Asarcus, KOCH, 1839
|
publication ID |
https://doi.org/ 10.1111/j.1096-3642.2008.00484.x |
|
persistent identifier |
https://treatment.plazi.org/id/03AF87FA-FF9C-FFE5-FC01-FB8FFAD163DE |
|
treatment provided by |
Felipe |
|
scientific name |
Asarcus |
| status |
|
ASARCUS KOCH, 1839 View in CoL
Asarcus Koch, 1839a: 16 View in CoL (desc.); 1839b: 68; Erichson, 1845: 2; Simon, 1879: 234; Sørensen, 1884: 616 (rdesc.); Roewer, 1913: 283 (key), 299 (cat., rdesc.); 1923: 509 (key), 515 (cat., rdesc.); Mello-Leitão, 1923: 168, 198 (key); 1926 (key): 359; Roewer, 1931: 106 (key), 112 (cat., rdesc.); Mello-Leitão, 1932: 391 (key), 409 (rdesc.); 1935b: 108; Kästner, 1937: 389; Mello-Leitão, 1941: 440; Soares & Soares, 1945b: 255 (= Bogdana Mello-Leitão, 1940 View in CoL ) (syst.); 1949: 225 (key) 230 (cat., rdesc.); Kury, 1989b: 5; 1994a: 97; 1994b: 351 (syst.); 2000: 5; 2003: 107 (cat.) (type species Asarcus longipes Kollar in Koch, 1839b View in CoL , by monotypy).
Opisthoplites Sørensen, 1884: 615 View in CoL (desc.); Roewer, 1913: 170 (key), 255 (rdesc.): 1923: 464 (key), 496 (cat., rdesc.); 1930: 348 (key), 382; Mello-Leitão, 1923: 154, 191 (key); Piza, 1940: 318; 1943: 43; Soares & Soares, 1949: 157 (key), 198 (cat., rdesc.); 1985: 194 (syst.); Kury, 1994b: 351 (syst.); 2003: 108 (cat.) (type species Opisthoplites ypsilon Sørensen, 1884 View in CoL , by monotypy). New synonymy.
Opysthoplites (lapsus): Mello-Leitão, 1926: 356 (key).
Opisthoplithes (lapsus): Mello-Leitão, 1935b: 102.
Bogdana Mello-Leitão, 1940: 27 View in CoL (desc.); Kury, 1994b: 351 (syst.); 2003: 107 (cat.) (type species Bogdana ingenua Mello-Leitão, 1940 View in CoL , by original designation). New synonymy.
Cnemoleptes Mello-Leitão, 1941: 440 View in CoL (desc.); Soares & Soares, 1949: 225 (key), 231 (cat., rdesc.); Kury, 1989b: 5; 1994b: 351 (syst.), 352; 2000: 1 (rdesc.), 5 (syst.); 2003: 108 (cat.) (type species Cnemoleptes passarellii Mello-Leitão, 1941 View in CoL , by original designation). New synonymy.
Diagnosis
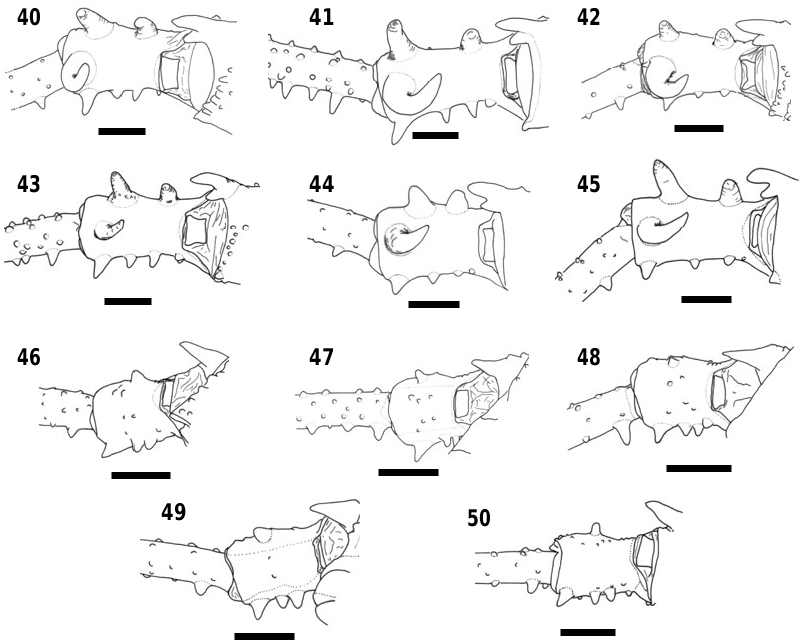
Male ( Figs 13–16 View Figures 13–16 , 32, 34, 36, 38 View Figures 32–39 , 51–54 View Figures 51–58 , 70–73, 95–102 View Figures 95–98 View Figures 99–102 , 114–117 View Figures 114–121 ): Body with lower and fewer granules than in Bourguyia . Dorsal scutum with three areas. Area III can have a median pair of tubercles. Dorsal scutum longer than wide. Posterior margin of dorsal scutum ends abruptly, without the extension present in Bourguyia . Posterior margin width much greater than prosoma width. Ocularium wider and lower than in Bourguyia , medially depressed, and more rectangular-shaped, with the length almost constant. Coxa IV narrow and never surpassing the posterior margin of the dorsal scutum, and without inner rows of tubercles near the articulation with the trochanter; apical ventral spine always well developed. Trochanter IV without the inner hook-shaped apophysis; distal dorsal apophysis always very reduced, like a tubercle. Femur IV with few granules and without large distal spines.
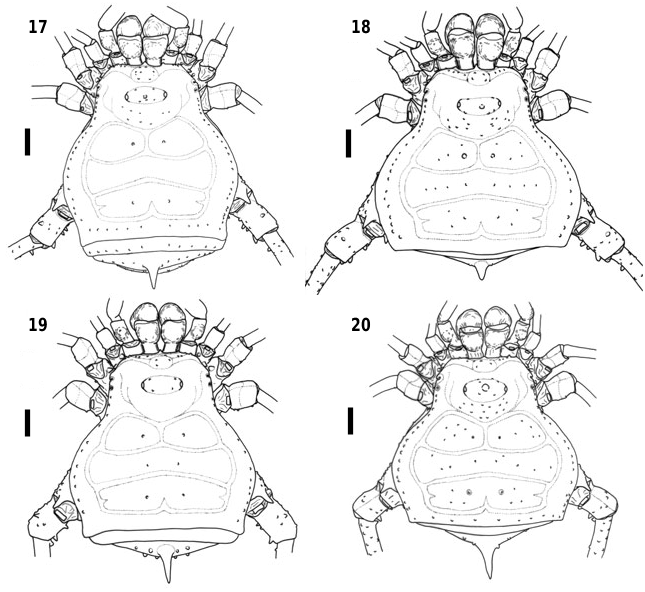
Female ( Figs 17–20 View Figures 17–20 , 33, 35, 37, 39 View Figures 32–39 , 46–50 View Figures 40–50 , 74–77, 118–121 View Figures 114–121 ): Body a little more flattened than in Bourguyia , median longitudinal line lower, almost same height as the lateral margins. Lateral margins of dorsal scutum and posterior margin of free tergites II and III with a row of small and low granules.
ASARCUS LONGIPES KOLLAR IN KOCH, 1839
( FIGS 13 View Figures 13–16 , 17 View Figures 17–20 , 32–33 View Figures 32–39 , 51, 55 View Figures 51–58 , 70, 74, 97–98 View Figures 95–98 , 114, 118 View Figures 114–121 )
Asarcus longipes Kollar in Koch, 1839a: 16 View in CoL (desc.); 1839b: 68 (rdesc.), pl. 234, fig. 569; Simon, 1879: 234 (rdesc.); Roewer, 1913: 299, 300 (key, rdesc.), fig. 120; 1923: 515 (key, cat., rdesc.), fig. 644; Mello-Leitão, 1923: 168 (cat.), 194 (key); Giltay, 1930: 239; Roewer, 1931: 112 (key); Mello-Leitão, 1932: 409 (key), 410 (rdesc.), fig. 274 (not fig. 224 as in text); Soares & Soares, 1949: 230 (cat.); Kury, 2003: 107 (cat.) [ Brazil; holotype ♂ (NHMW), lost].
Asarcus lutescens Sørensen, 1884: 617 View in CoL (desc.); Roewer, 1913: 300 (key), 303 (rdesc.), fig. 122; 1923: 515 (key), 516 (cat., rdesc.), fig. 646; Mello-Leitão, 1923: 168 (cat.), 193 (key); Roewer, 1931: 112 (key), 113 (cat.); Mello-Leitão, 1932: 409 (key), 410 (rdesc.), fig. 275; Soares & Soares, 1945b: 254 (= Asarcus ingenuus View in CoL ) (syst.); 1949: 231 (cat.); Kury, 2003: 107 (cat.) [ Brazil, H. Reinhardt leg.; holotype ♀ (ZMUC 90053), examined]. New synonymy.
Asarcus corallipes Simon, 1879: 235 View in CoL (desc.); Roewer, 1913: 299 (key), 301 (rdesc.), fig. 121; 1923: 515 (key), 516 (rdesc.), fig. 645; Mello-Leitão, 1923: 168 (cat.), 193 (key); 1932: 409 (key, rdesc.), fig. 273; Roewer, 1931: 112 (key), 113 (cat., rdesc.); Soares & Soares, 1949: 230 (cat.) [ Brazil; holotype ♀ (MNHN), examined from photo]. New synonymy.
Opisthoplites corallipes: Kury, 2003: 108 (cat., syst.).
Opisthoplites ypsilon Sørensen, 1884: 615 View in CoL (desc.); Roewer, 1913: 256 (rdesc.); 1923: 496 (cat., rdesc.); Mello-Leitão, 1923: 154 (cat.), 191 (key); Soares, 1945f: 361; Soares & Soares, 1949: 198 (cat.); 1970: 340; Kury, 2003: 107 (cat.) [ Brazil; holotype ♀ (ZMUC), examined]. New synonymy.
Asarcus pallidus Mello-Leitão, 1923: 168 View in CoL (desc.), 194 (key); Roewer, 1931: 113 (key, rdesc.); Mello- Leitão, 1932: 409 (key), 411 (rdesc.), fig. 276; Soares, 1944d: 289 (rdesc.), fig. 2; 1946: 510; Soares & Soares, 1949: 231 (cat.); Kury, 2003: 107 (cat.) [ Brazil, São Paulo, Piquete, 1896; two syntypes ♂ (MZSP 499), examined]. New synonymy.
Redescription
Male: Measurements (N = 5). Dorsal scutum: length, 6.2–6.7; width, 6.1–6.7; anterior margin width, 2.5– 3.0; posterior margin width, 4.2–4.6. Leg lengths: I, 15.2–19.3; II, 36.8–49.3; III, 26.6–34.4; IV, 57.1–73.7. Femora lengths: I, 14.5–19.3; II, 11.5–15.5; III, 8.7– 11.3; IV, 17.8–22.7.
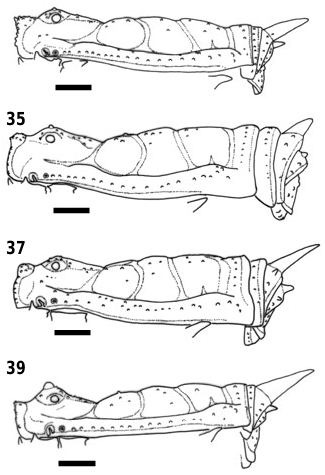
Dorsum ( Figs 13 View Figures 13–16 , 32 View Figures 32–39 , 114 View Figures 114–121 ). Ocularium with median tubercle very small, hard to see. Free tergites II and III with median spines, ad with the spine of free tergite II about twice the length of the spine of free tergite III.
Legs ( Figs 13 View Figures 13–16 , 51 View Figures 51–58 , 70, 114 View Figures 114–121 ). Coxa IV very narrow, covered almost entirely by dorsal scutum, only apex visible. Conspicuous granules present laterally. Trochanter IV with two small ventral median spines.
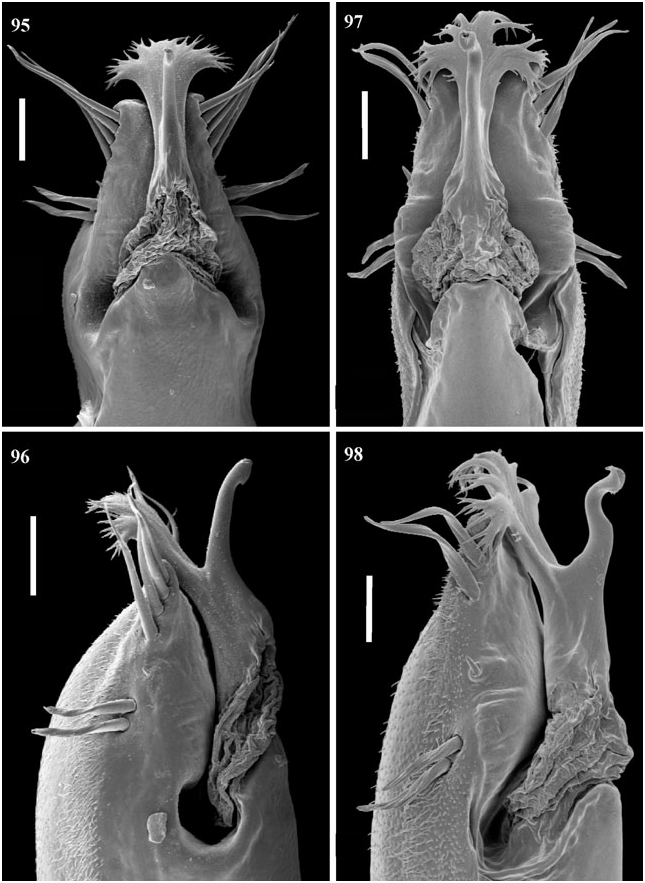
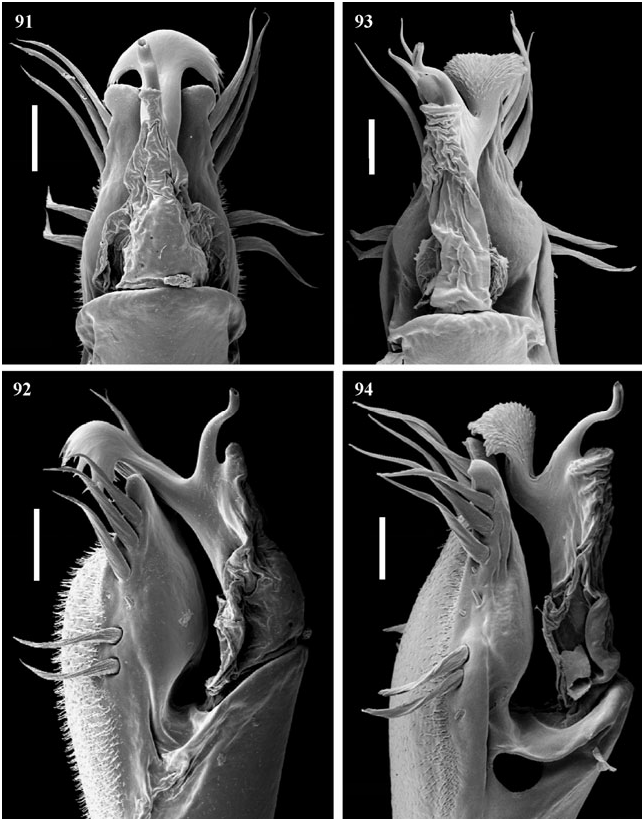
Penis ( Figs 95, 96 View Figures 95–98 ). Dorsal process absent. Ventral process slightly trilobed, fan-shaped over 180°. The apex is ramified: branches are thick and straight. Apex of stylus slightly dorsally curved.
Coloration (live) ( Fig. 114 View Figures 114–121 ). Dorsal scutum, free tergites, and chelicerae yellow, prosoma a little darker. Small black spots sparsely distributed on whole dorsum and femur IV, larger on femur. Dorsal scutum and free tergite margins darker. Pedipalpus dark yellow. Leg coxae yellow, light brown towards apex. Spines, apophyses, and granules darker.
Dry marks ( Fig. 114 View Figures 114–121 ). Very reduced, only a few white spots dispersed on dorsum. Y-shaped stain behind the ocularium can be reduced. One pair of stains on lateral margin of prosoma, at ocularium sides.
Female ( Figs 17 View Figures 17–20 , 33 View Figures 32–39 , 55 View Figures 51–58 , 74, 118 View Figures 114–121 ): Measurements (N = 5). Dorsal scutum: length, 6.0–6.8; width, 6.1– 6.9; anterior margin width, 2.4–2.7; posterior margin width, 5.0–5.6. Leg lengths: I, 11.2–13.3; II, 27.7– 32.9; III, 19.3–23.6; IV, 29.9–36.3. Femora lengths: I, 2.1–3.5; II, 7.8–9.4; III, 5.8–7.6; IV, 7.8–9.6. Free tergite II with median spine; free tergite III with small spine or median tubercle. Trochanter IV with two dorsal apophyses, with distal one a little larger than basal one. Femur IV almost straight, with two distal rows of ventral spines.
Comparison: Differs from other Bourguyiinae species in having a very narrow coxa IV ( Fig. 13 View Figures 13–16 ), reduced dry marks ( Fig. 114 View Figures 114–121 ), and the small median tubercle of ocularium ( Fig. 13 View Figures 13–16 ). Male differs from other Bourguyiinae species in having the spine of free tergites just a little curved ventrally ( Fig. 32 View Figures 32–39 ).
Material examined: Brazil, Rio de Janeiro, Teresópolis ( Colônia Alpina ), 1893, E.A. Göldi leg., two ♂ ( ZMUC) ; E.A. Göldi leg., one ♀ ( ZMUC 90029 View Materials ) ; E.A. Göldi leg., one ♂ ( ZMUC 90030 View Materials ) ; Teresópolis ( Sítio Décio ), 26 November 2000, Moraes Jr. & F. Décio leg., two ♂ and two ♀ ( MNRJ 4839 View Materials ) ; Itatiaia ( Rio Maromba ), February 2003, A.B. Kury, R. Pinto-da- Rocha & L. Mestre leg., one ♂ ( MZSP 1248 View Materials ) ; one ♀ ( SMF 5390 View Materials ) . São Paulo: three ♀ and one juvenile ( SMF 864 View Materials ) ; Mogi das Cruzes ( Rio Tietê ), July–August 1996, R. Martins leg., one ♀ ( IBSP 975 View Materials ) ; May 1997, C.T. Seixas Filho leg., one ♂ ( MZSP 16007 View Materials ) ; Santo André (E.B. Paranapiacaba), one ♂ and one juvenile ( MZSP 1509 View Materials ) ; Cotia (P.E. Morro Grande), August 2006, R. Pinto-da-Rocha leg. ( MZSP 27724 View Materials ) .
Remarks: The holotype of Asarcus longipes is lost. The species could be determined with the original description ( Koch, 1839a), redescription, and illustration of Koch (1839b), and with the redescription of Roewer (1913).
ASARCUS INGENUUS (MELLO- LEITÃO, 1940) ( FIGS 14 View Figures 13–16 , 18 View Figures 17–20 , 34–35 View Figures 32–39 , 52, 56 View Figures 51–58 , 71, 75, 97–98 View Figures 95–98 ,
115, 119 View Figures 114–121 )
Bogdana ingenua Mello-Leitão, 1940: 27 View in CoL (desc.), fig. 29; Kury, 2003: 107 (cat.) [ Brazil, São Paulo, Campos do Jordão; holotype ♂ (MNRJ 127), examined].
Asarcus ingenuus: Soares & Soares, 1945b: 252 View in CoL , 254 (syst.); Soares, 1945f: 367; 1946: 510; Soares & Soares, 1949: 230 (cat., syst.); Soares, 1972: 55.
Asarcus nigriconspersus Soares & Soares, 1945b: 254 View in CoL , 259 (desc.), figs 5–6; 1949: 231 (cat.); Kury, 2003: 107 (cat.) [ Brazil, São Paulo, São Francisco Xavier, December 1944, J. Damico leg.; holotype ♂ and one paratype ♀ (MZSP 827), two paratypes ♂ and two paratypes ♀ (MZSP 828), examined]. New synonymy.
Redescription
Male: Measurements (N = 6). Dorsal scutum: length, 6.4–7.3 (6.8); width, 6.5–8.0 (7.5); anterior margin width, 2.6–3.1 (2.9); posterior margin width, 4.8–5.9 (5.1). Leg lengths: I, 13.2–16.6 (14.0); II, 32.8–43.1 (34.6); III, 24.4–31.5 (25.2); IV, 62.1–80.3 (?, tarsus broken). Femora lengths: I, 3.3–4.0 (3.6); II, 9.8–12.8 (10.5); III, 8.0–10.0 (8.0); IV, 15.3–23.4 (17.0).
Dorsum ( Figs 14 View Figures 13–16 , 34 View Figures 32–39 , 115 View Figures 114–121 ). Ocularium with large median tubercle. Anterior margin of prosoma with low granules. Area I of mesotergum with a pair of median tubercles; areas II and III with equal-sized granules. Free tergites II and III with median spine very small and wide at base.
Legs ( Figs 14 View Figures 13–16 , 52 View Figures 51–58 , 71, 115 View Figures 114–121 ). Coxa IV wider at apex. Metatarsus IV with two distal ventral rows of small curved spines.
Penis ( Figs 92, 93 View Figures 91–94 ). Dorsal process absent. Ventral process smooth, apex trilobed and ramified, with long branches. Stylus sinuous and with enlarged apex.
Coloration (live) ( Fig. 115 View Figures 114–121 ). Dorsum, leg coxae, and Trochanters red/brown. Posterior margin of dorsal scutum and posterior margin of free tergites yellow. Pedipalpus and chelicera yellow, reticulated with green. All leg femora through tarsi dark brown, almost black near the articulations; femur IV red/ brown, lighter than the dorsum, with all granules dark, almost black. Apophyses of coxa and trochanter IV, and spines of free tergites, black. Anal plate with same colour as dorsum, with median white spots on posterior margin. Venter lighter than the dorsum. In alcohol, the whole dorsum can be a homogeneous light yellow.
Dry marks ( Fig. 115 View Figures 114–121 ). One pair of small white stains on lateral margins of prosoma, at ocularium sides. White median spots on anterior margins of areas II and III of dorsal scutum.
Female ( Figs 18 View Figures 17–20 , 35 View Figures 32–39 , 56 View Figures 51–58 , 75, 119 View Figures 114–121 ): Measurements (N = 5). Dorsal scutum: length, 6.0–7.0; width, 6.6– 7.9; anterior margin width, 2.4–2.7; posterior margin width, 5.2–6.2. Leg lengths: I, 10.3–12.1; II, 24.9– 29.7; III, 18.4–22.1; IV, 29.2–35.2. Femora lengths: I, 2.4–3.2; II, 7.3–8.3; III, 6.2–6.8; IV, 7.2–9.0. Metatarsus IV granulated, without the ventral rows of spines.
Comparison: Differs from other Bourguyiinae species in having metatarsus IV with two distal ventral rows of small curved spines, and posterior margin of dorsal scutum and free tergites yellow ( Fig. 115 View Figures 114–121 ). Differs from other Asarcus species in coloration (light reddish brown) of the dorsal scutum ( Fig. 115 View Figures 114–121 ).
Material examined: Brazil, Minas Gerais, Delfim Moreira , 1 November 2004, M.B. da Silva & R. Pintoda-Rocha leg., three ♀ ( MZSP 27723 View Materials ) . São Paulo, Campos do Jordão , February 1906, H. Luederwald leg., two ♀ ( MZSP 1508 View Materials ) ; December 1944, F. Lane leg., two ♀ ( MZSP 818 View Materials ) ; 1944, F. Lane leg., one ♂ ( MZSP 842 View Materials ) ; March 1945, P. Wygodzinsky leg., one ♂ and one ♀ ( MZSP 1675 View Materials ) ; March 1945, P. Wygodzinsky leg., three ♂ and one ♀ ( MZSP 1676 View Materials ) ; March 1945, P. Wygodzinsky leg., one ♂ and three ♀ ( MZSP 1684 View Materials ) ; March 1945, P. Wygodzinsky leg., one ♂ ( MZSP 1693 View Materials ) ; 3 January 1948, F. Lane leg., one ♂ ( MZSP 22736 View Materials ) ; July 1976, L.M. da Silva leg., one ♂ ( IBSP 192 View Materials ) ; May 1977, C.T. Seixas Filho leg., one ♂ ( MZSP 16007 View Materials ) ; 19 January 1978, P.C.M. Pelug leg., one ♂ ( IBSP 196 View Materials ) ; 7 September 1989, R. Marques et al. leg., one ♂ and one ♀ ( MNRJ 4807 View Materials ) ; 1 January 1989, A. Kury & L. Kury leg., one ♂ and two ♀ ( MNRJ 412 View Materials ) ; 15–19 October 1992, S. Ide leg., one ♂ ( MZSP 16019 View Materials ) .
ASARCUS PASSARELLII (MELLO- LEITÃO, 1941) COMB. NOV. ( FIGS 15 View Figures 13–16 , 19 View Figures 17–20 , 36, 37 View Figures 32–39 , 53, 57 View Figures 51–58 , 72, 76, 99, 100 View Figures 99–102 , 116, 120 View Figures 114–121 )
Cnemoleptes passarellii Mello-Leitão, 1941: 440 View in CoL (desc.); Soares, 1945f: 367; Soares & Soares, 1949: 232 (cat.); Kury, 2000: 2 (rdesc.), 6, figs 1–6; 2003: 108 (cat.) [ Brazil, Rio de Janeiro, Duque de Caxias (Serra do Barro Branco), A. Passarelli leg.; lectotype ♂ (MNRJ 5457), examined; same, eight paralectotypes ♂ and 19 paralectotypes ♀ (MNRJ 196), one ♂ and one ♀ examined, the remainder of the type material is lost].
Redescription
Male: Measurements (N = 2). Dorsal scutum: length, 5.2 (6.8); width, 5.3 (6.8); anterior margin width, 2.2 (2.6); posterior margin width, 3.9 (4.7). Leg lengths: I, 14.3 (21.5); II, 37.3 (59.4); III, 24.9 (37.2); IV, 47.5 (93.0). Femora lengths: I, 3.8 (5.7); II, 10.9 (18.0); III, 7.7 (12.1); IV, 13.9 (28.0).
Dorsum ( Figs 15 View Figures 13–16 , 36 View Figures 32–39 , 116 View Figures 114–121 ). Ocularium unarmed. Free tergites II and III with median spine; extremely narrow and long in free tergite II (twice the length of the spine of free tergite III).
Legs ( Figs 15 View Figures 13–16 , 53 View Figures 51–58 , 72, 116 View Figures 114–121 ). Coxa IV with a few small granules on each side. Trochanter IV with three small ventral median spines. Tibia IV with a row of ventral granules. Metatarsus IV with rows of ventral granules.
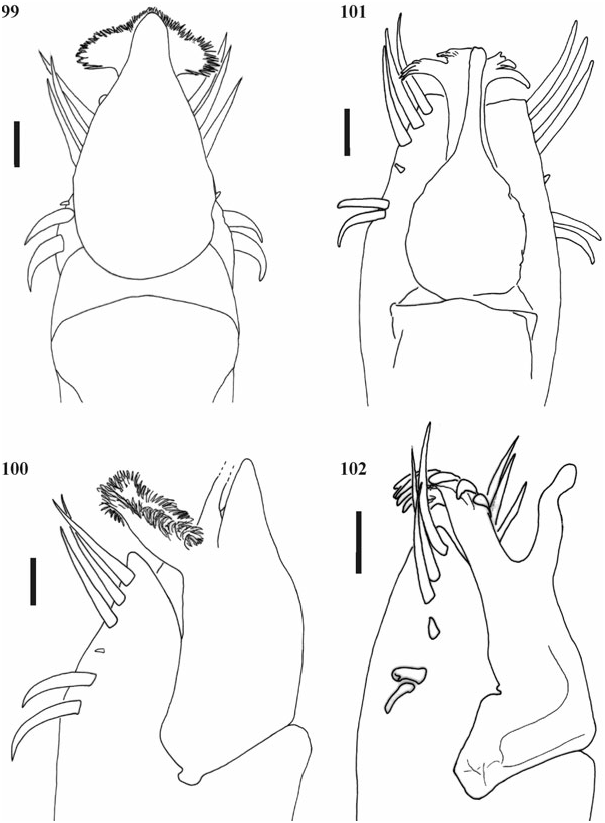
Penis ( Figs 99, 100 View Figures 99–102 ). Dorsal process well developed and contiguous with the sack, pointed upwards. Ventral process with apex very wide, opened to 180°, very ramified. Stylus straight.
Coloration (in alcohol) ( Fig. 116 View Figures 114–121 ). Homogeneous yellow in entire dorsal scutum. Posterior margin of coxa IV, entire trochanter, and femur IV a little darker. Dorsal basal apophysis of trochanter IV darker, almost red. Mesotergum and dorsum of coxa IV covered with small black spots. According to the original description ( Mello-Leitão, 1941), the body is homogeneous ‘burned’ yellow; sometimes the area IV and free tergites are black; stigmatic area with a black median line; femora reticulated with black; the other segments (of the legs) are black.
Dry marks ( Fig. 116 View Figures 114–121 ). Present on grooves, with two large stains laterally on area III. Stain in closed Y-shape behind ocularium.
Female ( Figs 19 View Figures 17–20 , 37 View Figures 32–39 , 57 View Figures 51–58 , 76, 120 View Figures 114–121 ): Measurements (N = 1). Dorsal scutum: length, 6.2; width, 6.6; anterior margin width, 2.6; posterior margin width, 5.7. Leg lengths: I, 14.0; II, 28.9; III, 23.1; IV, 37.5. Femora lengths: I, 4.0; II, 9.9; III, 7.3; IV, 10.9. Trochanter IV with dorsal distal apophysis and ventral spines larger than male. Femur IV with a row of small distal outer spines and two ventral rows of small distal spines, curved apicad. Tibia IV and metatarsus IV without the rows of ventral spines.
Comparison: Differs from other Bourguyiinae species in having a very long and narrow spine on free tergite II, with a basal tubercle on each side ( Figs 15 View Figures 13–16 , 116 View Figures 114–121 ). Differs from other Asarcus species in having an unarmed ocularium. Male differs from other Bourguyiine species in the dorsal process of the penis, which is very large and contiguous with the sac.
Remarks: Only the type series is known.
ASARCUS PUTUNABERABA SP. NOV. ( FIGS 16 View Figures 13–16 , 20 View Figures 17–20 , 38, 39 View Figures 32–39 , 54, 58 View Figures 51–58 , 73, 77, 101, 102 View Figures 99–102 , 117, 121 View Figures 114–121 )
Type material: Brazil, Minas Gerais, Alto Caparaó ( Parque Nacional do Caparaó ), 1–5 May 2002, Equipe Biota leg.; holotype ♂ ( IBSP 2849 View Materials ) ; same, one paratype ♀ ( IBSP 2861 View Materials ) ; same, one paratype ♀ ( MZSP 26077 View Materials ) .
Etymology: The specific name is a combination of the Tupi words putuna (night) and beraba (bright), in reference to the coloration of the dorsum.
Description
Male: Measurements (holotype). Dorsal scutum: length, 5.7; width, 6.2; anterior margin width, 2.4; posterior margin width, 4.1. Leg lengths: I, 13.7; II, 34.1; III, 25.3; IV, 53.9. Femora lengths: I, 3.8; II, 10.8; III, 7.7; IV, 15.5.
Dorsum ( Figs 16 View Figures 13–16 , 38 View Figures 32–39 , 117 View Figures 114–121 ). Ocularium very wide, with large median tubercle. Area I of dorsal scutum with one pair of median tubercles, area III only with small granules. Free tergite II with large median spine, very wide basally; free tergite III with small median spine.
Legs ( Figs 16 View Figures 13–16 , 54 View Figures 51–58 , 73, 117 View Figures 114–121 ). Coxa IV narrow, only the apex is visible from dorsal view. Apical ventral apophysis well developed. Dorsal basal apophysis of trochanter IV a little curved on the inner surface.
Penis ( Figs 101, 102 View Figures 99–102 ). Dorsal process absent. Ventral process smooth, apex trilobed and ramified, with long branches. Stylus sinuous and with extensive apex.
Coloration (live) ( Fig. 117 View Figures 114–121 ). Dorsal scutum entirely black, weakly reticulated with dark brown, mostly on prosoma. Grooves of mesotergum, posterior margin of dorsal scutum, and posterior margins of free tergites dark brown. Apex of legs lighter, almost yellow. Chelicera and pedipalpus dark green, almost black, with dark yellow spots.
Dry marks ( Fig. 117 View Figures 114–121 ). Many large white spots on entire dorsal scutum, anal plate, and abdominal sternites. A very large Y-shaped stain behind the ocularium, filled out and invading a small part of the ocularium. White stain on median anterior region of ocularium, and on inner and outer regions of coxa and trochanter IV, near the articulation.
Female ( Figs 20 View Figures 17–20 , 39 View Figures 32–39 , 58 View Figures 51–58 , 77, 121 View Figures 114–121 ): Measurements (N = 2). Dorsal scutum: length, 6.0–6.2; width, 6.2– 6.5; anterior margin width, 2.5; posterior margin width, 5.3–6.4. Leg lengths: I, 10.4–11.0; II, 26.2– 26.9; III, 19.2–19.6; IV, 29.3–29.6. Femora lengths: I, 2.1–2.6; II, 7.8–8.1; III, 5.6–6.0; IV, 7.5–7.6. Spine of free tergite II larger than males. Femur IV curved on inner surface. Coloration a little lighter than males, dark brown. Dry marks with same pattern, only weaker in anal plate and sternites.
Comparison: Differs from other Bourguyiinae species in the coloration of the body, which is black ( Fig. 117 View Figures 114–121 ). Differs from other Asarcus species in having welldeveloped dry marks, with many large spots on the entire dorsal scutum ( Fig. 117 View Figures 114–121 ).
Remarks: Only the type series is known.
BIOGEOGRAPHY
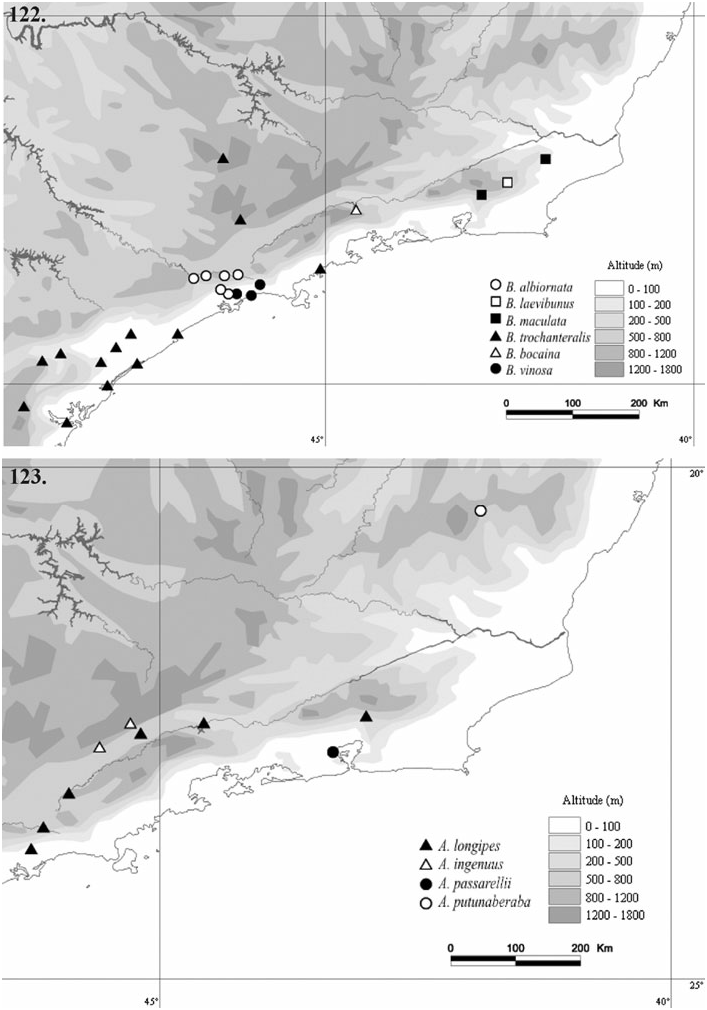
The species of the Bourguyiinae occur in the southeastern region of the Atlantic Rainforest (‘Mata Atlântica’), between the Serra da Mantiqueira and the Serra do Mar mountain ranges, in the states of Minas Gerais, Rio de Janeiro, São Paulo, and Paraná. The northernmost record is from Alto Caparaó, MG ( A. putunaberaba sp. nov.), and the southernmost record is from Paranaguá, PR ( B. trochanteralis ). Camanducaia, MG, is the farthest record from the coast ( B. trochanteralis ). All of the Bourguyiinae species occur from sea level ( B. vinosa sp. nov., Boracéia beach, São Sebastião, SP) up to an altitude of 1500 m a.s.l. ( B. bocaina sp. nov., Serra da Bocaina, São José do Barreiro, SP).
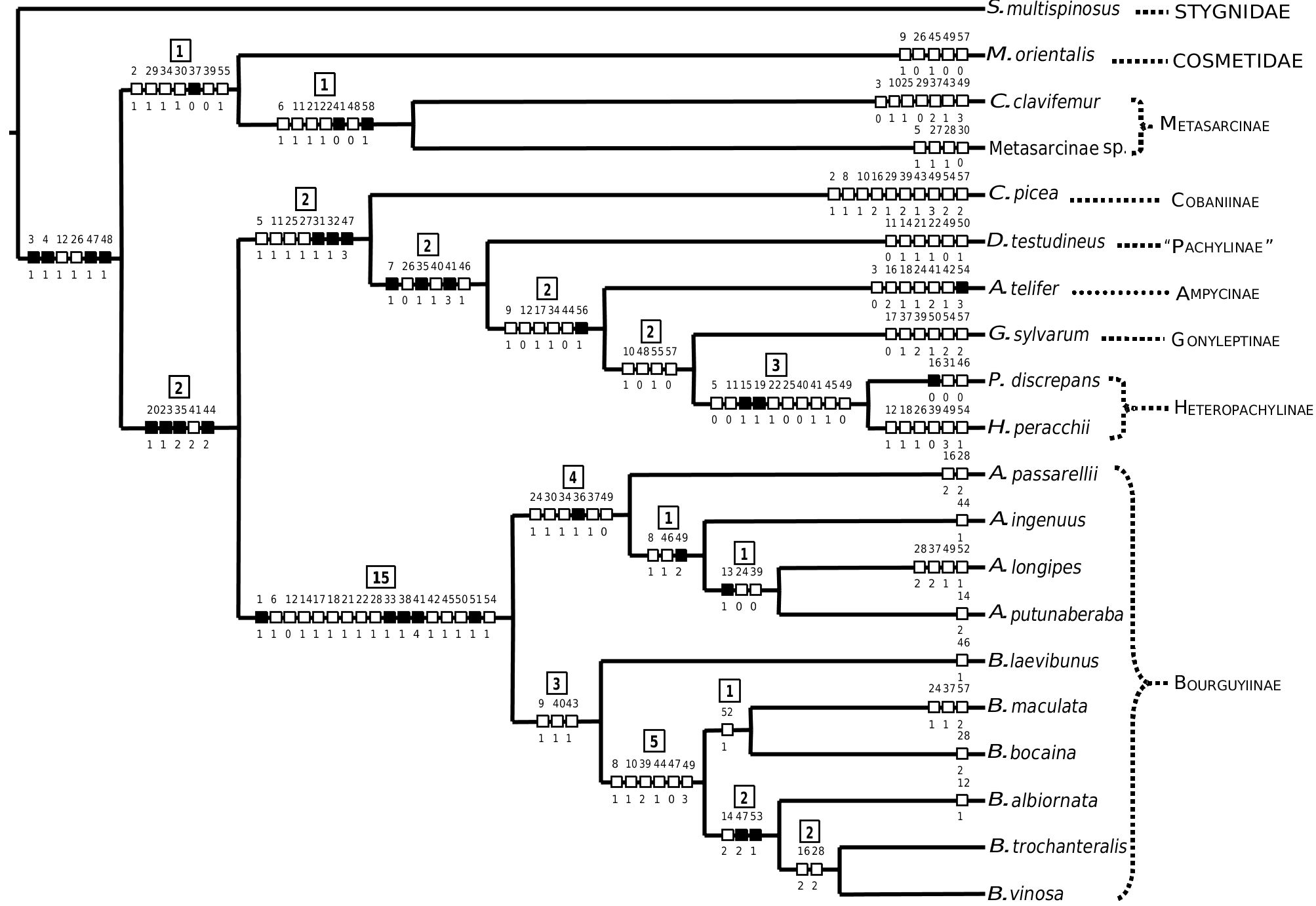
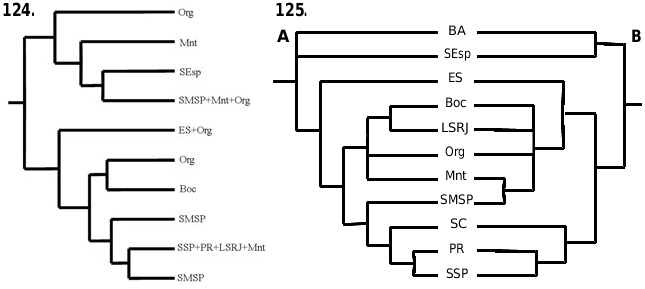
The Bourguyiinae is restricted to the forested formations, preferentially the Coastal Atlantic Rainforest. Four species are restricted to just one area of endemism proposed for harvestmen ( Figs 122, 123 View Figures 122–123 ) by Pinto-da-Rocha et al. (2005): A. ingenuus occurs in ‘Serra da Mantiqueira’, B. albiornata occurs in ‘Serra do Mar’, B. bocaina sp. nov. occurs in ‘Serra da Bocaina’, and B. vinosa sp. nov. occurs in ‘Serra do Mar’. Asarcus passarellii (‘Serra dos Órgãos’) and A. putunaberaba sp. nov. (‘Serra do Espinhaço’) are known only from the type series, and only three specimens of B. laevibunus are known (‘Serra dos Órgãos’). Two species are widespread: B. trochanteralis occurs in ‘Paraná’, ‘South of São Paulo’, ‘Coast of Rio de Janeiro’, and ‘Serra da Mantiqueira’, and A. longipes occurs in ‘Serra do Mar’, ‘Serra dos Órgãos’, and ‘Serra da Mantiqueira’ (see legend of Figure 125 View Figures 124–125 for detailed descriptions of the areas of endemism).
Pinto-da-Rocha et al. (2005) carried out extensive work on the historical biogeography of the harvestmen of the Atlantic Rainforest. The authors used 84 species from four subfamilies of Gonyleptidae (Goniosomatinae, Caelopyginae, Sodreaninae, and Progonyleptoidellinae), and defined 11 areas of endemism. They did a primary BPA (Brooks Parcimony Analysis) in order to test the historical relationship hypotheses among these 11 areas ( Fig. 125A View Figures 124–125 , strict consensus of three most parsimonious trees: L = 254; CI = 0.64; RI = 0.54). We added Bourguyiinae ( Fig. 1 View Figure 1 ) to the matrix of Pinto-da-Rocha et al. (2005) in order to propose a new hypothesis. The addition of Bourguyiinae changes the topology, and results in two most parsimonious trees ( Fig. 125B; L View Figures 124–125 = 281; CI = 0.62; RI = 051). The differences between the two trees are in the central areas (‘Serra da Bocaina’–‘Serra do Mar’). In one tree, the sister area of ‘Serra da Bocaina’ is ‘Coast of Rio de Janeiro’, and the sister area of ‘Serra do Mar’–‘Serra da Mantiqueira’ is ‘Serra dos Órgãos’. In the other tree, the sister area of ‘Serra da Bocaina’ is ‘Serra dos Órgãos’, and the sister area of ‘Serra do Mar’–‘Serra da Mantiqueira’ is ‘Coast of Rio de Janeiro’. The more significant difference is the inclusion of ‘Espírito Santo’ and ‘Serra do Mar’ into the group ‘Serra da Bocaina’–‘Serra da Mantiqueira’ of the Pinto-da-Rocha et al. (2005) hypothesis ( Fig. 125 View Figures 124–125 ). This happens because of the presence of A. longipes in ‘Serra do Mar’, ‘Serra da Mantiqueira’, and ‘Serra dos Órgãos’, making ‘Serra do Mar’ and ‘Serra da Mantiqueira’ sister areas ( Fig. 123 View Figures 122–123 ). However, ‘Serra da Mantiqueira’ belongs to the Serra da Mantiqueira mountain range, and ‘Serra do Mar’ belongs to the Serra do Mar mountain range, which are separated by the valley of the Paraíba do Sul river that, according to Pinto-da-Rocha et al. (2005), is supposed to be an important vicariance barrier for harvestmen.
On analysing the primary BPA for the five subfamilies ( Fig. 125B View Figures 124–125 ), we note some major vicariance events. The first is the separation of ‘Bahia’–‘Serra do Espinhaço’ from the southern areas of the Atlantic Rainforest. This may have occurred as a result of an uplifting of the Serra da Mantiqueira mountain range in the region of Serra do Espinhaço. The second is the separation of the group ‘Serra da Bocaina’–‘Serra do Mar’ to the north from group ‘Santa Catarina’–‘South of São Paulo’ to the south, which may have ocurred because of an interruption of the Serra do Mar–Serra da Mantiqueira mountain range. The third is the separation of ‘Espírito Santo’ to the north from the group ‘Serra da Bocaina’–‘Serra do Mar’ to the south, which may have resulted from the appearance of the valley of the Paraíba do Sul river.
The grouping of ‘Bahia’ and ‘Serra do Espinhaço’ as sister areas may be just a methodological problem, because no Bourguyiinae occur in ‘Bahia’. Amorim & Pires (1996) constructed a general area cladogram for the Neotropics using numerous groups of Diptera and Primates. The Brazilian areas that include ‘Bahia’ and ‘Serra do Espinhaço’ (NEBr and MGBA, respectively) are sister areas, but the authors did not exemplify events to explain the separation of these areas from areas of the Brazilian south-east.
We performed a primary BPA restricted to the Bourguyiinae species. No relationship hypothesis was found among the areas of endemism (strict consensus of 225 most parsimonious trees, no resolution; L = 46; CI = 0.41; RI = 0).
Comparing the individual area cladogram for Bourguyiinae ( Fig. 124 View Figures 124–125 ) with the general area cladogram for the five subfamilies ( Fig. 125B View Figures 124–125 ), we propose the following hypotheses for Bourguyiinae geographical history. The genus Bourguyia probably originally occurred in the areas ‘Serra da Bocaina’–‘Serra do Mar’, which are related to each other ( Fig. 125B View Figures 124–125 ). Subsequently, B. trochanteralis dispersed to the southern areas ‘South of São Paulo’ and ‘Paraná’. This hypothesis is more plausible than the assumption that Bourguyia originally occurred in all of these areas, as the areas ‘Santa Catarina’–‘South of São Paulo’ are not directly related to the areas ‘Serra da Bocaina’–‘Serra do Mar’. Besides, B. trochanteralis is the only species that occurs in the southern areas. The genus Asarcus originally occurred in areas ‘Serra dos Órgãos’–‘Serra do Mar’. This group appears monophyletic in one of the shortest trees. Later individuals of Asarcus would have dispersed to ‘Serra do Espinhaço’, originating A. putunaberaba sp. nov. (post-dispersal speciation). Therefore, the original distribution of the whole subfamily probably did not include ‘Serra do Espinhaço’, occurring from the north of Paraná to the north of Rio de Janeiro, and to the south-east of Minas Gerais, in the areas ‘Serra da Bocaina’–‘Serra do Mar’. The only vicariance event among these areas is the separation of ‘Serra da Mantiqueira’–‘Serra do Mar’ from the others, probably resulting from the appearance of the valley of the Paraíba do Sul river. However, this event apparently had no effect on the species of Bourguyiinae .
BIOLOGICAL ASPECTS
Important information regarding the biology of some of the species of Bourguyiinae was available from certain labels. However, in most of them there is no information about collection sites. Three species were found inside bromeliads: B. trochanteralis , B. vinosa sp. nov., and A. ingenuus . Machado & Oliveira (2002) recorded the oviposition of B. trochanteralis (cited as B. albiornata ) on bromeliad leaves. The flat body and ocularium, as well as the absence of dorsal armature in bourguyiines, could be related to a life within bromeliads, allowing them to move between the internal leaves. In addition, the coxa IV of Bourguyiinae is twisted on the longitudinal axis. Their outer apophyses are in a dorsal position, and their inner apophyses are in a ventral position ( Figs 2–7 View Figures 2–7 ); the femoropatellar articulation is on a horizontal plane (in most harvestmen it is vertical), thereby enabling movement inside bromeliads.
Machado & Oliveira (2002) studied the oviposition and maternal care of B. trochanteralis . They found 281 females caring for eggs inside the bromeliad Aechmea nudicaulis (Bromeliaceae) . Twenty-five males were found close to these females, whereas three males were found copulating with guarding females, and two others were attacked and repelled from the bromeliad by the female. Aechmea nudicaulis is a large bromeliad: its leaves (up to 50-cm high) are very close to each other, forming a tube in its interior, and this tube is used by females of B. trochanteralis for oviposition. The authors also verified the presence of seasonality in reproduction (February–October, activity peak in March–May), and the importance of maternal care for the maintenance of the eggs.
Adaptations for life within bromeliads have already been recorded in vertebrates and invertebrates, and they generally imply a flattening of the body, such as in spiders ( Dias & Brescovit, 2003), frogs ( Hedges, 1989), and lizards ( Vrcibradic & Rocha, 1996).
Asarcus longipes was found inside bamboo shoots in Teresópolis, RJ (MNRJ 4839) and in P.E. Morro Grande, Caucaia do Alto, SP (MZSP 27724, ♂). Bourguyia laevibunus specimens (MHNC 6391) were found in bamboo groves in Nova Friburgo, RJ. This kind of bamboo grove is not native to Brazil (P. T. Sano, pers. comm.). Harvestmen probably enter inside the bamboo through holes made by other animals, such as beetles or wasps. Lima (1914) described the beetle Erethistes lateralis ( Coleoptera : Curculionidae ) that eats bamboo marrow. Its eggs are deposited inside the bamboo through a small hole made by the female rostrum. The larvae eat bamboo marrow, and open a new and larger hole to leave. The author also reported the larva of the wasp Prodecatoma cruzi ( Hymenoptera : Chalcidioidea), which feeds on these beetle eggs. Immediately after oviposition by the beetle, the wasp deposits its egg on the beetle egg. The wasp larva, which hatches first, feeds on the beetle egg until turning into an adult. Thus, the adult wasp also opens a larger hole to leave the bamboo shoot. By entering holes like these, harvestmen can hide and maybe also eat bamboo marrow, or even eggs or larvae from beetles or wasps.
| ZMUC |
Zoological Museum, University of Copenhagen |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
Asarcus
| Yamaguti, Humberto Y. & Pinto-Da-Rocha, Ricardo 2009 |
Opisthoplites corallipes:
| Kury AB 2003: 108 |
Asarcus ingenuus: Soares & Soares, 1945b: 252
| Soares BAM 1972: 55 |
| Soares BAM & Soares HEM 1949: 230 |
| Soares BAM & Soares HEM 1945: 252 |
| Soares BAM 1945: 367 |
Asarcus nigriconspersus
| Kury AB 2003: 107 |
| Soares BAM & Soares HEM 1945: 254 |
Cnemoleptes Mello-Leitão, 1941: 440
| Kury AB 1994: 351 |
| Kury AB 1989: 5 |
| Soares BAM & Soares HEM 1949: 225 |
| Mello-Leitao CF 1941: 440 |
Cnemoleptes passarellii Mello-Leitão, 1941: 440
| Kury AB 2000: 2 |
| Soares BAM & Soares HEM 1949: 232 |
| Soares BAM 1945: 367 |
| Mello-Leitao CF 1941: 440 |
Bogdana Mello-Leitão, 1940: 27
| Kury AB 1994: 351 |
| Mello-Leitao CF 1940: 27 |
Bogdana ingenua Mello-Leitão, 1940: 27
| Kury AB 2003: 107 |
| Mello-Leitao CF 1940: 27 |
Opisthoplithes
| Mello-Leitao CF 1935: 102 |
Asarcus pallidus Mello-Leitão, 1923: 168
| Kury AB 2003: 107 |
| Soares BAM & Soares HEM 1949: 231 |
| Soares BAM 1944: 289 |
| Roewer CF 1931: 113 |
| Mello-Leitao CF 1923: 168 |
Opisthoplites Sørensen, 1884: 615
| Kury AB 1994: 351 |
| Soares BAM & Soares HEM 1949: 157 |
| Piza ST Jr. 1943: 43 |
| Piza ST Jr. 1940: 318 |
| Mello-Leitao CF 1923: 154 |
| Roewer CF 1913: 170 |
| Sorensen W 1884: 615 |
Asarcus lutescens Sørensen, 1884: 617
| Kury AB 2003: 107 |
| Soares BAM & Soares HEM 1945: 254 |
| Mello-Leitao CF 1932: 409 |
| Roewer CF 1931: 112 |
| Mello-Leitao CF 1923: 168 |
| Roewer CF 1913: 300 |
| Sorensen W 1884: 617 |
Opisthoplites ypsilon Sørensen, 1884: 615
| Kury AB 2003: 107 |
| Soares BAM & Soares HEM 1949: 198 |
| Soares BAM 1945: 361 |
| Mello-Leitao CF 1923: 154 |
| Roewer CF 1913: 256 |
| Sorensen W 1884: 615 |
Asarcus corallipes
| Soares BAM & Soares HEM 1949: 230 |
| Roewer CF 1931: 112 |
| Mello-Leitao CF 1923: 168 |
| Roewer CF 1913: 299 |
| Simon E 1879: 235 |
Asarcus
| Kury AB 1994: 97 |
| Kury AB 1994: 351 |
| Kury AB 1989: 5 |
| Soares BAM & Soares HEM 1945: 255 |
| Mello-Leitao CF 1941: 440 |
| Kastner A 1937: 389 |
| Mello-Leitao CF 1932: 391 |
| Roewer CF 1931: 106 |
| Mello-Leitao CF 1923: 168 |
| Roewer CF 1913: 283 |
| Sorensen W 1884: 616 |
| Simon E 1879: 234 |
| Erichson GF 1845: 2 |
| Koch CL 1839: 16 |
Asarcus longipes Kollar in Koch, 1839a: 16
| Kury AB 2003: 107 |
| Soares BAM & Soares HEM 1949: 230 |
| Mello-Leitao CF 1932: 409 |
| Roewer CF 1931: 112 |
| Giltay L 1930: 239 |
| Mello-Leitao CF 1923: 168 |
| Roewer CF 1913: 299 |
| Simon E 1879: 234 |
| Koch CL 1839: 16 |