyond the apex of the abdomen (exception Globocalynda cyrtocnemis ) and have a just weakly developed praeopercular organ. In accordance to the weakly developed praeopercular organ in ♀♀, which serves as a contact point for the ♂♂ genitalia during the copulation, ♂♂ of all genera have more or less distinctly elongated and incurved cerci (occasionally not very pronounced in Andeocalynda ) or a tectate anal segment that serves to grasp the abdomen of ♀♀ when mating. Eggs ( Figs. 98A–E, G View FIGURE 98 ) are ovoid with a rounded polar area, more or less notably compressed laterally and always possess a smooth to minutely coriaceous and glossy capsule surface. The colour usually ranges from uniformly brown to almost black and the dorsal surface may sometimes have a more or less distinctly lighter coloured area that surrounds the micropylar plate; they are distinctly mottled in only one case ( Laciphorus ). The sponge-like matrix operculum is small, roundly convex to obtusely conical and less than one-quarter of the capsule length, the dorsal surface usually showing radially arranged ridges and grooves.
Calynda ( Figs. 13–14 View FIGURE 13 View FIGURE 14 ) and Spinocloidea ( Figs. 77–78 View FIGURE 77 View FIGURE 78 ) share a very short median segment in both sexes, that is notably less than one-quarter the length of the median segment and wider than long. Females have a very long and lanceolate, acutely pointed subgenital that projects beyond the apex of the abdomen by much more than the length of the three terminal abdominal terga taken together. Males of these two genera are virtually identical morphologically and even chromatically and may only be distinguished by slight differences of the anal segment and cerci. A subgenital plate that is similarly long, lanceolate and apically pointed like in the two aforementioned genera is also seen in Andeocalynda ( Figs. 9–10 View FIGURE 9 View FIGURE 10 ), but this genus has a median segment that is more than one-quarter the length of the metanotum. Laciphorus ( Fig. 35 View FIGURE 35 ) and Globocalynda ( Figs. 20–23 View FIGURE 20 View FIGURE 21 View FIGURE 22 View FIGURE 23 ) have an even longer median segment that is up to two-thirds as long as the metanotum. Females of Calynda are characteristic for their cephalic armature, which consists of one or two pairs of closely spaced and transversely arranged spines between the eyes ( Figs. 14A–C View FIGURE 14 ). With the exception of the ♀♀ of two species of Globocalynda ( G. cornuta sp. n. and G. cyrtocnemis ) the heads of all other representatives of this group are unarmed, but variable in shape ranging from elongate and sub-cylindrical to almost spherical. Males of Globocalynda ( Fig. 23 View FIGURE 23 ) and Laciphorus ( Figs. 35D–F View FIGURE 35 ) share a remarkably similar morphology of the terminalia, which includes a very short and tectate anal segment that is hardly longer than wide or high, a bulgy poculum that more or less reaches ( Globocalynda ) or slightly projects beyond the tip of the abdomen ( Laciphorus ) and elongated, incurved and downward-directed cerci. A very distinctive and most certainly apomorphic character of the ♀♀ terminalia that is shared by Andeocalynda , Globocalynda and Laciphorus is the strikingly enlarged, laterally compressed and paddle-shaped gonoplacs, that mostly project beyond the posterior margin of the anal segment. These are most prominently enlarged in Laciphorus ( Fig. 35C View FIGURE 35 ) where they project over the abdomen by as much as the length of the anal segment. Whereas the gonapophyses VIII are strongly elongated, filiform and project the tip of the anal segment in ♀♀ of Spinocloidea , they are wholly or mostly concealed under the anal segment in all other genera.
The short median segment of two genera and the egg morphology ( Figs. 98A–E, G View FIGURE 98 ), which includes an ovoid general shape, almost smooth and glossy capsule surface, a lighter coloured area that surrounds the micropylar plate and an operculum with just a low central sculpturing resemble eggs of certain members of Diapheromerini s. str. (e. g. Diapheromera and Megaphasma ) and the genus Bacteria (“Bacteriformia”). Moreover, the rim of setae on the outer margin of the operculum in the eggs of Calynda and Spinocloidea is unique within Cladomorformia but otherwise present in eggs of the abovementioned three genera of Diapheromerini s. str. and “Bacteriformia”. These morphological characters emphasise on the close relation between Cladomorformia and the two mentioned lineages (compare Figs. 133A–B View FIGURE 133 ).
Two genera take on rather isolated positions within Cladomorphini and based on morphological characters cannot be attributed to any of the five generic groups outlined above. The majority of anatomical traits however appear to support affiliation to the Phanocles group.
Bostriana : This monotypic Bolivian genus combines morphological characters of various genera and of the Calynda and Phanocles groups. In general shape an appearance ♀♀ strongly resemble certain representatives of Diapheromerini s. str. (e. g. Diapheromera ) or Clonistria (“Bacteriformia”) by having a fairly short median segment, a small and scoop-shaped subgenital plate ( Figs. 12D–E View FIGURE 12 ) and wholly unarmed legs. But actually, there are no anatomical characters that would place Bostriana in affinity with the abovementioned taxa and also the distribution in Bolivia contradicts a close relation to these taxa. Stigmae VIII are placed at athe anterolateral angles of abdominal tergum VIII and there are well-developed thorn pads and a distinct vomer in ♂♂ and the cerci are not enlarged. As already pointed out by Hennemann et al. (2020) the short median segment that is less than one-third the length of the metanotum, apterous ♂♂ and egg morphology ( Fig. 98F View FIGURE 98 ), which includes an almost smooth and glossy, ovoid capsule of plain dark brown colour with a pale cream area that surrounds the micropylar plate as well as a rather small opercular protuberance that has radially arranged ridges and furrows, indicate affinity to the Calynda group. However, the distinct medio-longitudinal keel of the mesosternum ( Fig. 85A View FIGURE 85 ), distinct praeopercular organ of ♀♀ ( Figs. 12D–E View FIGURE 12 ) as well as the strongly deflexed and triangular lateral margins of abdominal tergum IX, roundly rectangular vomer ( Fig. 95G View FIGURE 95 ) and small cerci of ♂♂ ( Figs. 12F–H View FIGURE 12 ) clearly contradict a position within that group and would rather suggest affinity to taxa of the Phanocles group. Whereas the small and scoop-shaped subgenital plate of ♀♀ is also seen in two members of Phanocloidea (i. e. Ph. gracilis and Ph. ibaguena ) and the shape of abdominal tergum IX of ♂♂ resembles Phanocloidea , the medio-longitudinally keeled and posteromedially incised and bifid poculum ( Fig. 12H View FIGURE 12 ) is unique within Cladomorformia .
Ocreatophasma gen. n.: The three newly described Peruvian species of this strange genus are all only known from the ♀♀ ( Figs. 41–42 View FIGURE 41 View FIGURE 42 ). These are remarkable for a number of morphological characters that are unique within Cladomorformia and readily separate the genus from all other members of the clade. These include the flattened and scoop-shaped subgenital plate ( Figs. 42D–L View FIGURE 42 ), the prominently deflexed and uniformly lamellate, blade-like posterodorsal carina of the protibiae, the huge rounded dorsal lobe of the probasitarsus, the slender, laterally compressed and entirely unarmed mid and hind legs and the very distinct, sharp medio-longitudinal keel of the meso- and metasternum. A longitudinal median keel on the meso- and metasternum is also seen in other genera of the tribe (e. g. Lanceobostra or Phanoclocrania ) but neither genus has it raised to such an extreme extent as in Ocreatophasma ( Figs. 85K–M View FIGURE 85 ). The general shape and overall appearance of these ♀♀ and most of the abovementioned morphological peculiarities resemble certain members of the Oriental subfamily Lonchodinae (family Lonchodidae ), e. g. the genera Carausius Stål, 1875 or Leprocaulinus Uvarov, 1940 , why the examined insects were at the first moment believed to be wrongly labelled representatives of these Oriental genera. The fact however that there were three different species and that all specimens were quite recently collected by scientists and staff of the MNHN prompted a more detailed and careful investigation of these specimens. Actually, several morphological traits such as the flattened and rather scoop-shaped subgenital plate, notably elongated gonapophyses VIII and morphology of the profemora showed these distinctive members do not have affinity within the Oriental Lonchodinae but represent an as yet undescribed genus that belongs in the New World Cladomorformia , with the morphology of the genitalia resembling certain species of Phanocloidea . By all means Ocreatophasma is a superb example for stunning convergent evolution in two not closely related taxa of the New World Occidophasmata and Old World Oriophasmata. Although no live specimens of Ocreatophasma have been observed, the striking convergence suggests Ocreatophasma- species to exhibit similar habits, rest positions and camouflage as representatives of the Oriental Carausius , Leprocaulinus and allied genera. Due to the almost identical morphology of the thoracic sterna and legs it can be assumed that the mid legs are tightly laid alongside the concave lateral portions of the mesosternum with also the front and hind legs held in an axis to the body, which provides an impressive stick mimic.
Although the present paper described as many as seven new genera and 41 new species and provided descriptions and illustrations of the previously unknown ♂♂ of thirteen, ♀♀ of six and eggs of 26 species, our knowledge of the true diversity of Cladomorformia is still much incomplete. A few numbers might therefore help illustrating the gaps in our knowledge. As many as 81 of the 163 described species (49.7 %) are to date only known from a single sex and the majority of these species is even only known from the unique type specimens. What concerns to the eggs, only those of 40 species have so far become known, which is less than one-quarter (24.5 %). Nonetheless, the numerous new descriptions presented herein have fundamentally helped in delimiting genera, attributing species to these and splitting the two previously highly polyphyletic genera Bacteria and former Bostra . The latter genus has unnecessarily been replaced by Bostranova by Villet (2023) and all species that were attributed to Bostranova could be placed in other genera. Bacteria however still remains a polyphyletic genus that in addition to its Caribbean type-species B. ferula (Fabricius, 1793) and the supposedly congeneric B. donskoffi ( Langlois & Lelong, 1998) from the island of Guadeloupe still contains a good number of species that are not congeneric and whose true generic affiliation still deserves clarification.
Although the Cladomorphini mostly comprises large to very large phasmatodeans that exhibit a wide range of morphological traits and may at first glance appear easy to handle in means of taxonomy, this particular clade represents an earnest challenge not only for morphology-based approaches. Also, molecular approaches (e. g. Bank & Bradler, 2022; Forni et al., 2022) have not yet been able to provide us with satisfying results to a full extend, which however is due to the still not sufficiently broad sampling. Moreover, there are still several unclarified discrepancies between morphological traits and molecular-based phylogenetic approaches towards explaining relationships within Diapheromerinae and the two sub-ordinate tribes Diapheromerini and Oreophoetini , some of which have been subjected to a critical discussion herein (→ 4.). Undoubtedly, also the still fractional knowledge of the true diversity of Cladomorformia taxa outlined above yet hinders a more comprehensive understanding of inter- and intrageneric relationships within the Diapheromerini . In aspect of the fact that about half of the known species diversity of Cladomorformia is only known from a single sex, there are morphological traits in some genera that seriously complicate the matching of sexes and represent a considerable difficulty for morphology-based studies. The genus whose taxonomy is by far most seriously affected by this problem is Phanocles , which is the most speciose taxon of the entire clade and currently contains as many as 35 known species. This genus shall here serve as an example to outline some of the challenges and hassle for morphology-based taxonomic work within most genera of Cladomorformia .Apart from that there is extreme sexual dimorphism between the ♀♀ and ♂♂ of individual species in Phanocles there are hardly any morphological characters that would allow a reliable matching of sexes and there appears to be almost no congruence between certain characters in ♀♀ and the corresponding ♂♂. For instance, if ♀♀ of a certain species have a globose and distinctly bi-cornute head this does not necessarily mean that the respective ♂♂ also have a globose and armed head and vice versa. A species that very well illustrates this problem is the Mexican Phanocles saussurei . The ♀ and ♂ of this species could only be matched because the two specimens examined were reared from eggs that were laid by an individual wild-caught ♀. While the ♀ has a fairly globose head with a roundly convex and sparsely nodulose vertex that bears a pair of prominent, laterally compressed and carinated auriform horns, the head of the ♂ is entirely different being sub-cylindrical in shape, somewhat narrowing towards the posterior and has a flat and wholly smooth vertex. Such different head morphologies between the sexes of one individual species like they can be seen in some representatives of Phanocles are unusual within Phasmatodea and therefore particularly remarkable. The same issue is observed in the armature of the extremities, which however is always much less developed in ♂♂. Quite frequently ♀♀ with prominent sub-basal ventral lobes on the two outer ventral carinae of the meso- and metafemora and dorsal lobes on the corresponding tibiae have ♂♂ with wholly unarmed legs. This is also true for the basitarsi, which may bear dorsal lobes in the ♀♀ but are slender and non-lobed in the corresponding ♂♂. Also, the length relation of the median segment and metanotum may vary between the sexes of individual species within Phanocles and is no reliable anatomical trait to allocate ♂♂ and ♀♀, especially if the ♂♂ are winged. In such cases the ♂♂ usually have a very longer median segment that is several times longer than the metanotum, whereas it is just scarcely longer than the metanotum in the respective ♀♀. The issue concerning the length relation of the metanotum and median segment can to a somewhat lesser extent also be observed in certain species of Phanocloidea , which have apterous ♂♂ in which the median segment is slightly longer than in the respective ♀♀ (e. g. Ph. muricata or Ph. venezuelica sp. n.). Finally, also chromatic traits are mostly of little assistance for allocating sexes because also the colouration if affected by intraspecific variability and may be very different between the ♂♂ and ♀♀ of individual species. Thus, reliable matching of ♂♂ and ♀♀ of individual species in the genera Phanocles and Phanocloidea in particular is a serious difficulty and actually only possible if specimens from the same locality and ideally even the same collecting event are available, specimens were found on the same host plant or in copula or if both sexes were obtained by breeding in captivity. DNA barcoding may constitute a useful tool for the distinction and delimitation of similar and cryptic species but good numbers of specimens are needed for reliebale results. A taxon, whose anatomy, egg-morphology and distribution suggest a cryptic species is Phanocles keratosqueleton from the Lesser Antilles. Populations from different islands appear to show fairly consistent anatomical differences and it is hoped that an integrative approach that comprises morphological and molecular data may render clarification of this hypothesis. The authors have examined a considerable number of further specimens from these two genera that were not included in the present study. Although most of these are likely to represent as yet unknown species it can due to the reasons summarized above not be ruled out with certainty that they merely represent the opposite sex of already described species. Hence, it was considered wiser to refrain from describing these at this point and rather wait for the availability of more material in the future. As for all Phasmatodea the eggs of Cladomorphini have proven a very helpful tool for distinguishing similar species or delimiting the range of intraspecific variability in variable species. Thus, if available the eggs should always be taken into account in future taxonomic studies within Cladomorphini taxa.
It has been shown that there are still considerable gaps in our knowledge of the so-called “giant stick insects” of the New World and that to date there is a great likelihood of several large and spectacular as yet undescribed species to be found. This is not only true for lesser prospected areas of the Neotropics. As examples have shown i. e. by the discovery of Phanocles maximus sp. n., which is the longest extant insect of the Americas new giant species may also be discovered in supposedly well explored areas. The large number of new species described in the present study, leave no doubt that with certainty still a good number of new species of Cladomorformia are to be found if more extensive surveys are being conducted. In aspect of the fact that about half of the species are only known from one sex and that the eggs of about three-quarters of the known species have yet remained unavailable and undescribed there still is a lot of work to deal with in order to complete our knowledge of this spectacular group of New World phasmatodeans. Apart from the taxonomy and diversity also our knowledge of the biology, habits, habitats and natural host plants can yet be regarded as very limited with sufficient information so far available for only a very few species, thus still representing wide fields of research for scientists. It is therefore hoped that future studies on the Cladomorformia will not only restrict to the descriptions of new taxa but will also provide recognition of the many so far unknown sexes and eggs, new precise records and distribution data to understand the distributional patterns of the genera and species, and also information to broaden our knowledge on the biology, ecology and dietary preferences. The quickly proceeding destruction of tropical forests throughout the neotropics, which are the main habitats of these insects, is the most severe problem not only for the diversity of the Cladomorformia but for most Phasmatodea and must be seen with great dismay. Therefore, it is very much hoped that increasing interest and scientific research on the New World Phasmatodea and the discoveries of more stunning species will help in drawing the responsible authorities’ attention in the various countries of this part of the world on the imperative to save these sensitive ecosystems and complex habitats for sustaining their irretrievable biodiversity for future generations.
“Man is the most insane species. He worships an invisible God and destroys a visible Nature. Unaware that this Nature he’s destroying is this God he’s worshipping.” Hubert Reeves (French Canadian astrophysicist)
8.1. List of new taxa
Cladomorformia tax. n.
Globocrania gen. n.
Hirtuleiodes gen. n.
Lanceobostra gen. n.
Ocreatophasma gen. n.
Parotocrania gen. n.
Phanoclocrania gen. n.
Spinocloidea gen. n.
Cladomorphus pleuracanthus sp. n. [ Brazil]
Globocalynda cornuta s p. n. [ Ecuador]
Globocalynda marcapatae s p. n. [ Peru]
Globocalynda ruficollis sp. n. [ Bolivia]
Hirtuleius peruanus sp. n. [ Peru]
Jeremiodes costaricensis sp. n. [ Costa Rica]
Jeremiodes ecuadoricus sp. n. [ Ecuador]
Jeremiodes peruanus sp. n. [ Peru]
Lanceobostra chapalaense sp. n. [ Mexico]
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Hirtuleiodes
| Hennemann, Frank H. & Conle, Oskar V. 2024 |
Phibalosoma michaelis
| Redtenbacher, J. 1908: 427 |
Xylodus adumbratus
| Redtenbacher, J. 1908: 427 |
| Saussure, H. de 1859: 62 |