Bopyrione synalphei Bourdon and Markham, 1980
|
publication ID |
https://doi.org/ 10.1080/00222933.2020.1842535 |
|
persistent identifier |
https://treatment.plazi.org/id/03D987BD-FF88-FFF4-FEA5-FD7AAB8EF95F |
|
treatment provided by |
Carolina |
|
scientific name |
Bopyrione synalphei Bourdon and Markham, 1980 |
| status |
|
Bopyrione synalphei Bourdon and Markham, 1980 View in CoL
Figures 1 View Figure 1 (b), 2(b), 4; Tables 1 and 2
?bopyrid. – Coutière, 1909: 52 [Unspecified locality, parasitising Synalpheus brevicarpus ].
‘branchial bopyrid’. – Chace, 1972: 95, 104 [ Tobago, parasitising S. minus ; Bahía de la Ascensión, Quintana Roo, Mexico, parasitising S. towsendi ].
‘bopyrid isopods’. – Hoetjes et al. 1976: 33 [ CuraÇao, parasitising ‘snapping shrimp’].
Bopyrione synalphei Bourdon and Markham, 1980: 221–228 View in CoL , figs. 1–3 [type-locality Golfe de Gonáve, Haiti, parasitising S. bousfieldi View in CoL , S. pectiniger View in CoL , Synalpheus sp. (near S. longicarpus View in CoL ) and Synalpheus sp. (near S. goodei View in CoL ); St. Michielsbaai, CuraÇao, parasitising Synalpheus sp. ; west of Egmont Key, Florida, U.S.A., parasitising S. goodei View in CoL ]. – Markham, 1982: 351, 354. – Bourdon, 1983: 863. – Markham 1985: 60, 61, figs. 25, 26 [Florida, U.S.A. parasitising S. longicarpus View in CoL and S. minus View in CoL ; Enriquillo, Dominican Republic, parasitising S. pectiniger View in CoL ; southwest of Jamaica, parasitising S. pectiniger View in CoL ; Bahía de la Ascensión, Quintana Roo, Mexico, parasitising S. towsendii ].– Markham, 1988: 57 table 1. – Kensley and Schotte, 1989: 110 table 2, 267 table 6. – Salazar- Vallejo and Leija-Tristan, 1990: appendix 1. – Markham and Donath-Hernández, 1990: 243. – Markham et al. 1990: 416. – Duffy, 1992: 133. – Camp et al. 1998: 133. – Román-Contreras, 2008: table 2. – Schotte et al. 2009: 981. – Román-Contreras and Martínez-Mayén, 2011: 1145–1146, 1148 [Laguna de Términos, Campeche, Mexico, parasitising S. apioceros View in CoL ]. – An et al. 2015: 37, 39, 40, 41 in key. – Romero-Rodríguez and Martínez-Mayén, 2018: 1191 table 2.
‘cf. Probopyrus pandalicola View in CoL ’. – Westinga and Hoetjes 1981: 141 table 1 [not Probopyrus pandalicola View in CoL ].
Material examined
One adult female, three ovigerous females, one paired with a male (YUC-CC-255-11- 006555), parasitising four males of Synalpheus elizabethae ( Ríos and Duffy, 2007) (YUC- CC-255-11-000206), J . A . Duarte det . host; Arrecife Alacranes , Yucatán, Mexico (22º29 ʹ 16.12”N, 89º41 ʹ 5.89”W); N GoogleMaps . Simões coll GoogleMaps .; 8 June 2008. Three adult females paired with males and two ovigerous females, only one paired with a male (YUC-CC-255-11- 006556), parasitising five males of Synalpheus brooksi Coutière, 1909 (YUC-CC-255-11- 000185), J . A . Duarte det . host; Arrecife Alacranes , Yucatán, Mexico (22º35 ʹ 12.7”N, 89º44 ʹ 41.1”W); N GoogleMaps . Simões coll GoogleMaps .; 24 April 2011.
Distribution
Both coasts of Florida, U.S.A., Quintana Roo, Mexico, and the West Indies to CuraÇao ( Markham 1985).
Remarks
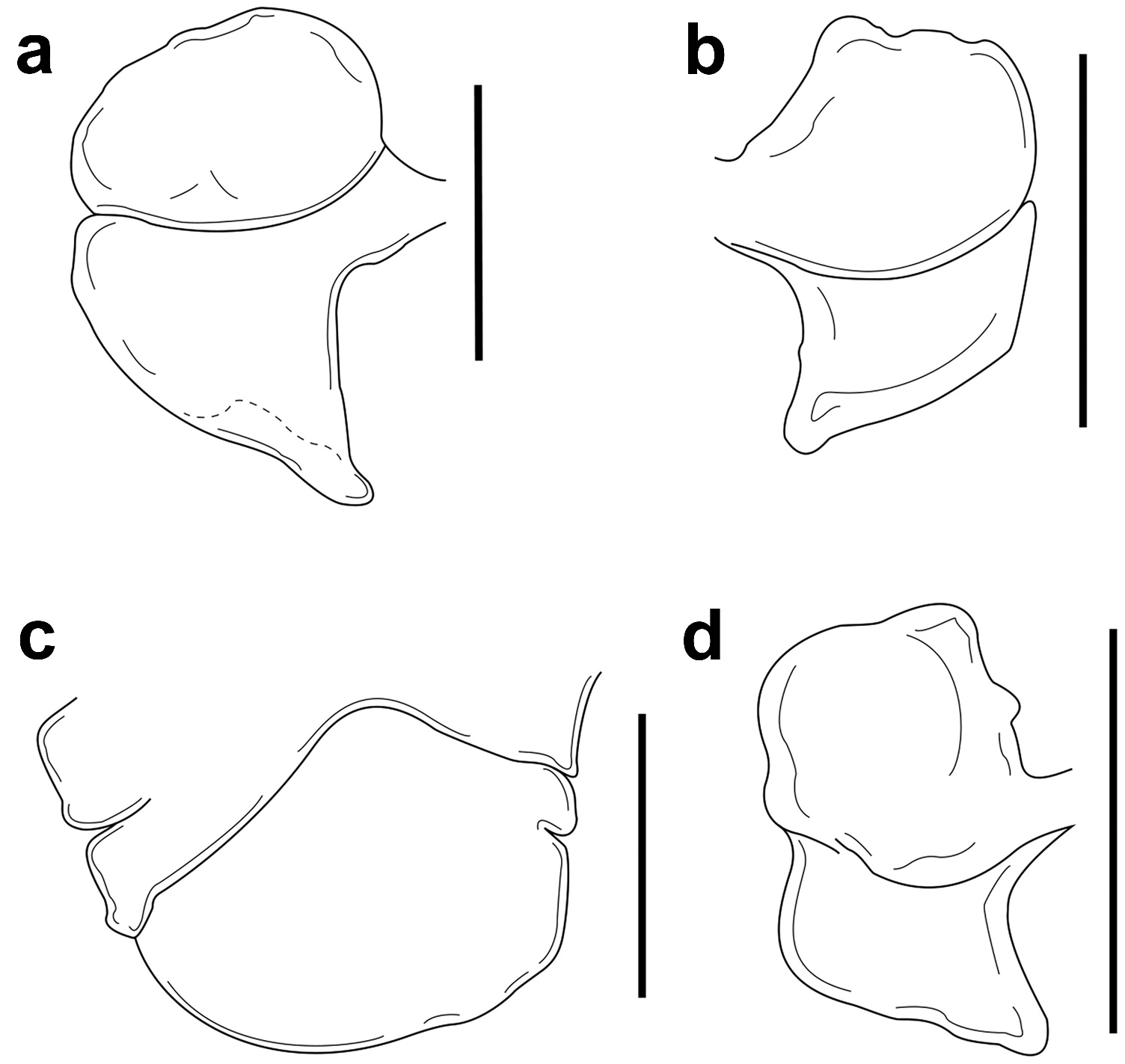
The frontal lamina of the female head was variable in form, including being absent, anterior margin slightly creased (two females), similar to that reported by Bourdon and Markham (1980, Figure (a)), to absent but with smooth anterior margin (two females), to a narrow, well-defined frontal lamina (three females) ( Figure 1 View Figure 1 (b)), and two females with just a small piece of frontal lamina on a corner of the head. The eyes in all females are conspicuous and located near to the anterior margin of head ( Figure 1 View Figure 1 (b)). The shape of the pereomeres, coxal plates and dorsolateral bosses fit the description of Bourdon and Markham (1980), but the number of pereomeres with these traits were consistently greater on the short side of body ( Figure 1 View Figure 1 (b)). Overall, the shape of the first pair of oostegites ( Figure 4 View Figure 4 (a)) agrees with previous descriptions ( Bourdon and Markham 1980, Figure 2 View Figure 2 (d); Markham 1985, Figure 25E, F) but usually the oostegites were larger along the short side of the body than in the longer side. Three females that parasitised S. brooksi had the posterior segment of the first oostegite rectangular in shape ( Figure 4 View Figure 4 (b,c)). The pleon was 1.59 ± 0.19 times broader than long (range 1.27–1.97), similar to the interval (1.30–1.64) proposed by Bourdon and Markham (1980). The pleon shape in five of the nine females differed from previous descriptions, having straight lateral margins on the shorter side instead of rounded ones ( Bourdon and Markham 1980, Figure 1 View Figure 1 (a); Markham 1985, Figure 25(a, g)); and in three of them, the lateral margin of first pleomere was clearly indented ( Figure 4 View Figure 4 (d)).
The head of the bopyrid males was oval-shaped, smaller than the first pereomere and with conspicuous eyes located on the posterolateral margins of head ( Figure 2 View Figure 2 (b)). The number of segments and extension of the antennae, as well as the characteristics of the pereomeres, pereopods, and pleomeres fits well those mentioned by Bourdon and Markham (1980) for B. synalphei .
All the shrimps parasitised by B. synalphei were males. Host mean size was 2.68 ± 0.19 mm CL (range 2.40–2.83 mm) for S. elizabethae (n = 4) and 3.51 ± 0.49 mm CL (range 3.07–4.15 mm) for S. brooksi (n = 5). The parasite was attached more often in the host’s right branchial chamber (n = 8) than to the left one (n = 1). The sizes of the female and male of B. synalphei are shown in Table 1. Five ovigerous parasite females were recorded with a mean size of 1.75 ± 0.48 mm LT (range 1.31–2.42 mm), three with an evident embryo loss (one with 20 embryos on egg stage and two with less than 15 epicaridium larvae). Fecundity and embryo sizes of the females with a more compact brood mass are indicated in Table 2.
Bopyrione synalphei had been recorded from Bahía de la Ascensión, Quintana Roo ( Markham 1985; Markham and Donath-Hernández 1990; Markham et al. 1990) and Laguna de Términos, Campeche, ( Román-Contreras and Martínez-Mayén 2011); therefore, this is a new locality record. Synalpheus brooksi was reported as host of B. synalphei on reefs of the Panamanian Caribbean ( Duffy 1992) and parasitised by undetermined abdominal and branchial bopyrids in Antigua Islands, Tobago Cays and Mexican Caribbean ( Chace 1972). Synalpheus elizabethae was reported with a remarkably high incidence of unidentified abdominal and branchial bopyrids in Panama ( Ríos and Duffy 2007), whilst McGrew and Hultgren (2011) identified the branchial parasite of S. elizabethae as Bopyrione sp. , now we can confirm this alpheid as host for B. synalphei , increasing to nine the known hosts for this parasite, all belonging to Synalpheus : S. bousfieldi Chace, 1972 , S. brevicarpus (Henrrick, 1891) , S. brooksi , S. goodei Coutière, 1909 , S. longicarpus ( Herrick, 1891) , S. minus , S. pectiniger and S. townsendii Coutière, 1909 .
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |
Bopyrione synalphei Bourdon and Markham, 1980
| Romero Rodríguez, Jesús & Álvarez, Fernando 2021 |
Bopyrione synalphei
| Romero-Rodriguez J & Martinez-Mayen M 2018: 1191 |
| An J & Boyko CB & Li X 2015: 37 |
| Roman-Contreras R & Martinez-Mayen M 2011: 1145 |
| Schotte M & Markham JC & Wilson DF 2009: 981 |
| Camp DK & Lyons WG & Perkins TH 1998: 133 |
| Duffy JE 1992: 133 |
| Kensley B & Schotte M 1989: 110 |
| Markham JC 1988: 57 |
| Markham JC 1985: 60 |
| Bourdon R 1983: 863 |
| Markham JC 1982: 351 |
| Bourdon R & Markham JC 1980: 228 |