Cytherideinae Sars, 1925
|
publication ID |
https://doi.org/ 10.11646/zootaxa.3899.1.1 |
|
publication LSID |
lsid:zoobank.org:pub:D78F2010-08E1-45C0-86FF-7F2D3601070D |
|
persistent identifier |
https://treatment.plazi.org/id/017587FE-FFA9-FFCB-71F4-DBA6FACBFD5D |
|
treatment provided by |
Felipe |
|
scientific name |
Cytherideinae Sars, 1925 |
| status |
|
Subfamily Cytherideinae Sars, 1925 View in CoL
Genus Cyprideis Jones, 1857
Type species: Candona torosa Jones, 1850
4.1. Remarks to supraspecific taxonomy
Whatley et al. (1998) emended the diagnosis of Cyprideis and placed several genera into its synonymy. Among them are the following taxa, founded on fossil Amazonian species: Amazonacytheridea Purper, 1979 , Botulocyprideis Sheppard & Bate, 1980 (emended by Purper & Pinto, 1983), Chlamydocytheridea Purper, 1979 , Nealecythere Purper & Pinto, 1983, Paulacoutoia Purper, 1979 , Pseudoparakrithella Purper, 1979 and Sohnicythere Purper & Pinto, 1983 . In addition, several Amazonian species, originally assigned to Cytheridea Bosquet, 1852 , were transferred to Cyprideis by these authors (see also Muñoz-Torres et al. 1998). In particular, the smooth, vestibulate species—formerly attributed to Amazonacytheridea , Chlamydocytheridea , Paulacoutoia and Pseudoparakrithella (with the possible synonyms Botulocyprideis and Nealecythere)—diverge significantly from Cyprideis as defined by earlier authors (e.g. Kollmann 1960; Benson et al. 1961; Van Morkhoven 1963; Sandberg 1964a). Since a discussion of this supraspecific concept is beyond the scope of the present investigation and subject of upcoming works, we apply it here with some reservation.
4.2. Conceptual notes to species identification herein
As usual, fossil species are based exclusively on morphologic characters, in this case on all features observable on the ostracods’ valves. Typically, a combination of traits is used for species definition (multidimensional species definition; Sbordoni 1993).
We are aware that specimens obtained from our samples are time-averaged to some degree and, e.g. seasonal or interannual variations (abundance, size, etc.) are blurred (e.g. Heip 1976; Schweitzer & Lohmann 1990). While disadvantageous on one side (e.g. obscuration of evolutionary patterns; increase of phenotypic variance of species), such fossil populations comprise an array of biologic generations and “random”, short-term variations are advantageously suppressed ( Neil 2000; Bush et al. 2002; Hunt 2004, 2010; Hohenegger 2014).
In the present case, we usually found various ontogenetic stages of taxa within the samples, which signalise insignificant relocation or alteration of the assemblages. Therefore, we assume the ostracod faunas of each sample to represent a fossil biocoenosis (autochthonous thanatocoenosis; Whatley 1988; Boomer et al. 2003).
Recent Cyprideis reproduce sexually and—as ostracods in general—moult during their ontogeny. When females, males and as far as possible juvenile valves can be related to one morphospecies, we consider them as genetically isolated, sympatric population/species with reference to other morphotypes co-occurring in the same sample (best documented with both sexes and juveniles alike; Schweitzer & Lohmann 1990).
Shell characters, which show gradual transitions within one sample and/or vary (“randomly”) between samples (in this case in time), while the cluster of other features remains constant, are regarded as intraspecific variability. Some valve characters of modern Cyprideis species (best studied in Cyprideis torosa (Jones, 1850)) display substantial, environmentally cued variability, which complicates the recognition of phenotypic or species diagnostic traits ( Gross et al. 2008; Ligios & Gliozzi 2012; for soft parts compare Wouters 2002, 2003):
Shape. The general shape (ovate, subrectangular, subtrapezoidal, etc.) of valves (especially of their outline in lateral view) is an essential character for ( Cyprideis ) species discrimination (among many others: Sandberg 1964a). This has been demonstrated successfully with geometric morphometric methods equally. Nonetheless, if outlines are very similar also quantitative methods fail and other traits must be considered ( Gross et al. 2008; Ligios & Gliozzi 2012). Intraspecific variations of the outline—apart from sexual and ontogenetic polymorphism—largely concern the development of the females’ brood pouch ( Kollmann 1960; Sandberg 1964a). For extant C. torosa, Carbonnel (1983) reported minor variations in shape correlated with different salinities, which, however, could not be confirmed by Frenzel et al. (2011).
Size. C. torosa varies significantly in size. This has been found to be related to salinity (e.g. Van Harten 1975, 1996) or not ( Kilenyi 1971; Vesper 1972a; Frenzel 1991) or discussed to be linked (additionally) to other parameters like organic contents (food supply; Vesper 1972a; Van Harten 1975). Lately, Boomer & Frenzel (2011) reported that C. torosa is larger below 8–9 psu and smaller above this, probably physiologically controlled threshold ( Aladin 1993). However, the relative size of Cyprideis valves can be a valuable diagnostic feature, especially in cases where more than one species co-occur within one sample (e.g. Sandberg 1964a; Keen 1982; Gross et al. 2008; Ligios & Gliozzi 2012). Conversely, absolute dimensions of Cyprideis species —like recent C. torosa —fluctuate noticeably between localities (spatial) and as shown in the current study between samples (strata/ temporal). Thus, absolute dimensions of species may significantly overlap and are a useful discriminating character on population level only (within one locality or one palaeontologic sample respectively).
Ornament. The ornament of Cyprideis is subject to serious intraspecific variation, ranging from smooth, punctated to reticulated surfaces. Parameters like calcium carbonate content ( Van den Bold 1976), salinity ( Sandberg 1964b) or the Mg/Ca ratio of the water ( Carbonel 1982)—indirectly related to salinity—are under discussion to cause this variability. Compared to modern Cyprideis , we observed pronounced variations in ornamentation, which should not be over-interpreted ( Sandberg 1964a, b). However, by examining a large number of valves, some stable ornamentation patterns can be recognized within species (e.g. for the current work about one thousand SEM-photographs of Cyprideis were taken, supplemented by the same amount of light microscopic pictures). Hence, under the assumption that variability is adequately covered, basic traits of the ornament (including the sulcus) can thoroughly form a diagnostic feature (note that keys to recent Cyprideis species likewise use the valves’ ornament as a diagnostic character; e.g. Smith & Delorme 2010; Karanovic 2012).
Nodes. Frequently, nodes (hollow protuberances) are developed on Cyprideis ’ surface, which gave rise to long lasting debates about their taxonomic value (for reviews see e.g. Sandberg 1964a; Vesper 1972 b, 1975; Van Harten 2000). Lately, the phenomenon of nodosity has been explained by Keyser & Aladin (2004) and Keyser (2005) to be a pathologic effect caused by osmoregulatory problems of the animals during moulting. However, the abundance of noded valves can be a useful proxy for salinity (noded valves dominate at a salinity below 7–8 psu) and/or Ca 2+ contents of the water (a low amount of calcium leads to increased nodosity; Frenzel et al. 2012). Interestingly, not a single noded Cyprideis valve was found among the current material (~12,000 valves; ESM 1).
Normal pores. All Cyprideis species of the present investigation bear roundish normal pores of sieve type (a depressed sieve plate with a small, eccentric pore). The position and number of normal pores in C. torosa is rather stable and supposed to be an additional, genetically fixed, diagnostic character ( Rosenfeld 1982). On the other hand, their shape varies along the salinity gradient (rounded shapes predominate in low saline settings), which is valuable for palaeosalinity calculations (e.g. Rosenfeld & Vesper 1976; Medley et al. 2008; Pint et al. 2012). No obvious differences in shape have been observed within the current specimens. Sometimes their investigation is hampered due to adhering sediment. For these reasons, the analysis of normal sieve pore pattern (shape, position, size, number) and their possible taxonomic as well as palaeoecological relevance is not considered in the present study.
Marginal denticulations and spines. Conversely to the assumption of Wouters (2002; for C. torosa ), the basic pattern of anteromarginal and posteroventral spines/denticles (e.g. presence/absence, position, shape) is found several times to be an effective diagnostic character in Cyprideis (e.g. Kollmann 1960; Sandberg 1964a; Schweitzer & Lohmann 1990; Gross et al. 2008; Ligios & Gliozzi 2012; for recent Cyprideis see e.g. Smith & Delorme 2010; Karanovic 2012). Nevertheless, variations (e.g. strength, number) occur, which need to be as carefully examined as the preservation of valves. Marginal spines can be a useful character for species delineation, but should not be used as a single diagnostic trait and in a too tight manner (e.g. relying on the exact number of spines). The fundamental pattern of marginal spines is already developed in juvenile individuals, but the number of spines is frequently reduced in the adult stage ( Sandberg 1964a; Gross et al. 2013; and present investigation).
Inner lamella. Intraspecific variability of the inner lamella (e.g. width, course, position of the selvage) as well as of marginal pore canals (shape, course, number) has been barely studied systematically in recent Cyprideis . Sandberg (1964a) noted only slight interspecific differences in the width of the anterior inner lamella (for a general statement see Van Morkhoven 1962). However, for some species with significantly narrower or wider inner lamella it can be an (additionally) characterising feature. For marginal pore canals Sandberg (1964a) observed minor differences in shape (note: his genus concept does not include the presence of a vestibulum) but mentioned that the density of (anterior) marginal pores can be a species diagnostic trait. In view of the Amazonian species and genera included by Whatley et al. (1998) in Cyprideis , striking interspecific differences in the inner lamella and (obviously linked with that) in marginal pore canal patterns are evident, which provide a useful diagnostic trait. Subordinate intraspecific variations of the vestibulum are observed between left and right valves as well as between both sexes ( Whatley et al. 1998; and present work).
Hinge. Concerning recent Cyprideis little is known about the intraspecific variability of its hinge. Only Sandberg (1964a) noticed some variations in the crenulation of the (postero-)median element. Although the general pattern of the hinge (e.g. proportions of hinge elements, expression of teeth or denticles) is mainly of supraspecific significance (e.g. Kollmann 1960; Wouters & Martens 1992; Tibert et al. 2003), in combination with additional characters it can be of species diagnostic value (e.g. Gross et al. 2008; Ligios & Gliozzi 2012). Occasionally, within fossil Cyprideis material reversed valve overlap and inverse hinges respectively are documented ( Krstić 1968a, b; Van den Bold 1976; Purper & Pinto 1983, 1985; Whatley et al. 1998; Gross et al. 2013; and herein). The taxonomic or environmental importance of this feature remains unclear ( Kollmann 1960; Krstić 1968a, b, 1971; Van den Bold 1976; Purper & Pinto 1985; Whatley et al. 1998).
Central muscle scars. Variability is low in Cyprideis’ central muscle scar pattern. Only the shape of the upper mandibular scar seems to be useful to some degree for species discrimination ( Sandberg 1964a; Schweitzer & Lohmann 1990). No substantial intra- and interspecific differences in central muscle scar arrangement or shape were observed in our material.
Bringing the discussion above to the point: more than a single character is necessary for proper Cyprideis species definitions and species must be defined with a cluster of traits (“Gesamthabitus”; Kollmann 1960: 139).
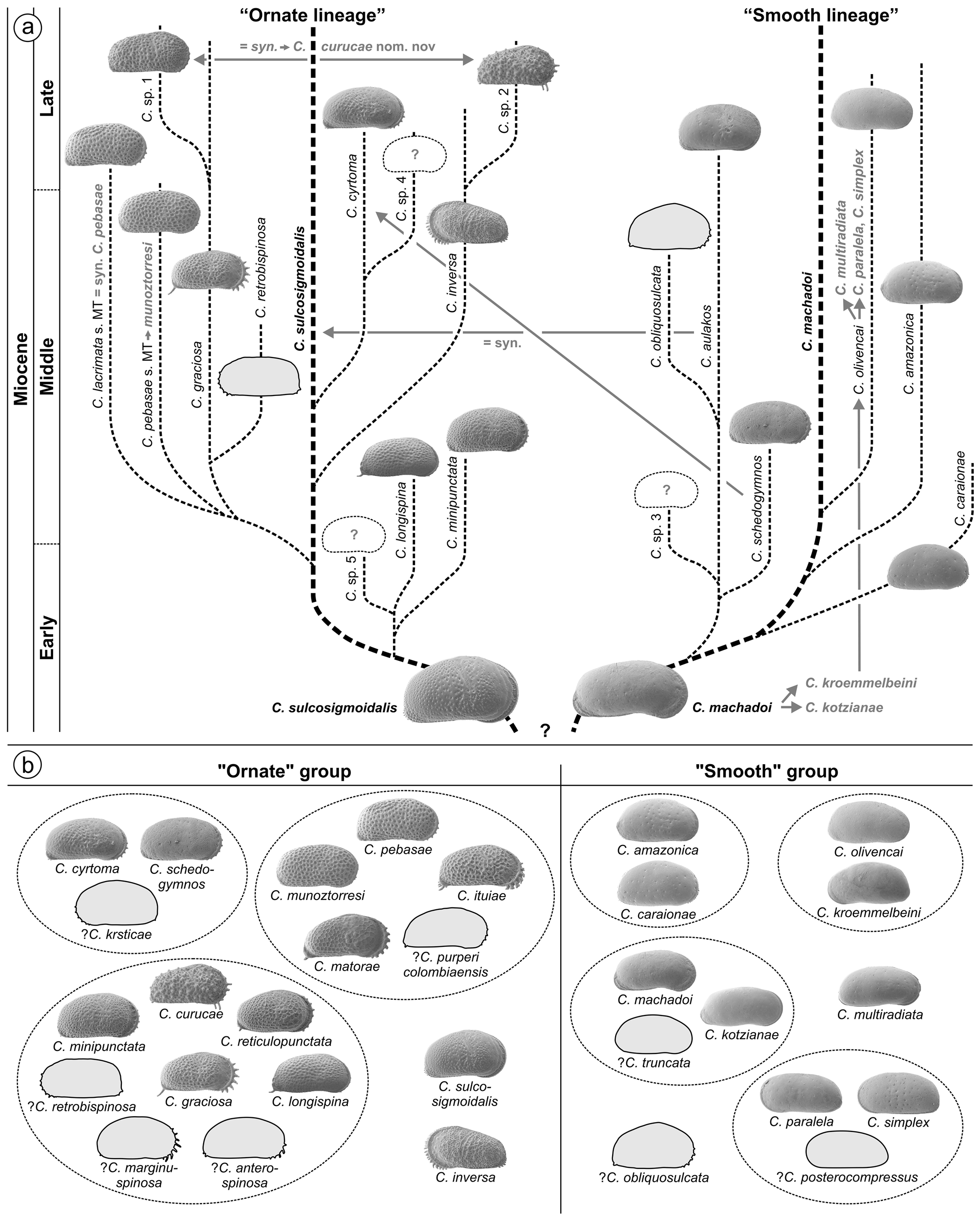
4.3. Western Amazonian Cyprideis species groupings
Whatley et al. (1998) and Muñoz-Torres et al. (2006) proposed a phylogenetic scheme for endemic western Amazonian Cyprideis based on stratigraphic occurrence as well as on comparative morphology ( Fig. 3a View FIGURE 3 ). These authors introduced two main lineages: the “ornate” and the “smooth” lineage with C. sulcosigmoidalis and C. machadoi as “core” species, respectively. Both are characterised by their surface ornament (ornamented vs. smooth). In addition, members of the “smooth” lineage generally have a wide inner lamella, principally bi- or polyfurcating (anterior) marginal pore canals and all develop a vestibulum. In “ornate” species, the inner lamella is comparably narrow; they lack polyfurcated radial pore canals as well as a vestibulum.
Our results confirm this generally applied grouping, here used for the basic arrangement of taxa (see chapter 4.5. and 4.6.), instead of a strictly alphabetic order, which would hamper a comparison between similar species. However, based on the current investigation some regroupings were necessary as well as the addition of some characters to refine both groups. The traits “size” (in both groups from small to large), “normal pores” (in all species of sieve type and roundish) and “central muscle scar pattern” (in all species generotypic; also the upper mandibular scar throughout round to elongate-oval) have no significance for this grouping.
4.3.1. Nominal species and emended characteristics of the “smooth” Cyprideis group
Species (generic assignment sensu Whatley et al. 1998; alphabetic order; *not among the current material): Cyprideis amazonica Purper, 1979 ; * Cyprideis caraionae Purper & Pinto, 1985 ; Cyprideis kotzianae ( Purper & Ornellas, 1991) ; Cyprideis kroemmelbeini ( Purper, 1979) ; Cyprideis machadoi ( Purper, 1979) [syn. Otarocyprideis elegans Sheppard & Bate, 1980 ;?syn. * Cyprideis truncata Purper, 1979 ; re-examination pending]; Cyprideis multiradiata ( Purper, 1979) ; Cyprideis olivencai ( Purper, 1979) ; Cyprideis paralela ( Purper, 1979) [?syn. * Cyprideis posterocompressus ( Purper & Pinto, 1983) ; re-examination pending]; Cyprideis simplex ( Sheppard & Bate, 1980) ; questionably: * Cyprideis obliquosulcata Muñoz-Torres, Whatley & Van Harten, 1998 .
Characters. Shape: subovate, elongated-ovate or subtrapezoidal. Ornament: smooth (except normal pore openings); asulcate (except * C. obliquosulcata ). Marginal spines/denticles: without marginal spines (except C. amazonica and juveniles of C. machadoi plus * C. caraionae and * C. obliquosulcata ). Inner lamella: very to moderately wide; all with vestibulum (except C. simplex , * C. obliquosulcata and * C. caraionae according to original description). Marginal pore canals (anterior): short to long; basically bifurcated or polyfurcated, some simple. Hinge: generotypic; some with very short median element ( C. multiradiata ); sometimes ( C. kotzianae ) or constantly ( C. kroemmelbeini , C. paralela , C. simplex ) inverse hinges.
4.3.2. Nominal species and emended characteristics of the “ornate” Cyprideis group
Species (generic assignment sensu Whatley et al. 1998; alphabetic order; *not among the current material): Cyprideis curucae nom. nov. [= Sohnicythere tuberculata Purper & Pinto, 1983 ; syn. Cyprideis sp. 1 –2 of Muñoz- Torres et al. 1998 = Cyprideis sp. 2 –3 of Whatley et al. 1998]; Cyprideis cyrtoma Muñoz-Torres, Whatley & Van Harten, 1998 ; Cyprideis graciosa ( Purper, 1979) ; Cyprideis inversa ( Purper & Pinto, 1983) ; Cyprideis ituiae n. sp.; * Cyprideis longispina ( Purper, 1979) ; Cyprideis matorae n. sp. [?syn. Cyprideis sp. 3 of Linhares et al. 2011]; Cyprideis minipunctata ( Purper & Ornellas, 1991) [?junior syn. Cyprideis purperi purperi Sheppard & Bate, 1980 ; re-examination pending]; Cyprideis munoztorresi nom. nov. [= Cyprideis lacrimata Muñoz-Torres, Whatley & Van Harten, 1998 sensu Ramos 2006 ]; Cyprideis pebasae ( Purper, 1979) [syn. Cyprideis lacrimata Muñoz-Torres, Whatley & Van Harten, 1998 ]; Cyprideis reticulopunctata ( Purper, 1979) ; Cyprideis schedogymnos Muñoz-Torres, Whatley & Van Harten, 1998 ; Cyprideis sulcosigmoidalis ( Purper, 1979) [syn. Cyprideis aulakos Muñoz-Torres, Whatley & Van Harten, 1998 ; pars syn. Cyprideis purperi purperi Sheppard & Bate, 1980 ]; probably: * Cyprideis anterospinosa Purper & Ornellas, 1991 ; * Cyprideis krsticae Purper & Pinto, 1985 ; * Cyprideis marginuspinosa ( Purper & Ornellas, 1991) , * Cyprideis retrobispinosa Purper & Pinto, 1983 [for all re-examination pending]; status unclear: * Cyprideis purperi colombiaensis Sheppard & Bate, 1980 ; re-examination pending].
Characters. Shape: subrectangular, subordinately subtriangular, subtrapezoidal or subovate. Ornament: punctate to reticulate; some with reduced ornament (* C. krsticae , C. schedogymnos , variants of C. cyrtoma and C. sulcosigmoidalis ); mainly sulcate. Marginal spines/denticles: essentially all with anteromarginal spines/denticles on both valves (in C. sulcosigmoidalis sometimes reduced); all with posteroventral spines in right valves (except C. inversa , * C. krsticae and * C. retrobispinosa ; due to reversed overlap in left valves); posteroventral spines in both valves in some species ( C. graciosa , C. matorae , C. reticulopunctata ). Inner lamella: moderately wide (except C. curucae where it is rather wide); all avestibulate. Marginal pore canals: simple, bifurcate (never polyfurcate). Hinge: generotypic; sometimes inverse hinges ( C. inversa plus * C. krsticae , * C. retrobispinosa ).
4.4. Western Amazonian Cyprideis species subgroups
Several “sublineages” were suggested by Whatley et al. (1998), later modified by Muñoz-Torres et al. (2006; Fig. 3a View FIGURE 3 ).
“Ornate lineage”: C. sulcosigmoidalis gave rise to the following subgroups: i) C. longispina , C. minipunctata and Cyprideis sp. 5 (the latter never described or illustrated); ii) C. graciosa , C. lacrimata , C. pebasae , C. retrobispinosa , Cyprideis sp. 1 , iii) C. inversa , Cyprideis sp. 2 ; and, iv) C. cyrtoma , Cyprideis sp. 4 (the latter never described or illustrated).
“Smooth lineage”: From C. machadoi evolved: i) C. caraionae , C. amazonica ; ii) C. aulakos , C. schedogymnos , C. obliquosulcata , Cyprideis sp. 3 (the latter never described or illustrated); and, iii) C. olivencai .
Due to the current taxonomic revision, these sublineages are challenged (for details see taxonomic treatment of species in chapters 4.5. and 4.6.). For instance: C. aulakos (= synonym to C. sulcosigmoidalis ) and C. schedogymnos (very similar to C. cyrtoma ) are transferred to the “ornate” group here. C. olivencai sensu Muñoz- Torres et al. (1998) and sensu Whatley et al. (1998) comprises the valid taxa C. multiradiata , C. paralela and C. simplex . Among C. machadoi , C. kotzianae and C. kroemmelbeini were included, which are valid species; and, C. kroemmelbeini is virtually much closer to C. olivencai . Cyprideis sp. 1 (placed in the “ graciosa ” sublineage) and Cyprideis sp. 2 of Muñoz-Torres et al. (1998; referred to the “ inversa ” sublineage) actually belong to the same species ( C. curucae ). While C. lacrimata of Muñoz-Torres et al. (1998) and Whatley et al. (1998) is synonymous with C. pebasae , C. pebasae Muñoz-Torres et al. (1998) and sensu Whatley et al. (1998) is a discrete species, renamed here as C. munoztorresi . Apparently, these taxonomic changes affect not only possible phylogenetic relations but also the biostratigraphic concept of Muñoz-Torres et al. (2006; see chapter 5.1.).
As far as the current stratigraphy is settled down, core 1AS-10-AM comprises only late Middle and, possibly, early Late Miocene sediments (see chapter 5.1.). Thus, a short time-range (probably <2 Ma) of the entire evolutionary history of Amazonian Cyprideis is covered here, which hampers phylogenetic considerations. Hence, a revision of the currently available phylogeny for Amazonian Cyprideis is beyond our possibilities. Nevertheless, we decided to arrange the found species into (sub-)groups, exclusively based on their morphologic similarity ( Fig. 3b View FIGURE 3 ). The “smooth” group comprises the amazonica , machadoi , olivencai and paralela subgroups, and the “ornate” Cyprideis species the cyrtoma , graciosa and pebasae subgroups. Among the “smooth” taxa, C. multiradiata as well as among the “ornate” species C. inversa and C. sulcosigmoidalis were not attributed to subgroups due to their impartial morphology.
4.5. “Smooth” Cyprideis group
4.5.1. amazonica subgroup
Species. C. amazonica and C. caraionae (the latter not among the present material).
Characters. Subovate; medium sized; smooth, asulcate; with or without anteromarginal denticles, short posteroventral denticles in right valves; moderately wide inner lamella, narrow anterior vestibulum; anterior simple and some bifurcated marginal pore canals; hinge with short antero- and long posteromedian element.
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.
|
Kingdom |
|
|
Phylum |
|
|
Class |
|
|
Order |
|
|
Family |