Cladomorformia
|
publication ID |
https://doi.org/ 10.11646/zootaxa.5444.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:5DE4A9DD-99F7-4E23-AD50-58DC491BB75E |
|
persistent identifier |
https://treatment.plazi.org/id/03FD87D9-FFB0-D87E-FF55-F4082A84E682 |
|
treatment provided by |
Plazi |
|
scientific name |
Cladomorformia |
| status |
|
5. Cladomorformia tax. n.
Type-genus: Cladomorphus Gray, 1835: 15 .
Cladomorphi Brunner v. Wattenwyl, 1893: 98 .
Cladomorphini View in CoL Bradley & Galil, 1977: 189 (in part).
Otte & Brock, 2005: 32 (in part).
Hennemann & Conle, 2007: 2 (in part).
Hennemann & Conle, 2010: 101.
Hennemann, Conle & Perez-Gelabert, 2016: 15, figs. 6–12, 50, 409
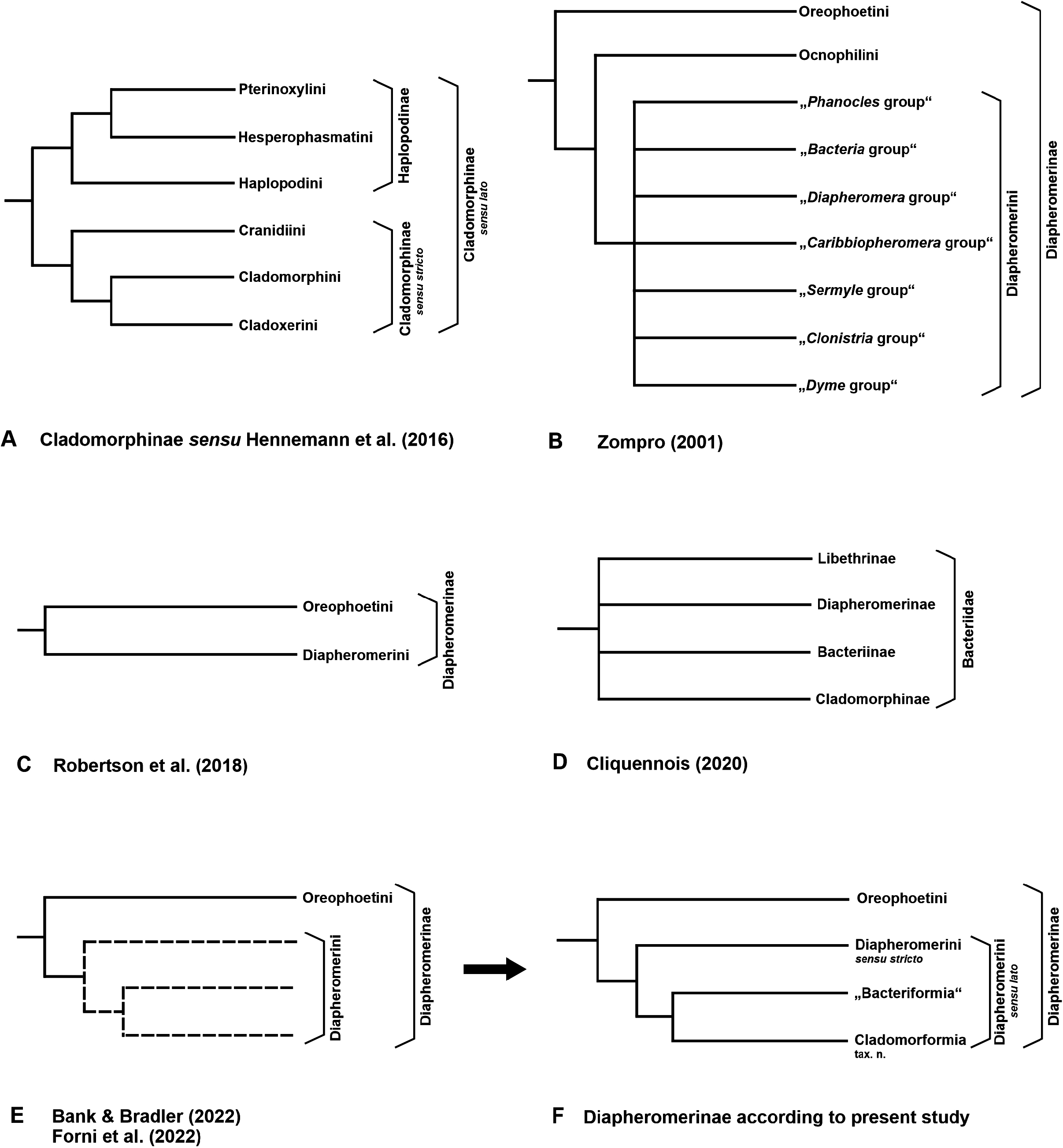
Cladomorphinae, Cliquennois, 2020: 414 View in CoL , tab. 1.
Cladoxerinae Karny, 1923: 237 (in part).
Cranidiini Günther, 1953: 557 View in CoL .
Zompro, 2004b: 134 (in part).
Otte & Brock, 2005: 32 (in part).
Hennemann, Conle & Delfosse, 2007: 358 (in part).
Hennemann, Conle & Perez-Gelabert, 2016: 17, figs. 13–19, 49.
Craspedoniini Bradley & Galil, 1977: 187. [Unnecessary replacement name for Cranidiini Güntrher, 1953 ]
Diapheromerini, Zompro, 2001: 192 (in part—only “ Phanocles group”, “ Bacteria group” (in part) and “ Clonistria group (in part)).
Robertson et al., 2018: 11 (in part).
Phibalosomini Günther, 1953: 557 (in part).
Phibalosomatini (Sectio V: Phibalosomata) Redtenbacher, 1908: 399 (in part).
Taxa included. The rank-free Cladomorformia tax. n. comprises all genera formerly attributed to the tribe Cladomorphini Brunner v. Wattenwyl, 1893 ( Hennemann & Conle, 2010; Hennemann et al., 2016 b), the genera Cranidium (tribe Cranidiini Günther, 1953 ) and Otocrania as well as all genera of Zompro’s (2001a) “ Phanocles group”, two genera of the “ Bacteria group” and one genus of the “ Clonistria group” ( Trychopeplus Shelford, 1909 ) of the tribe Diapheromerini sensu Robertson et al. (2018) . It also includes the genera Calynda Stål, 1875 and Alienobostra Zompro, 2002 but not Phantasca Redtenbacher, 1906 . More detailed justifications and a full list of genera are provided below.
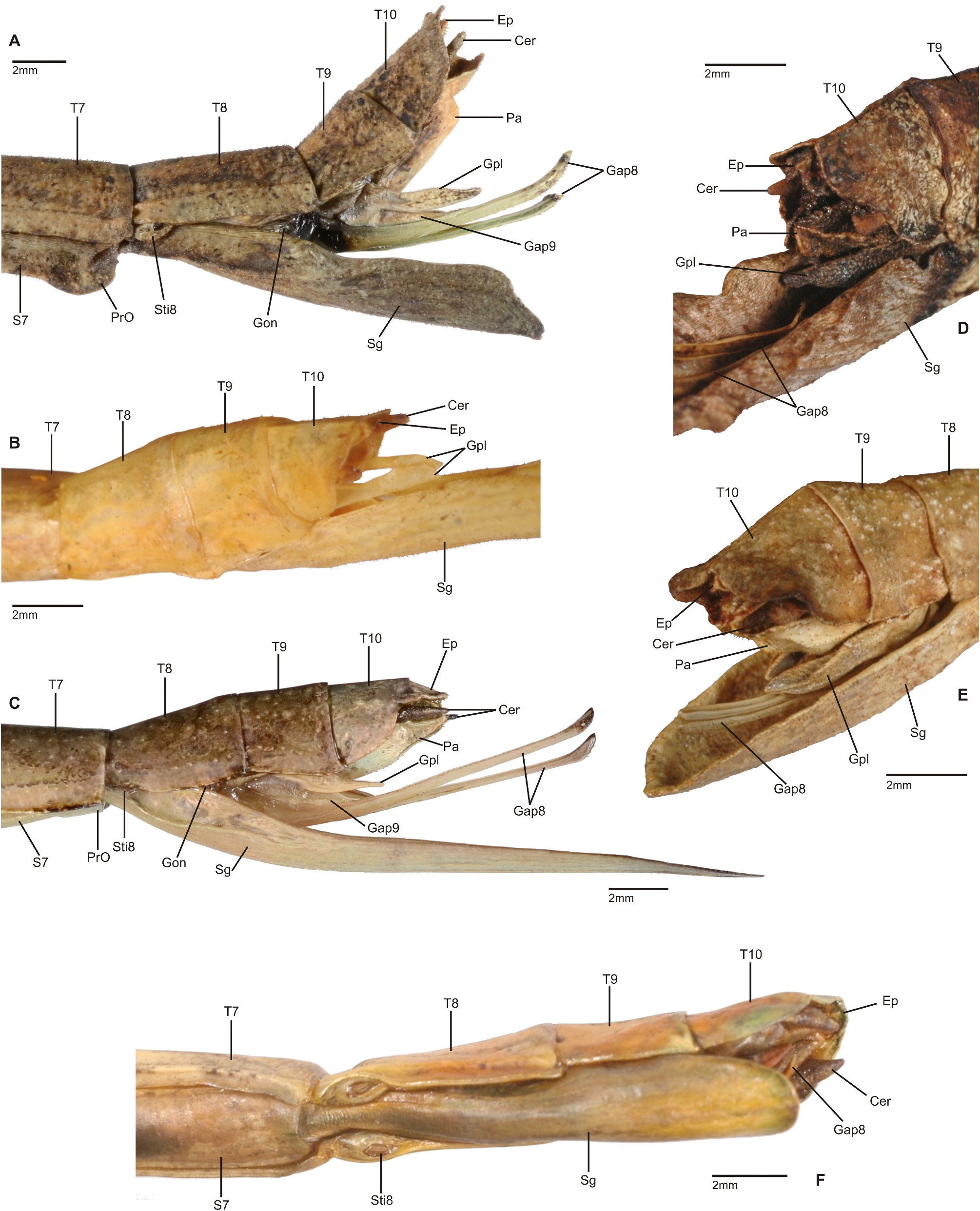
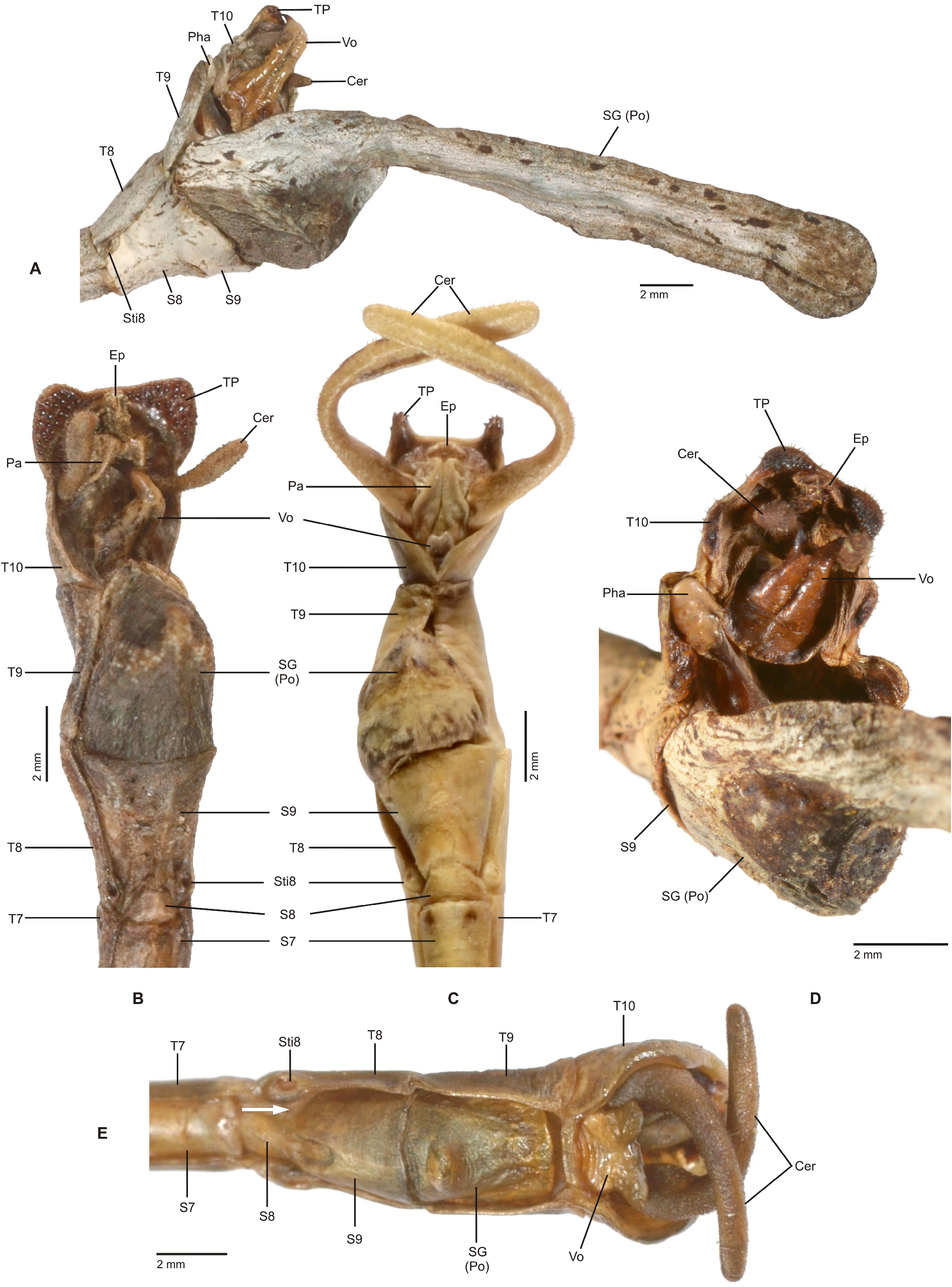
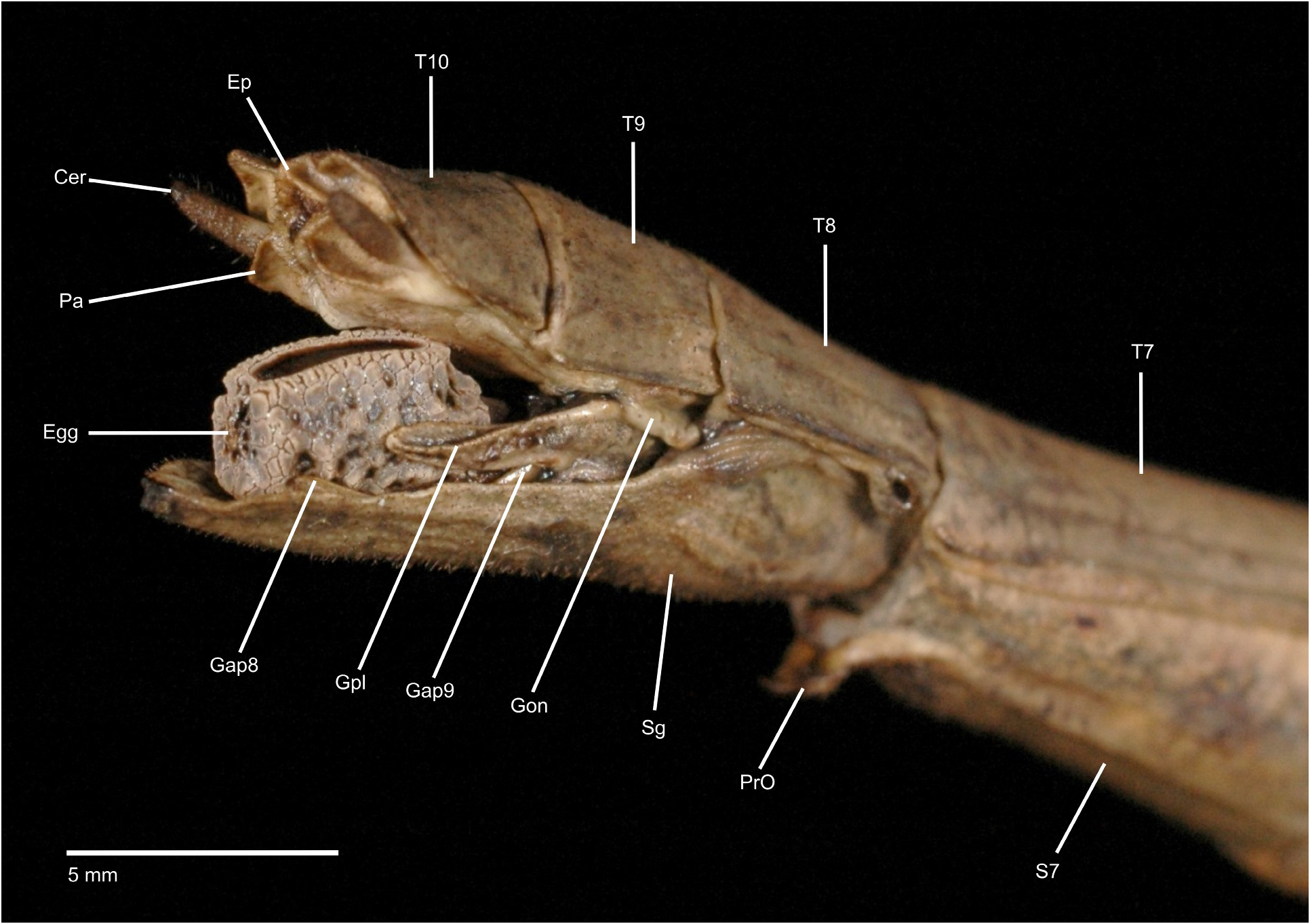
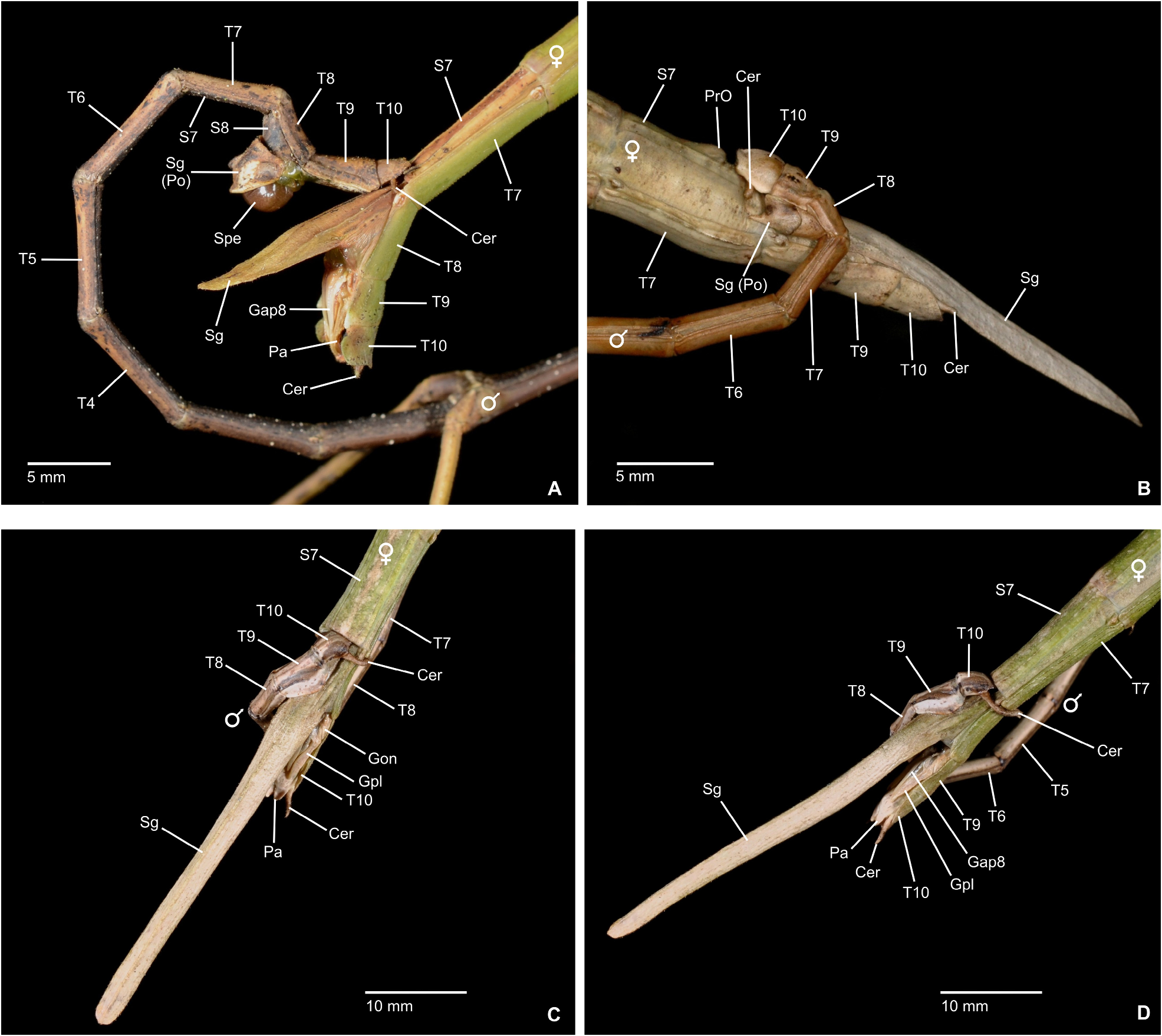
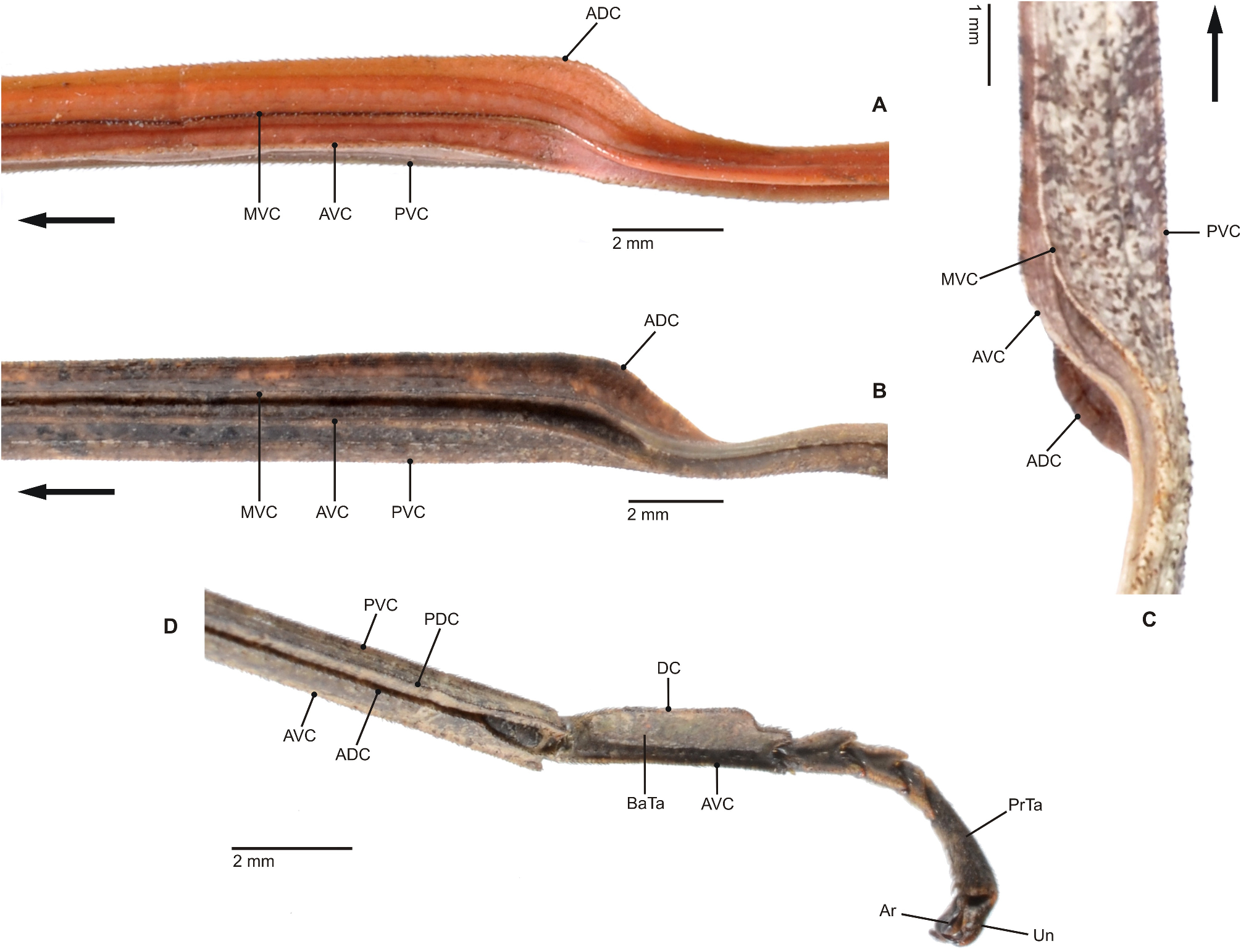
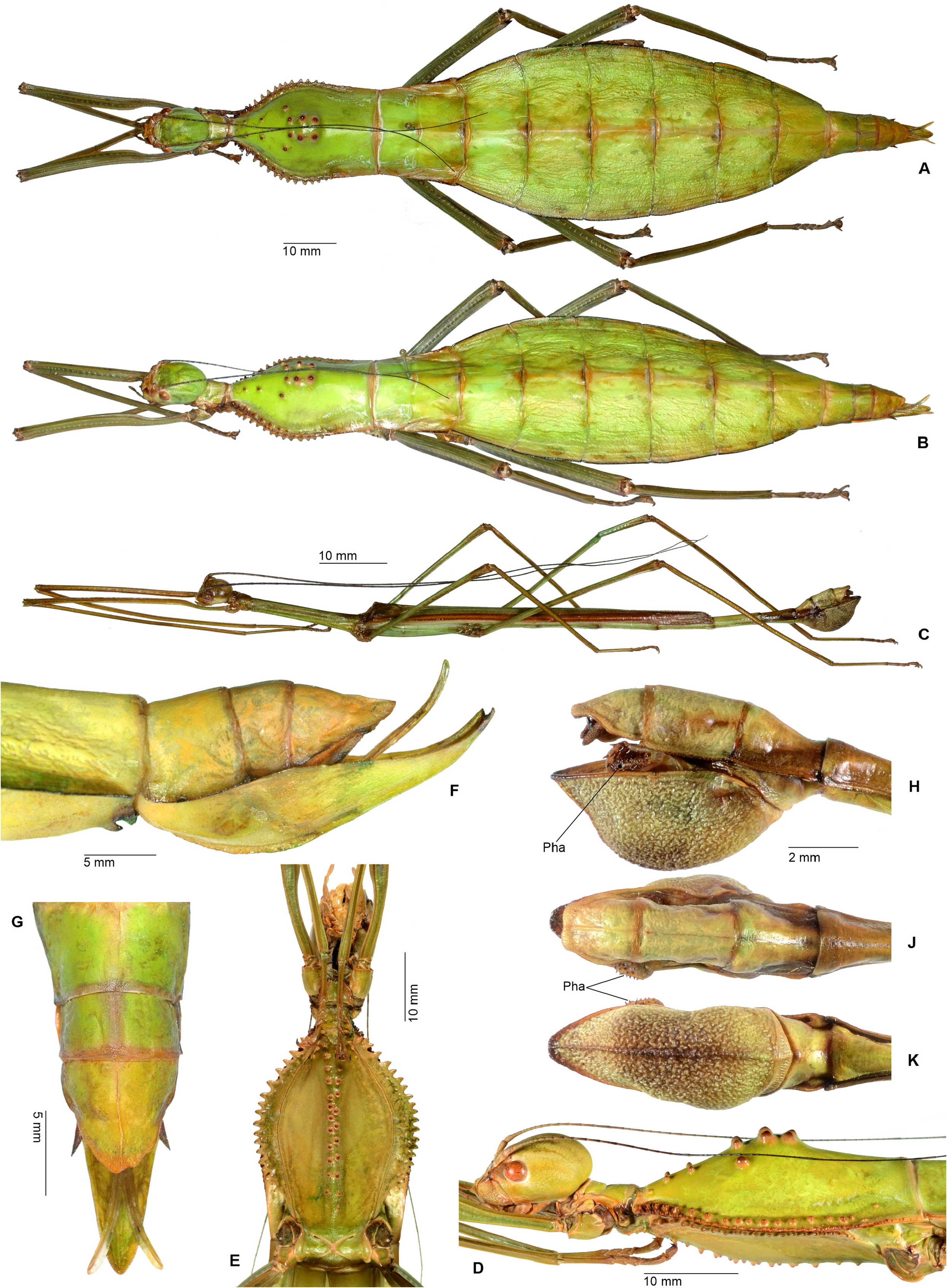
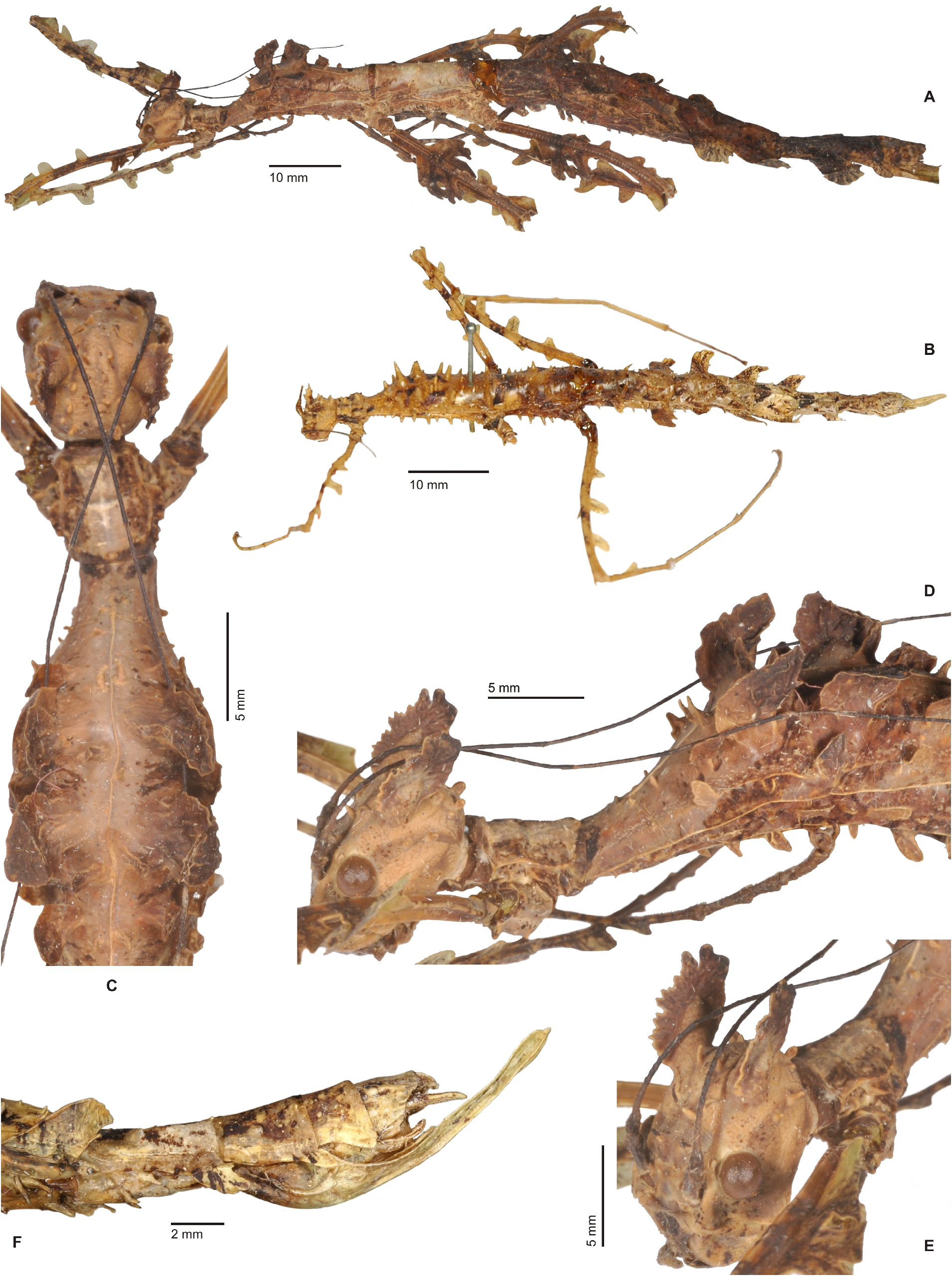
Characteristics. Medium-sized to very large (body length ♂♂ 59.0–159.0 mm, ♀♀ incl. subgenital plate 87.5– 285.0 mm), ± slender and stick-like, anareolate Cladomorphinae . Body cylindrical to oval in cross-section (♀♀), always cylindrical in ♂♂. Females apterous, ♂♂ apterous or fully alate; the length of the alae variable. Anal fan of alae (if present) hyaline or transparent grey. Head unarmed but often with a pair of cephalad tubercles, spines, horns or lobes. No ocelli. Gula present but small and transverse. Antennae fililform, longer than thorax and consisting of> 40 antennomers. Mesothorax elongate and slender, 3x longer than prothorax. Thoracic segments smooth, granulose or tubercular and more rarely spinose (♀♀); mostly smooth and rarely granulose or tubercular in ♂♂. Metathorax <two-thirds the length of mesothorax. Mesosternum (occasionally also metasternum) often with a medio-longitudinal carina or keel. Tegmina of ♂♂ (if present) small, oval or spatulate in shape and hardly longer than metanotum. Median segment variable in length, ranging from considerably shorter to much longer than metanotum (taxa with alate ♂♂ in particular). Abdomen longer than thorax. Females with a praeopercular organ near posterior margin of sternum VII; often very distinct and shaped by a pair or swellings, tubercles, spines or lobes. Tergum VII in ♀♀ often with lateral margins deflexed and forming a ± distinct lobe. Stigmae of segment VIII at anterolateral angles of segment. Terminalia of ♀♀ ( Figs. 3A–E View FIGURE 3 ): Cerci small and shorter than anal segment, cross-section round to oval but may also be distinctly compressed laterally. Paraprocts ± enlarged and carinate. Gonapophyses VIII ± elongated and considerably longer than gonapophyses IX; often extending noticeably over apex of abdomen ( Figs. 3A, C View FIGURE 3 ). Gonoplacs well-developed and> as long as gonapophyses IX; sometimes distinctly enlarged, laterally compressed and ± paddle-shaped ( Andeocalynda , Globocalynda and Laciphorus ) and even slightly longer than gonapophyses VIII ( Figs. 3A–E View FIGURE 3 ). Gonangulum present. Subgenital plate very variable in length and ± keeled medio-longitudinally; ranging from short and scoop-shaped to very long, ± lanceolate, spatulate or naviculate and extending considerably beyond apex of abdomen. Terminalia of ♂♂ ( Fig. 4A–D View FIGURE 4 ): With various specialisations of tergum IX, anal segment (= tergum X), cerci ( Fig. 6C View FIGURE 6 ), vomer and /or poculum (= sternum IX). Tergum IX open ventrally, not tubular. Anal segment open ventrally, the posterolateral angles ventrally with a distinct pair of thorn-pads. Vomer distinct and variable in shape, mostly elongate with a single upcurved terminal hook, or two apices ( Figs. 4A, D View FIGURE 4 ). Poculum ± bulgy, convex and cup-shaped, considerably larger than basal portion of sternum IX and often with a ± distinct central protuberance or spines and sometimes indented or excavated postero-medially; in one case ( Otocrania ) spatulate with the apex elongated and extending over apex of abdomen and in three genera ( Cladomorphus , Otocraniella and Xylodus ) forming a long tubular posterior appendix that considerably projects beyond the apex of the abdomen ( Figs. 4A, D View FIGURE 4 ). Cerci variable in size and shape, mostly small and simple but sometimes elongated ( Calynda and Spinocloidea gen. n.) or greatly enlarged and distinctly incurved ( Alienobostra ( Fig. 4C View FIGURE 4 ), Aplopocranidium , Jeremia and Jeremiodes ). No ‘area apicalis’ at apex of tibiae ( Fig. 7D View FIGURE 7 ). Profemora not serrated; medioventral carina distinct, ± lamellate and noticeably displaced towards anteroventral carina; anterodorsal carina ± raised ( Figs. 7A–C View FIGURE 7 ). Meso- and metafemora and all tibiae trapezoidal in cross-section with the dorsal carinae ± approaching each other ( Fig. 7D View FIGURE 7 ). Medioventral carina of meso- and metafemora indistinct, smooth or with a variable number of apical teeth or spines. The two outer ventral carinae often with a ± distinct expansion, tooth or lobe sub-basally (♀♀ in particular). Mid and hind legs in particular often with single tooth-like expansions or lobes (♀♀ in particular). Basitarsi longer than following three tarsomeres combined, carinate dorsally, either slender and or with the dorsal carina ± crested; the two dorsal carinae fused with another ( Fig. 7D View FIGURE 7 ).
Eggs ( Figs. 98–100 View FIGURE 98 View FIGURE 99 View FIGURE 100 ). Ovoid to angular, the polar-area often flattened or impressed. Anterodorsal portion of capsule ± strongly angular and convex. Capsule surface ranging from smooth and glossy to strongly sculptured and sometimes covered with hairy structures and/or fringes. Micropylar plate oval, longer than wide and> one-third the length of capsule. Micropylar cup placed at posterior end of plate. Internal micropylar plate open. Median line present. Operculum with an open matrix (non-stalked) capitulum; a ± strongly, conically or roundly elevated, hollow structure that is formed by extensions of the outer margin; the anterior portion open, porous or with numerous impressions.
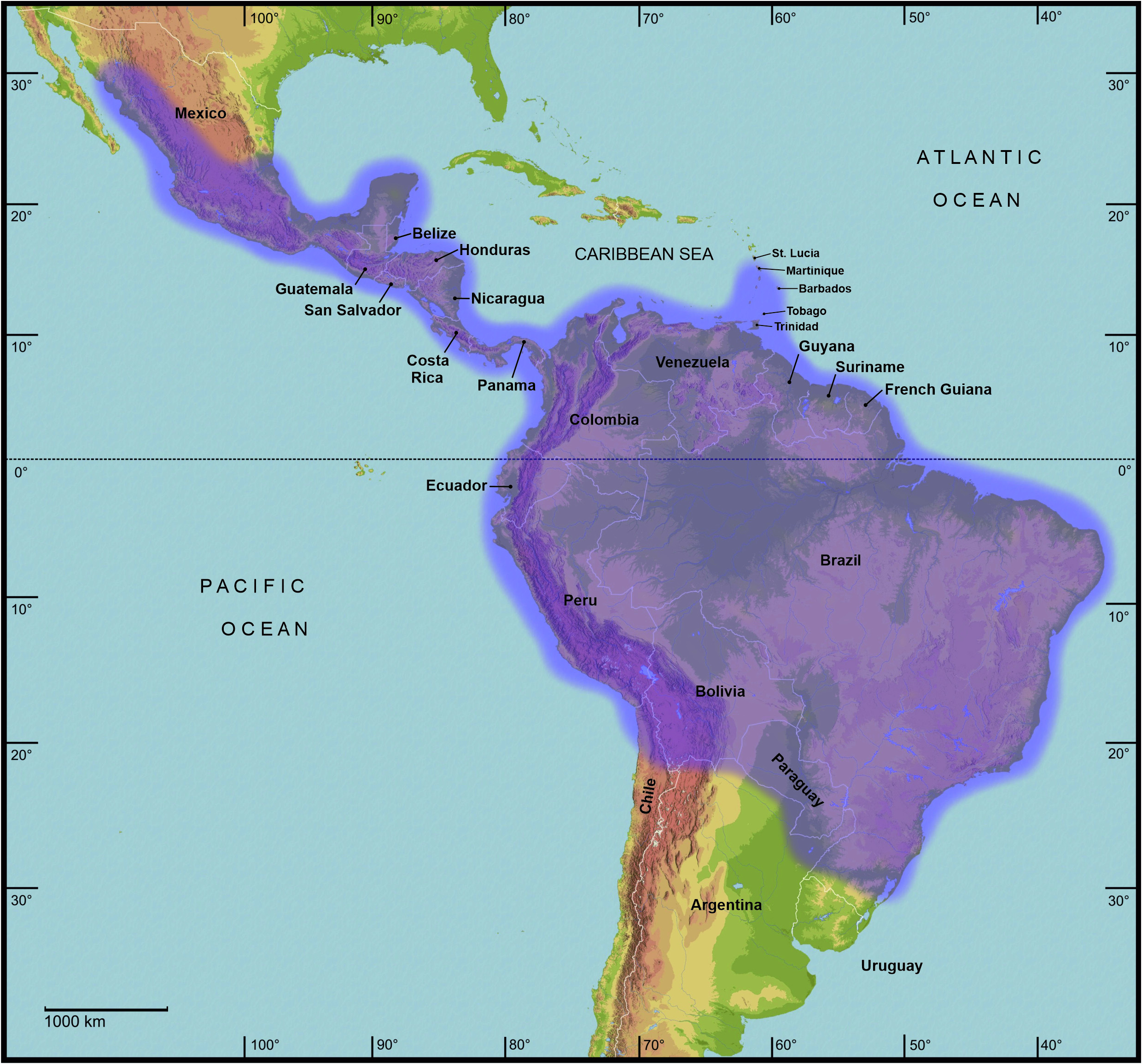
Differentiation. Both sexes of Cladomorformia tax. n. may be separated from Diapheromerini s. str. by having the stigma of abdominal segment VIII positioned at the anterior angle of abdominal tergum VIII, which is the normal condition in Phasmatodea and certainly the plesiomorphic character stage ( Bradler, 2009; Figs. 3A–E View FIGURE 3 , 4A–C View FIGURE 4 ). In Diapheromerini s. str. the stigmae VIII are more or less distinctly shifted towards the posterior and away from the anterior angle of abdominal tergum VIII, which has exclusively been regarded an autapomorphy of Diapheromerini s. str. or Eusermyleformia respectively (e. g. Bradler, 2000; Robertson et al., 2018). Cladomorformia frequently show dorsally lobed or crested basitarsi (though rarely in ♂♂), while in Diapheromerini s. str. the basitarsi are exceptionally slender and never bear a dorsal lobe or crest. A recognition of ♂♂ of Cladomorformia and separation from ♂♂ of Diapheromerini s. str. is possible by a combination of the following characters of the terminalia ( Figs. 4A–D View FIGURE 4 ): 1) Vomer well-developed and with one or two straight or upcurved terminal hooks; 2) Anal segment (tergum X) always open ventrally and posterior margin with a pair of distinct thorn-pads ventrally; 3) Tergum IX and sternum IX always distinctly separated from another; 4) Apex of cerci never forked and cerci never with an intero-basal tooth, protrusion or spine; 5) Poculum equal in size or larger than sternum IX. Some genera of Diapheromerini s. str. may exhibit one or two of the aforementioned characters, but lack the others. Consistent characters that separate ♀♀ of Cladomorformia from those of Diapheromerini s. str. are more difficult to determine. In contrast to ♀♀ of Diapheromerini s. str. there almost always is a distinct praeopercular organ near the posterior margin of abdominal sternum VII and the subgenital plate almost always extends beyond the apex of the abdomen and is often strongly elongated and lanceolate or spatulate. If the subgenital plate does not surpass the apex of the abdomen then there is a distinct praeopercular organ and vice versa. Thus, Cladomorformia ♀♀ with a weakly developed praeopercular organ ( Aplopocranidium , Calynda and Spinocloidea gen. n.) have a strongly elongated and lanceolate subgenital plate and ♀♀ with a short subgenital plate which does not extend beyond the apex of the abdomen (e. g. Bostriana ; Figs. 12 View FIGURE 12 D-E) exhibit a distinct praeopercular organ. The ♂♂ of taxa with a weakly developed praeopercular organ in ♀♀ usually have elongated cerci that serve as an anchorage during copulation by grasing the abdomen of ♀♀. Two characters frequently seen among members of Cladomorformia are strongly elongated and filiform gonapophyses VIII that often considerably project beyond the posterior margin of the anal segment ( Figs. 3A, C View FIGURE 3 ) and notably enlarged variably shaped gonoplacs (e.g. Andeocalynda , Cladomorphus , Globocalynda , Laciphorus or Xylodus ; Figs. 3A–E View FIGURE 3 ). Both characters are never to be observed within Diapheromerini s. str. While Cladomorformia are distributed throughout almost all of the tropical regions of the New World and are absent in the Nearctic region ( Fig. 2 View FIGURE 2 ), Diapheromerini s. str. have most strongly radiated in northern Central America (e. g. Pseudosermyle Caudell, 1903 ) and the southern portions of the Nearctic region and even have representatives in the temperate zones of Northern America (genera Diapheromera , Megaphasma and Manomera ).
A distinction of Cladomorformia tax. n. from genera of the mostly Caribbean lineage here provisionally referred to as “Bacteriformia” (→ see comments on genera incertae sedis below; Figs. 1F View FIGURE 1 , 133A–B View FIGURE 133 ) is more difficult, mostly because the latter group deserves more detailed investigation for delimitation by identifying anatomical apomorphies. A vomer is also present in the genera that belong to the “Bacteriformia” lineage, but then either the stigmae VIII are notably shifted towards the posterior ( Bacteria Berthold, 1827 , Fig. 4F View FIGURE 4 , 5E View FIGURE 5 ), abdominal tergum IX and sternum IX are sometimes partly or fully fused with another and/or the poculum is notably smaller than the basal portion of sternum IX or even asymmetrical (some species of Arumatia and Phantasca ), or segment IX is closed ventrally and fully fused with VIII to form a closed tube ( Caribbiopheromera Zompro, 2001 and some species of Phantasca ; see Bradler, 2009; Hennemann et al., 2018). In contrast to Cladomorformia , ♀♀ have a small and short, mostly scoop-shaped subgenital plate that never extends beyond the apex of the abdomen and only conceals the bases of the gonapophyses, the head of neither sex bears spines or horns and the basitarsi are slender without a dorsal lobe or crest.
Distribution ( Fig. 2 View FIGURE 2 ). Almost entire Neotropical Region, expanding from Central Mexico to the north over Central America and the Lesser Antilles to Paraguay and Argentina to the south.
Remarks on Cladomorformia tax. n.
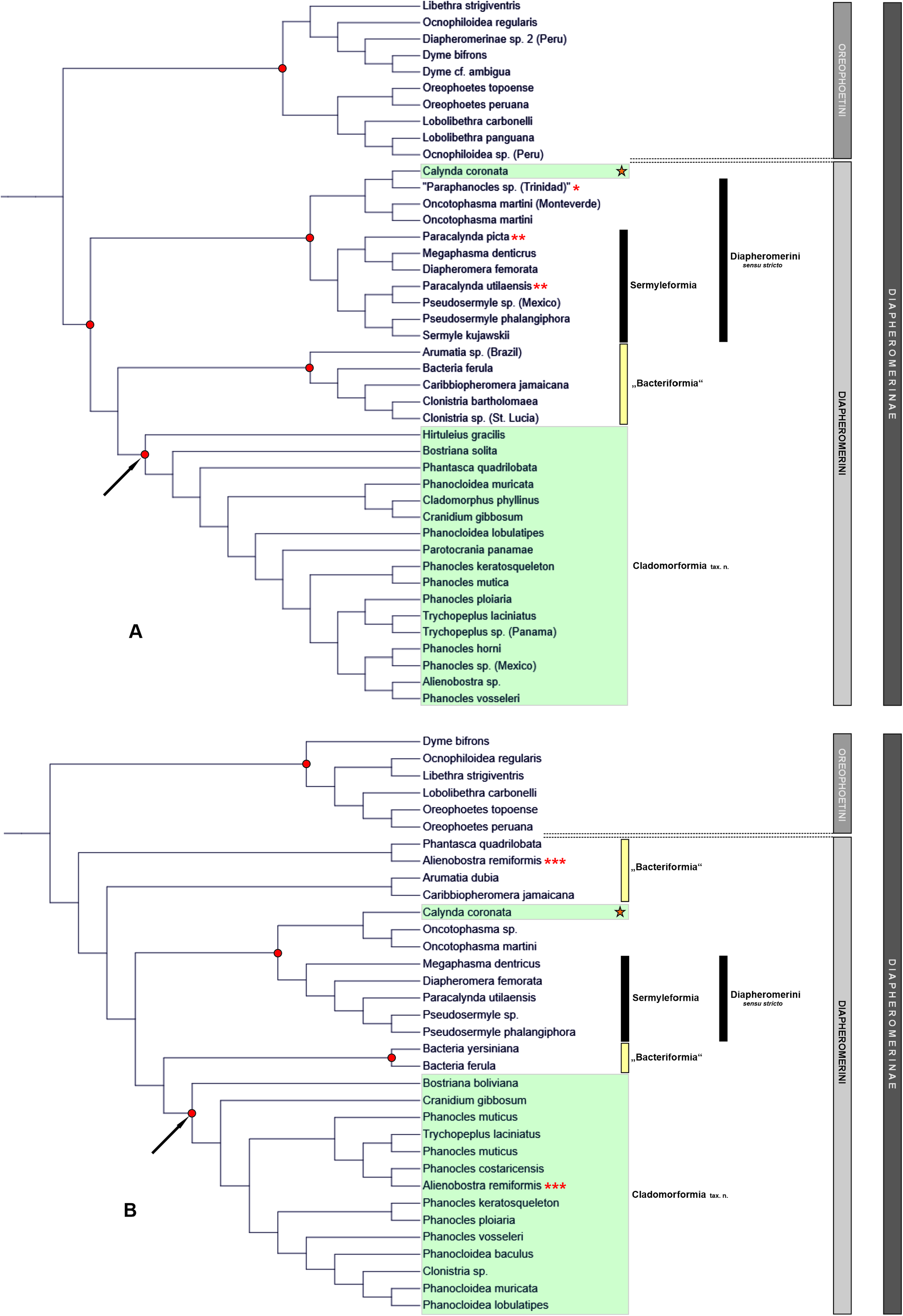
The Cladomorformia may represent a monophyletic subordinate clade among the Diapheromerini sensu Robertson et al., 2018 , which is basically supported by molecular results ( Robertson et al., 2018; Bank & Bradler, 2022; Forni et al., 2022). A few taxa however are questionable and here considered incerte sedis because they result with opposed phylogenetic positions in the latest and most comprehensive molecular analyses by Robertson et al. (2018), Bank & Bradler (2022; Fig. 133A View FIGURE 133 ) and Forni et al. (2022: Fig. 133B View FIGURE 133 ), or morphological characters do not certainly support either phylogenetic placement (→ see comments on genera incertae sedis below). A detailed description of Cladomorformia is provided above but an abbreviated summary of its main diagnostics may help understanding the reasoning for the introduction of this rank-free taxon. The Cladomorformia is characterised by the strongly lamellate medioventral carina of the profemora which is strongly displaced towards the anteroventral carina. Females possess a distinct praeopercular organ on abdominal sternum VII ( Figs. 3A, C View FIGURE 3 , 5 View FIGURE 5 ), the gonapophyses VIII are often enormously elongated and the gonoplacs are much enlarged ( Figs. 3A–E View FIGURE 3 ). The subgenital plate is almost always elongated and projects noticeably beyond the apex of the abdomen in the great majority of taxa. Occasionally, it is strongly elongated and lanceolate in shape. Males often show specialisations of the terminalia ( Figs. 4A–D View FIGURE 4 ), e. g. an apical projection of the poculum (= subgenital plate; Fig. 4A View FIGURE 4 ), enlarged and hook-like cerci ( Fig. 4C View FIGURE 4 ), a strongly enlarged and tube-like phallus ( Fig. 4D View FIGURE 4 ) or a vomer with two (sometimes asymmetrical) terminal hooks ( Fig. 4D View FIGURE 4 ). The vomer is always present and well-developed ( Figs. 4A–D View FIGURE 4 ). While females are exclusively apterous, ♂♂ show the entire range of wing anatomy ranging from apterous to fully winged but with the tegmina always small, scale-like and notably shorter than the alae. If the alae are developed, the anal fan is monochromatic and hyaline to transparent grey. Eggs show a wide range of morphological variability ( Figs. 98–100 View FIGURE 98 View FIGURE 99 View FIGURE 100 ) and always bear a hollow, either conical or crown-like matrix (non-stalked) capitulum. The capsule sculpturing varies from almost smooth over distinctly pitted or rugose to being all over covered with hairy structures.
The inclusion of four genera in Cladomorformia deserves explanation, because these have either not ( Calynda ) or not frequently ( Alienobostra ) been revealed as belonging to this clade in the available molecular analyses ( Bank et al., 2021; Bank & Bradler, 2002; Forni et al., 2022; Figs. 133A–B View FIGURE 133 ), or because they may anatomically not key out as Cladomorformia at first glance ( Cranidium and Trychopeplus ). Some annotations are therefore provided below to allow better understanding of the reasons that yield the taxonomic placement of these genera proposed herein.
Alienobostra : The genus Alienobostra was suggested to be the possible adelphotaxon of Sermyleformia by Bradler (2009: 101), who mentioned the enormously elongate, 90° incurved cerci of ♂♂, which functionally replace the vomer during copulation, as a likely synapomorphy. Moreover, Bradler interpreted the reduced vomer of this genus as a possible intermediate step towards an entire loss of this organ in the Sermyleformia. However, although these two characters readily separate Alienobostra from all other genera now contained in Cladomorformia , several morphological characters are shared with other members of this tribe, violate the apomorphies of Sermyleformia and clearly place Alienobostra within Cladomorformia . The analysis by Bank & Bradler (2002) shows Alienobostra to be deeply nested within Cladomorformia , whereas in the tree presented by Forni et al. (2022, Supplemental Figure 1 View FIGURE 1 ) Alienobostra appears twice, namely nested within Cladomorformia but also as the sister taxon of Phantasca within Diapheromerini s. str. ( Fig. 133B View FIGURE 133 ). Only the position within Cladomorformia is here clearly supported by morphological traits. The vomer is reduced and spatulate with two blunt apices ( Fig. 5C View FIGURE 5 , 95H View FIGURE 95 ), but it is well sclerotised and not functionally replaced but merely supported by the long, incurved cerci as an anchorage during the copulation ( Figs. 7C, D View FIGURE 7 ). Although the cerci are strongly enlarged and incurved ( Fig. 5C View FIGURE 5 ), they lack the intero-basal protuberance or spine typical for members of Sermyleformia. The anal segment is open ventrally with the posterolateral angles protruded and ventrally bearing distinct thorn-pads ( Fig. 5C View FIGURE 5 ). Also, the stigmae VIII are positioned at the anterolateral angles of abdominal tergum VIII. Additional characters which support the position of Alienobostra in Cladomorformia are the position of the distinct medioventral carina of the profemora of botrh sexes, the long median segment ( Fig. 9A View FIGURE 9 ), long and lanceolate subgenital plate of ♀♀ ( Figs. 8D–H View FIGURE 8 ), large poculum of ♂♂ ( Figs. 5C View FIGURE 5 , 9J View FIGURE 9 ), as well as the large hollow opercular structure of the eggs ( Figs. 98L, M View FIGURE 98 ).
Calynda : In molecular studies ( Bank & Bradler, 2022; Forni et al., 2022) the Central American Calynda Stål, 1875 resulted as a member of Diapheromerini s. str., namely among genera of Sermyleformia, Eusermyleformia as well as Oncotophasma Rehn, 1904 ( Figs. 133A–B View FIGURE 133 ). There is however no support for the position of Calynda within Diapheromerini s. str. from a morphological point of view. The only trait of the ♂♂ genital anatomy that would at first glance suggest close relation to members of Eusermyleformia are the long and gently incurved cerci that may bear a few minute denticles intero-basally. However, in all other aspects the anatomy of the terminalia of ♂♂ violate the striking autapomorphies of Eusermyleformia. The stigmae VIII are positioned at the anterolateral angles of abdominal tergum VIII ( Figs. 14M, O, Q View FIGURE 14 ), the vomer is well-developed ( Fig. 95A View FIGURE 95 ) and the anal segment bears prominent paired thorn-pads at its posterolateral angles ( Fig. 15N View FIGURE 15 ). Moreover, the morphology of the terminalia is almost identical to that of Spinocloidea gen. n., a genus that is morphologically very close to Phanocloidea Zompro, 2001 (→ 5.22.). Moreover, also the ♀♀ of Calynda provide no morphological support for a subordinate position within Diapheromerini s. str. but clearly place the genus within Cladomorphini instead. For instance, the subgenital plate is enormously elongated, lanceolate and projects considerably beyond the apex of the abdomen in ♀♀ of Calnyda ( Figs. 14F–J View FIGURE 14 ), a trait that is commonly seen in members of Cladomorformia but not observed in any taxa of Diapheromerini s. str. Thus, Calynda is here attributed to Cladomorformia .
Cranidium : The monotypic tribe Cranidiini Günther, 1953 and its only genus Cranidium have been discussed in detail by Hennemann et al. (2007) and Hennemann et al. (2016). The latter study has revealed the close affinity of Cranidium to taxa of Cladomorphini s. str. according to Hennemann et al. (2016), which was supported by the molecular analyses of Bank & Bradler (2022) and Forni et al. (2022) both of which revealed Cranidium as a member of Diapheromerini that clusters with taxa of Cladomorformia ( Figs. 133A–B View FIGURE 133 ). Morphologically, this striking genus is very well recognised among all other genera by the wholly unarmed extremities and indistinct medioventral carina of the profemora of both sexes, which is roughly central on the ventral surface of the profemur, strongly flattened and laterally dilated, leaf-like body of ♀♀ ( Figs. 19 View FIGURE 19 A-B), which is rhomboidal in cross-section, as well as the spinulose phallus of ♂♂ ( Figs. 19H–K View FIGURE 19 ) and eggs that have a narrowing of the posteromedian notch of the micropylar plate before it widens into the gap as well as a distinctly separated median line ( Hennemann et al., 2016: 18, fig. 49). All these distinctive characters are unique within the whole Diapheromerini s. l. and are most likely autapomorphies of Cranidium . Thus, as Cranidium is here included in Cladomorformia the tribe Cranidiini currently becomes obsolete.
Trychopeplus : In all trees obtained on the basis of molecular data the moss-mimicking Trychopeplus resulted as nested deep within Diapheromerini ( Bank & Bradler, 2022; Forni et al., 2022; Figs. 133A–B View FIGURE 133 ). According to both analyses Trychopeplus is found among different species of Phanocles , which would thereby place Trychopeplus as a synonym of Phanocles . This however appears highly implausible in aspect of the striking anatomy of the genus. Also, biological observations and data obtained by the authors during field trips in Costa Rica, Panama and Ecuador showed the habits and dietaries of Trychopeplus to differ significantly from Phanocles -species in the same habitats in various aspects and do not support a synonymy of Trychopleplus under Phanocles but demonstrate the validity of the genus instead. Morphological traits corroborate the position of Trychopeplus within Diapheromerini s. str. and close affinity to Phanocles , thus the genus clearly keys out as belonging to Cladomorformia . Characters which support this systematic position are the anatomy of the profemora, dorsally crested basitarsi, as well as the distinct praeopercular organ, strongly elongated gonapophyses VIII and elongated subgenital plate of ♀♀ ( Figs. 79E–G View FIGURE 79 , 80F View FIGURE 80 ), and characters of the terminalia of ♂♂, such as a well-developed vomer, paired thorn-pads of the anal segment ( Fig. 96L View FIGURE 96 ) and strongly bulgy poculum ( Fig. 79H View FIGURE 79 ). There are however several morphological specialisations that clearly separate the genus from Phanocles and all other members of Cladomorformia (→ 5.23). These specialisations are most certainly autapomorphies of Trchyopeplus and represent adaptions to the natural habitats of these remarkable insects, which live on mossy branches and stems in moist and mossy forests where the moss-mimicking crypsis provides an impressive camouflage ( Fig. 124A View FIGURE 124 ). This camouflage is achieved by numerous appendages and excrescences of the head and entire body as well as huge, irregularly foliaceous lobes and teeth of the extremities. The eggs are characteristic for having the capsule wholly covered with long hair-like fringes ( Fig. 100D View FIGURE 100 ). Neither of these characters is seen in any other genus of Cladomorformia .
Genera incertae sedis
In addition to Alienobostra and Calynda , whose inclusion in Cladomorformia is explained above, there are some genera within Diapheromerini sensu Robertson et al., 2018 (= Diapheromerini s. l.) that are remarkable for having contrary phylogenetic positions in the latest phylogenetic analyses based on melocular data ( Bank & Bradler, 2022; Forni et al., 2022; Figs. 133A–B View FIGURE 133 ) and which are not included in Cladomorformia herein. Namely, Arumatia Ghirotto, 2022 , Bacteria , Caribbiopheromera , Clonistria Stål, 1875 and Phantasca are here excluded from Cladomorformia and considered incertae sedis. In the molecular analyses of Bank & Bradler (2022) these genera cluster together in a mostly Caribbean lineage that is the sister group to Cladomorformia ( Fig. 133A View FIGURE 133 ). This is in contradiction to the result by Forni et al. (2022) where the two non-Caribbean genera Arumatia (as Echetlus ) and Phantasca as well as the Jamaican Caribbiopheromera cluster among genera of Diapheromerini s. str. ( Fig. 133B View FIGURE 133 ). The Caribbean Bacteria however, has the same position as sister to Cladomorformia in both analyses. Ghirotto et al. (2022) have summarized similarities and shared features of the terminalia of ♂♂ of Arumatia and Phantasca and have thus suggested close relationship between these two genera. The systematic position of both genera however still deserves clarification and is considered incertae sedis. Another example of contradictive phylogenetic positions between the two aforementioned molecular analyses is the exclusively Caribbean Clonistria . This genus is found to cluster among the above-mentioned mostly Caribbean lineage revealed by Bank & Bradler (2022; Fig. 133A View FIGURE 133 ), whereas the single Clonistria -species sampled by Forni et al. (2022) resulted as deeply nested within Cladomorformia ( Fig. 133B View FIGURE 133 ). Clonistria is a fairly speciose and apparently to date only partially known genus that deserves further work for proper characterisation and delimitation from closely related taxa. Therefore, the phylogenetic position of Clonistria is also considered incertae sedis. Besides, the same uncertain state applies to three supposedly closely related Caribbean genera, which have no yet been sampled in any molecular approach, but are very likely to belong to the same lineage, this is Jamanistria Bellanger et al., 2023 , Paraclonistria Langlois & Lelong, 1998 and Pseudoclonistria Langlois & Lelong, 2010 . Consequently, this entire lineage here provisionally referred to as “Bacteriformia” is considered incertae sedis and is in need of further investigation. Since much more in depth research is necessary no characterisation or delimitation is attempted at this point A few annotations and morphological observations on some of the concerned genera however appear useful for future studies that aim evaluating phylogenetic relationships within Diapheromerini and are given below.
Bacteria : The systematic position of the Caribbean genus Bacteria (sensu stricto, see Zompro, 2001a: 203) remains unclarified. Zompro (2001) placed Bacteria in his “ Bacteria group” along with not closely related genera, that are here shown to belong either in Cladomorformia ( Calynda and Globocalynda ) or in Diapheromerini s. str. ( Paracalynda ) as revealed by molecular analyses ( Bank & Bradler, 2022; Forni et al., 2022) and shown by morphological characters of the terminalia (Hennemann & Conle, 2012). Cliquennois (2020: 414) suggested a placement of Bacteria in Bacteriinae along with Caribbiopheromera and Trychopeplus , which however is not supported by molecular data nor morphological characters. Bacteria is remarkable for a combination of distinctive morphological characters. Both sexes have a prominent apical ventral spine on the meso- and metafemora, which is not positioned on the anteroventral carina but slightly displaced towards the medioventral carina. The spine resembles that seen in certain members of Diapheromerini s. str. (e. g. Diapheromera , Megaphasma and Manomera ) but in contrast to Bacteria these three genera have the spine placed exactly on the medioventral carina and not aside as in Bacteria . The cerci of ♂♂ are strongly enlarged and incurved in Bacteria , but like in Alienobostra they lack the intero-basal protuberance or spine that is typical for members of Diapheromerini s. str. ( Fig. 4E View FIGURE 4 ). Abdominal tergum IX of ♂♂ is not closed ventrally, and in contrast to Diapheromerini s. str. the vomer is developed, being prominent, sclerotised and roundly rectangular in shape with one or two distinct conical ventro-posterior protuberances ( Fig. 4E View FIGURE 4 ). The shape of the vomer is peculiar to Bacteria and unique within the whole of Diapheromerini and even Occidophasmata. The ♂ anal segment has the posterior margin considerably widened and swollen and bears a few minute ventral teeth at the outer angles, which may be interpreted as secondarily reduced thorn-pads. The poculum is larger than the basal portion of abdominal sternum IX, symmetrical and bears a conical central protuberance ( Fig. 4E View FIGURE 4 ). Both traits resemble Cladomorformia rather than Diapheromerini s. str. The stigmae VIII however are lightly shifted off the anterolateral angles of abdominal tergum VIII in both sexes ( Figs. 3F View FIGURE 3 , 4E View FIGURE 4 ). The position of stigmae VIII and the short, scoop-shaped subgenital plate of ♀♀ ( Fig. 3F View FIGURE 3 ) again resemble members of Diapheromerini s. str., as do the eggs which have a very elongate and slender micropylar plate and bear a rim of setae on the anterior margin of the capsule to surround the operculum. Since the morphology of Bacteria rather supports a position within Diapheromerini s. str. than Cladomorformia , the genus is here excluded from the latter clade and its position considered as incertae sedis.
Caribbiopheromera : This peculiar monotypic genus is only known from the Lesser Antillean island of Trinidad and Tobago ( Bellanger et al., 2023b) and is remarkable for several traits of the terminalia of ♂♂, that do not agree with the terminal morphology of Cladomorformia neither Diapheromerini s. str. Also, the phylogenetic position has varied throughout molecular analyses with the genus either resulting as sister to Cladomorformia + Bacteria ( Robertson et al., 2018) , sister to Arumatia but nested within Diapheromerini s. str. ( Forni et al., 2022; Fig. 133B View FIGURE 133 ) or clustered among the mostly Caribbean lineage here provisionally referred to as “Bacteriformia” together with Arumatia , Bacteria and Clonistria ( Bank & Bradler, 2022; Fig. 133A View FIGURE 133 ). In ♂♂ of Caribbiopheromera abdominal tergum VIII and IX are fully fused with another to form a tube-like structure, stigmae VIII are very slightly shifted off the anterior margin of segment VIII, the cerci are small and not elongated to form a clasping organ and the vomer and thorn pads of the anal segment are developed ( Bradler, 2009: 102, Figs. 26a View FIGURE 26 , 27c View FIGURE 27 ). The poculum is much smaller than the unusually swollen and hemispherical basal portion of sternum IX. In ♀♀ the subgenital plate is rather small and scoop-shaped and hardly reaches to the tip of the anal segment, the latter has the apex triangularly protruded, and there is a well-developed praeopercular organ near the posterior margin of abdominal sternum VII. In contrast to all other genera that supposedly belong in this mostly Caribbean lineage the probasitarsus of ♀♀ has the dorsal carina notably raised and weakly rounded. The eggs strongly resemble those of Bacteria but lack the rim of setae on the anterior margin of the capsule. Since the anatomy of the terminalia of ♂♂ strongly contradicts that of Cladomorformia , and the genus has taken on different systematic positions throughout the available phylogenetic analyses, Caribbiophermora is here excluded from Cladomorformia and considered incertae sedis.
Phantasca , Clonistria and Jamanistria : The 17 known species ( Lelong et al., 2022) of Phantasca are distributed throughout the northern half of South America ( Hennemann et al., 2018). The genus has contradictive positions in the two latest molecular analyses, resulting either as a member of Cladomorformia ( Bank & Bradler, 2022; Fig. 133A View FIGURE 133 ) or Diapheromerini s. str. ( Forni et al., 2022; Fig. 133B View FIGURE 133 ). The position yielded in the tree by Bank & Bradler (2022) with Phantasca deeply nested within Cladomorformia also contradicts the possible relationships and placement suggested by Hennemann et al. (2018) based on morphological characters. Mostly on the basis of the anatomy of the ♂♂ terminalia Hennemann et al. (2018: 4) have assumed possible close affinity to the strigiventris species group of the genus Libethra Stål, 1875 (see Günther, 1932) and some Jamaican species of the Caribbean genus Clonistria Stål, 1875 (e. g. C. annulipes Rehn & Hebard, 1938 , C. bicoloripes Rehn & Hebard, 1938 , C. latebricola Rehn & Hebard, 1938 , C. monticola Rehn & Hebard, 1938 ). Two of these Jamaican Clonistria -species have recently been shifted to the genus Jamanistria ( Bellanger et al., 2023a) . Close relation between Phantasca and Libethra (tribe Oreophoetini ) is corroborated by neither of the abovementioned molecular approaches and therefore unlikely. Indeed, characters such as the position of stigmae VIII, which are more or less strongly (much more distinct in ♂♂) shifted off the anterior angle of abdominal tergum VIII in Phantasca and at least the mentioned Jamaican representatives of Clonistria and Jamanistria (see Bellanger et al., 2023a, Figs. 93a–d View FIGURE 93 , 97a–d View FIGURE 97 ), suggest close relation to Diapheromerini s. str. Unfortunely however, none of the concerned species has so far been sampled or included in any phylogenetic analysis. Anatomical traits of the ♂♂ terminalia in particular rather support a position of Phantasca within Diapheromerini s. str. as found by Forni et al. (2022) and reject the position within the lineage here named as Cladomorformia tax. n. as shown in the tree presented by Bank & Bradler (2022, Fig. S1 View FIGURE 1 ). In addition to the position of the stigmae VIII, characters of the terminalia of ♂♂ in Phantasca that fundamentally violate the characteristics of Cladomorformia are the often partly or fully fused abdominal tergum and sterum VIII, increasingly approaching and sometimes fused lateral margins of tergum IX, as well as the small and sometimes asymmetrical poculum. The striking differences in the anatomy of the terminalia between Phantasca , Jamanistria and certain species of Clonistria on one side and Cladomorformia and the other, as well as the fact that the mentioned genera cluster in a separate lineage together with Bacteria , Caribbiopheromera and Arumatia , which is sister to Cladomorformia (see Bank & Bradler, 2022), preclude their inclusion in Cladomorformia . As yet, the relationships of Phantasca remain vague and the phylogenetic position is considered incertae sedis. The incertae sedis status also applies to Clonistria and the supposedly closely related and strictly Caribbean genera Jamanistria , Paraclonistria and Pseudoclonistria . More work is needed to reveal the relationships and phylogenetic positions of these genera and Clonistria in particular is in need of a revision at the species level.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
Cladomorformia
| Hennemann, Frank H. & Conle, Oskar V. 2024 |
Cladomorphinae
| Cliquennois, N. 2020: 414 |
Cladomorphini
| Bradley, J. C. & Galil, B. S. 1977: 189 |
Cranidiini Günther, 1953: 557
| Gunther, K. 1953: 557 |