Barbarophryne brongersmai ( Hoogmoed 1972 )
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3661.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:448C4455-5A22-4C99-AA04-6FAF6DAFB879 |
|
persistent identifier |
https://treatment.plazi.org/id/B64F87EA-2073-FFFD-FF20-2C5E59D7B81B |
|
treatment provided by |
Felipe |
|
scientific name |
Barbarophryne brongersmai ( Hoogmoed 1972 ) |
| status |
|
Barbarophryne brongersmai ( Hoogmoed 1972) View in CoL
Figs. 13D–F View FIGURE 13 .
Background information. While the validity of B. brongersmai was shortly doubted after its description (Benhachem et al. 1989), subsequent data on larval morphology, karyology, osteology and bioacoustic data confirmed its specific status ( Grillitsch et al. 1989; Herrero et al. 1993; Delfino et al. 2009; Doglio et al. 2009). The origin and interfamily relationships of B. brongersmai in regard to other bufonid species remain unresolved (see Results section of the present paper). The current calibration suggests that separation from other Eurasian Bufonidae would have occurred during the Oligocene, between 20 and 30 mya. The mountain ranges of the Western Palearctic were formed throughout that period as a result of the Alpine orogeny ( Rosenbaum et al. 2002). Being one of the main vicariant events during the Late Mesozoic and Cenozoic, the Alpine orogeny likely had profound impact on divergence within the Bufonidae (see also Van Bocxlaer et al. 2009, 2010). Indeed, most contemporary bufonid genera diverged throughout that period ( Van Bocxlaer et al. 2009). Initial separation of Barbarophryne gen. nov. during the Oligocene therefore seems a likely scenario, although comprehensive evidence is still lacking.
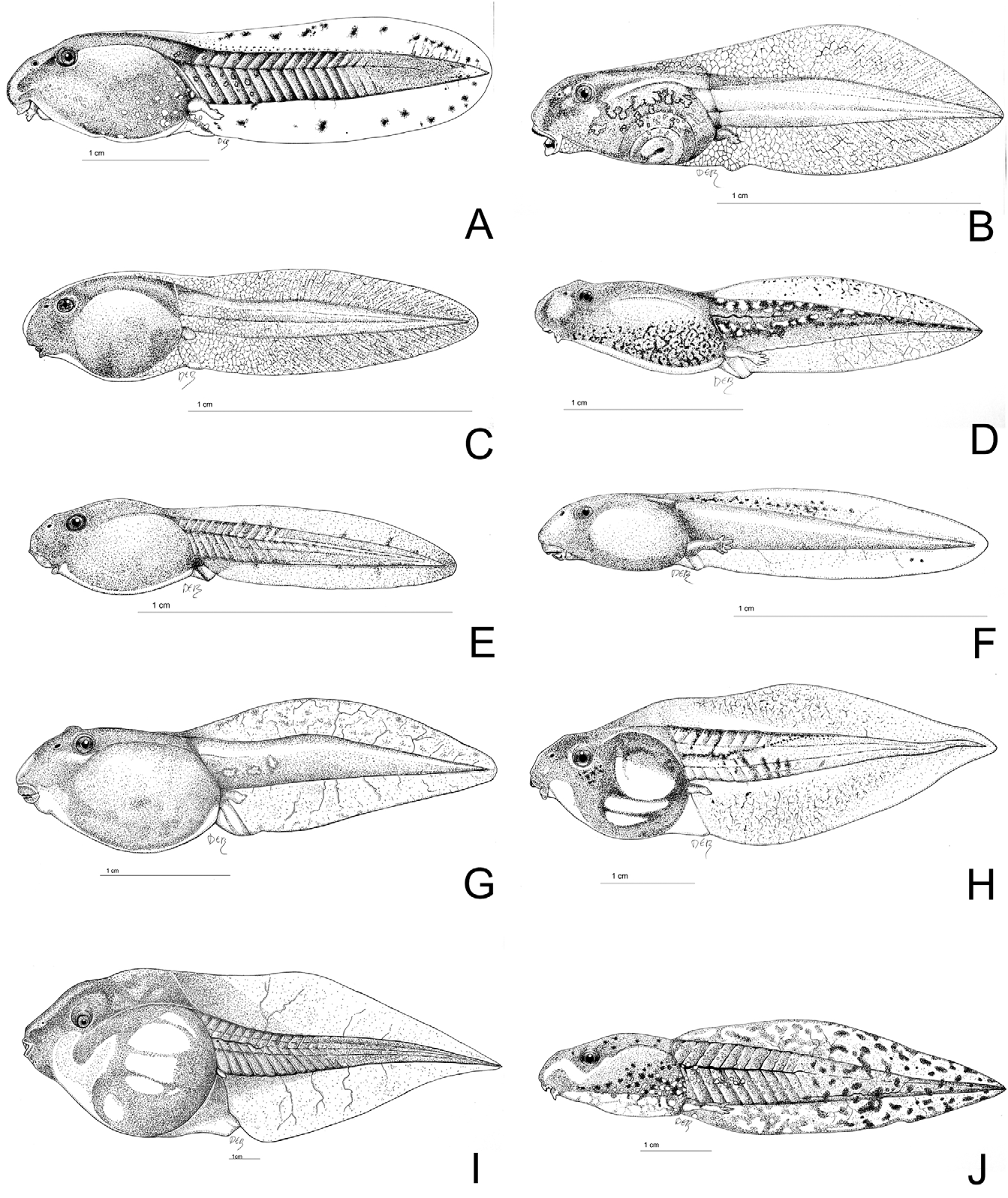
Tadpole. The following diagnosis is a summary of the elaborate descriptions given by Hoogmoed (1972) and Grillitsch et al. (1989). A drawing of the tadpole is shown in Fig. 12E View FIGURE 12 . Fully developed tadpoles are characterized by their flattened body shape and generally small interorbital space. Eyes positioned dorsally on head. Interorbital space varying from equal to nearly 1½ times as wide as the internarial space ( Grillitsch et al. 1989). Oral disk approximately 1½ times as wide as the interorbital space. Spiracle sinistral, pointing straight backwards or slightly upwards. Body 1¼ times as wide as deep, 1 2 / 3 times as long as wide. Tail less than four times as long as deep. Dorsal tail fin occasionally higher than ventral tail fin, ending posterior of the muscular base of the tail. Tail tip rounded. Oral disc ventral, about one and a half times as wide as interorbital space. Clusters of papillae at the corners of the mouth, upper and lower lip free of papillae. Two tooth rows on the upper and three on the lower labium. Lower supralabial row with median gap. Colour uniform dark brown to black, ventral side turning slightly lighter during ontogeny. Tail fins unspotted.
Hoogmoed (1972) described the interorbital space to be equal to the distance between the nostrils, and considered this as one of the most important characters to separate B. brongersmai from B. boulengeri . However, as shown by Grillitsch et al. (1989) this distance varies considerably, nearly approaching the distance observed in B. boulengeri (interorbital space 1½ times the distance between the nostrils). As the dorsal tail fin can be higher than the ventral tail fin in both species while they present similar colours, identification in the field is troublesome. The interorbital space in relation to both the internarial space and the oral disk, as well as presence/absence of spots on the tail are the most informative characters for identification.
Bioacoustics. Hoogmoed (1972) described that a male B. brongersmai emitted several ‘short squeaks’ from beneath a large stone lying at the edge of a pond, but did not assume that this represented an advertisement call as it was highly different from the call of other Bufonidae species that he was familiar with. Bogaerts (2001) described the advertisement call, based on captive individuals, as consisting of several short pulses lasting for approximately 1–2 seconds, thus confirming the observation of Hoogmoed (1972). Bioacoustics of B. brongersmai have been analysed first by Gallix (2002) and then by Doglio et al. (2009). The call is variable and composed of a train of 3 to 8 pulses, with a fundamental frequency ranging from 1217 to 1890 Hz. The call length varies between 0.53 to 3.11 seconds. Pulse length ranges from 0.03 to 3.11 seconds. Interpulse length varies from 0.09 to 0.14 seconds, while the pulse rate ranges from 6.39 to 6.73 seconds. Intervals between calls are irregular. The frequency of release calls is higher, while the length of this call and number of pulses are highly variable. The advertisement call analysed herein ( Fig. 7B View FIGURE 7 ) was recorded at 22.7ºC (34% humidity) and consisted of 5 notes composed of a series of 5 to 7 pulses (median = 7), with a dominant frequency ranging from 1217 to 1276 Hz. Note length varied between 0.77 to 1.10 seconds (average 0.98 ± 0.145). Pulse length ranged from 0.055 to 3.11 seconds (average 0.064 ± 0.004, n = 32). Interpulse length varied from 0.09 to 0.14 seconds (average 0.106 ± 0.011, n = 27,), while the pulse rate ranged from 6.39 to 6.73 seconds (average 6.52 ± 0.126).
Natural history. Barbarophryne brongersmai is an explosive breeder dependent on incidental periods of rain during spring, which uses all kinds of small, temporary water bodies such as rain puddles, temporary streams and ponds and occasionally man-made water reservoirs ( Gallix 2002; García-Muñoz et al. 2009) for reproduction. Site fidelity is relatively strong. Reproduction has been observed between mid-March and the beginning of April ( Hoogmoed 1972; Guillon et al. 2004). Eggs are deposited in small clutches, and attached onto stones or vegetation underwater ( Bogaerts 2001). Despite the general aridity, which characterizes the distribution of B. brongersmai , the larval period is rather long ( Gallix 2002). Recently metamorphosed juveniles have been observed in May (García- Muñoz et al. 2009).
Distribution. Initially, B. brongersmai was only known from the southern Moroccan Atlantic coast near Tiznit, and the adjacent Western Sahara ( Hoogmoed 1972). Destre et al. (1989) provided considerable range extensions along the entire sub-Atlas region of Morocco, and northwards onto the arid plain of Marrakech. Additional records have been published by Guillon et al. (2004) and Doglio et al. (2009). The distribution map ( Fig. 14B View FIGURE 14 ) is composed of records from Bons and Geniez (1996), Brito (2003), Herrmann and Herrmann (2003), Guillon et al. (2004), Harris et al. (2008), Ramos and Díaz-Portero (2008) and Doglio et al. (2009). New records fill in gaps within the sub-Atlas distribution of the species, which seems to be (nearly) continuous. The new record (see also Fig. 13F View FIGURE 13 ) north of Mechra-Benâbbou is close to that of De la Riva (1992), but significant due to its location north of the Oued Rbiaa.
National Red List Status. Near Threatened.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.