Cyanomethia pseudothonalmus Philips and Ivie, 1998
|
publication ID |
https://doi.org/ 10.1649/0010-065X-72.4.691 |
|
publication LSID |
urn:lsid:zoobank.org:pub:620DF9F5-99A0-4A62-9F58-1F49078C4B57 |
|
persistent identifier |
https://treatment.plazi.org/id/03C987B8-4724-5667-FF08-FD770AC5FABC |
|
treatment provided by |
Diego |
|
scientific name |
Cyanomethia pseudothonalmus Philips and Ivie, 1998 |
| status |
|
Cyanomethia pseudothonalmus Philips and Ivie, 1998 View in CoL
( Figs. 1 View Fig , 2 View Fig )
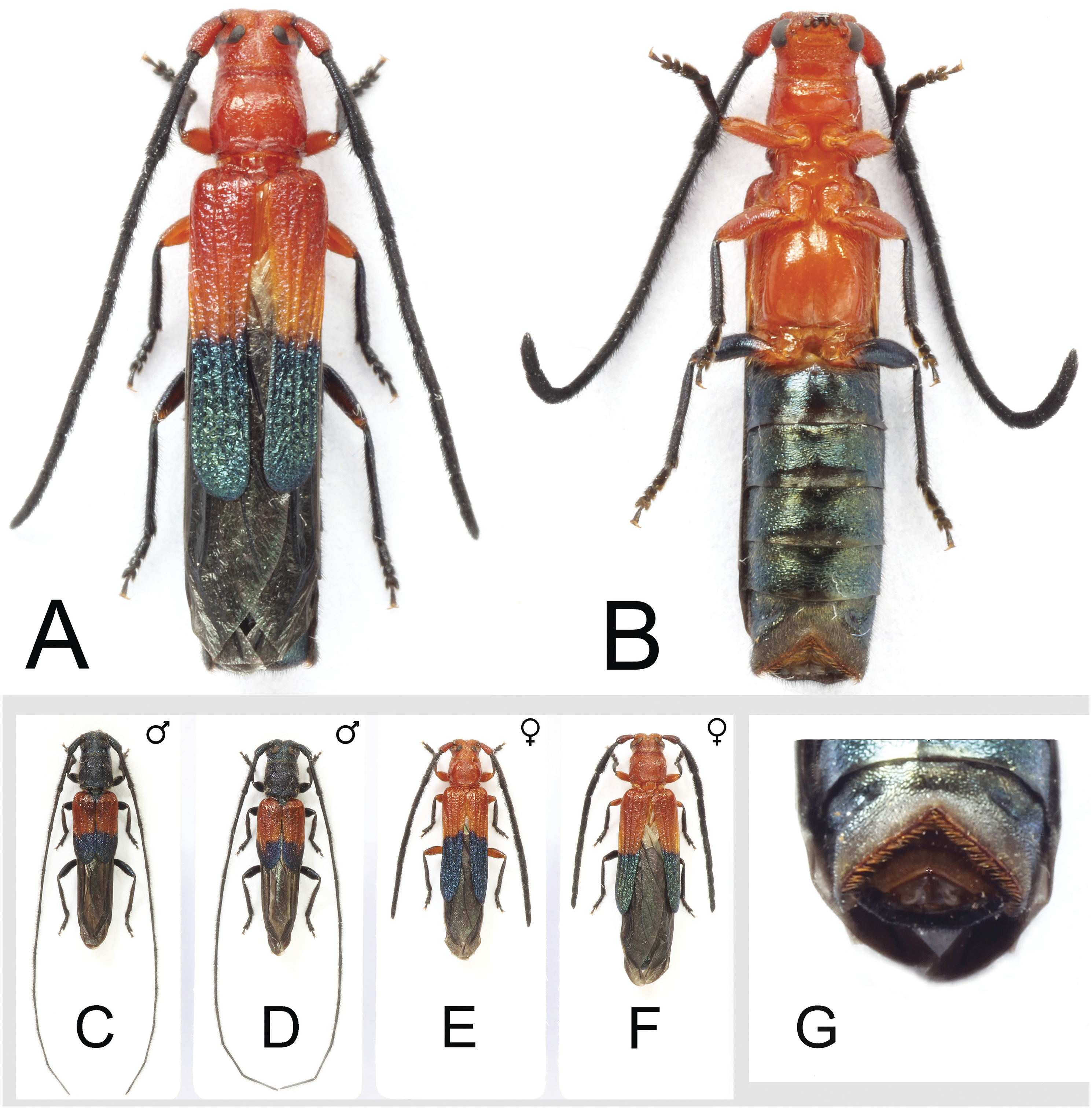
Description. Female ( Figs. 1A, B, E, F, G View Fig , 2E View Fig ). Length: 9.8–13.7 mm. All characters as in male except the following: Head and thorax entirely testaceous-orange (black with blue or purple reflections in male); scape, pro- and mesofemora also testaceous-orange, scape rarely black in some specimens (generally dark, black, brown, or testaceous-brown in male); testaceous-orange elytral base (slightly lighter in color than in male, Fig. 1C, D View Fig ); antenna short, robust, not exceeding abdominal apex, flattened at sides, covered with short, dense, suberect to depressed setae (long in male, extending about 5 segments beyond abdominal apex, slender, round, with brush of long, erect hair on the outer side and sparse, suberect to depressed hair on the inner side); pronotum slightly broader than long (as broad as long in male, pronotal disc and lateral glabrous areas slightly more inflated and less punctate in female); elytra abbreviated, about 2.5X basal width, extending beyond middle of abdomen (elytra distinctly shorter in male, about 1.5X basal width, extending approximately to 2 nd visible abdominal segment); abdomen robust, apical opening large, fringed with both tapered and clavate setae ( Fig. 1G View Fig ) (normally tapered in the male).
Biology. The larvae girdle living terminal twigs, typically less than 1 cm in diameter, of unidentified tropical hardwoods. Girdled twigs were found in November, and adults emerged 3–4 months later. A newly hatched larva produces a flat, non-spiral girdle ( Fig. 2A View Fig ) just under the bark. This severs the twig almost entirely, leaving just a very thin bark connection with the tree. The small larva enters the severed twig, usually through the center of the girdled end, and plugs the tunnel with a small, fibrous plug ( Fig. 2A View Fig ). Later, the bark breaks under the weight of the twig. These girdled twigs can be quite conspicuous on the forest floor during summer. The twigs contain small feeding larvae at the time they fall, and these larvae eventually devour most of the twig. Pupation occurs in the thicker part of the twig. Before building the pupal cell, the larva again girdles the twig, this time from the inside, separating the mostly consumed terminal portion from the mostly intact basal portion. The second girdle contains a large, conspicuous fibrous-frass plug in its center ( Fig. 2B View Fig ), through which the adult beetle will eventually emerge. Occasionally, instead of making the second girdle, the larva creates long openings on opposite sides of the twig ( Fig. 2C View Fig ). Doubly girdled twigs containing larvae in their pupal cells can be found during the fall. Adults emerge the following year. This girdling behavior is very similar to that of the unrelated Ropalopus spinicornis (Abeille de Perrin, 1869) (Vlasak and Rejzek 1998) .
Twenty-seven individuals (14 ♀♀ and 13 ƋƋ) were successfully reared, either from exposed larvae that were transferred into rearing vials or emerged directly from twigs; the 28 th specimen was a female that eclosed with a misformed antenna and did not emerge from its twig. All twigs produced adult beetles, no larval or pupal mortality was noted, and no parasitism occurred. Two females laid six and 10 eggs each, approximately 1.2 × 0.6 mm ( Fig. 2D View Fig ), in their rearing vials. Other females laid similar eggs on the twigs ( Fig. 2E View Fig ) in the rearing container. This contrasts with Philips and Ivie’ s (1998) report of 118 and 127 eggs inside two female M. necydalea . These eggs were considerably smaller, at 0.9 × 0.5 mm. Recently, one reared M. necydalea from Antigua laid 132 eggs of an equivalent size in a rearing container ( JV, unpublished data), similar to the earlier data. Although the differences in these measurements seem small, the difference in egg volume between comparably sized females is important as an indication of female investment. Using the two length/breadth measurements and a simple ovoid model, the egg of C. pseudothonalmus has a volume approximately nearly double (1.9X) that of M. necydalea (0.226 mm ³ vs. 0.117 mm ³, respectively) .
As noted in the original description, but now far more obvious with the female known, C. pseudothonalmus seems to be clearly an excellent mimic of the West Indian endemic genus Thonalmus Bourgeois, 1884 ( Coleoptera : Lycidae ). This follows the pattern documented by Darlington (1938) in Cuba. The level of detail in the resemblance of these two species is too great to be coincidence. A problem is that although Thonalmus occurs to the west of the Virgin Islands in Puerto Rico and to the south on Montserrat, no Virgin Islands specimens have been found. All historic records for Thonalmus from the Virgin Islands have been rejected as mistakes ( Miller 1984; MAI unpublished data). It follows that either Cyanomethia Philips and Ivie evolved on another island where Thonalmus occurs and then dispersed to St. John or that Cyanomethia evolved alongside Thonalmus in the Virgin Islands and Thonalmus has been extirpated there. It is also possible that, given the need for specialized collecting, Cyanomethia occur more widely than known and co-occur with Thonalmus on other islands, most likely Puerto Rico which was connected by land to St. John during the Pleistocene (Heatwole and MacKenzie 1967; Heatwole et al. 1981).
Another point suggesting this species evolved in the presence of Thonalmus is the difference in egg number and size, as well as the relative abundance of the adults, of M. necydalea and C. pseudothonalmus on St. John. Cyanomethia pseudothonalmus is simply rare, and each egg has a larger investment than those of the abundant M. necydalea . Each of a small number of large eggs is placed individually on a twig, and in over 200 years of collecting in the northern Virgin Islands only a single adult specimen has been detected. The M. necydalea female deposits a large number of smaller eggs. Many larvae occur together in single twigs (JV unpublished data). Literally hundreds of adults can be taken on a single night when this species is flying, and it was among the first species described from the Virgin Islands ( Fabricius 1798). This is a classic r/K dichotomy, with a large female investment resulting in few offspring of C. pseudothonalmus versus lower female investment by M. necydalea resulting in a large number of offspring (MacArthur and Wilson 1967). Since in the basic mimicry scenario a Batesian mimic must be less common than the model ( Wickler 1968; Quicke 2017), a K-selected population model would be predicted.
The alternative to Batesian mimicry is that C. pseudothonalmus is aposematic. For an aposematic model to avoid predation, the population must be large enough to educate the predator community through sacrificed individuals ( Wickler 1968; Quicke 2017). Such a rare K-selected species would not be expected to be aposematic in the absence of other Müllerian mimics, which do not seem to occur on St. John (Absence is a difficult thing to measure, this statement is based on MAI’ s 40 years of work on the fauna of the Virgin Islands.). These speculations are not conclusive, but the hypothesis is hereby laid out to be tested that C. pseudothonalmus is a Batesian mimic, therby indicating a ghost population of Thonalmus sp. was once associated with it as an aposematic model. One test would be to seek girdled twigs indicative of C. pseudothonalmus in places where Thonalmus occurs, most importantly in Puerto Rico.
Material Examined. Holotype, Caneel Bay , Lind Pt.; St. John, Virgin I.; Jan. 11,1966; Coll. R.T. Bell ( NMNH). 14 ♀♀ and 13 ƋƋ: coll. J. Vlasak; 23-30Nov2015; St. John US Virgin Islands; larva in girdled twig ( CMNH, DJHC, JVPC, TKPC, WIBF).
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |
|
|
Genus |