Daphnia hrbaceki, Juračka, Petr Jan, Kořínek, Vladimír & Petrusek, Adam, 2010
|
publication ID |
https://doi.org/ 10.5281/zenodo.199782 |
|
DOI |
https://doi.org/10.5281/zenodo.5623697 |
|
persistent identifier |
https://treatment.plazi.org/id/0D62F421-907A-FFE6-FF27-F9EAFDFA56FE |
|
treatment provided by |
Plazi |
|
scientific name |
Daphnia hrbaceki |
| status |
sp. nov. |
Daphnia hrbaceki sp. nov.
(Figs 1–8)
Etymology. The new species is dedicated to the eminent Czech hydrobiologist Jaroslav Hrbáček (1921–2010), who initiated complex ecological studies of Daphnia populations in the former Czechoslovakia. The name in the Czech language also reflects the hunched body shape of some individuals.
Type locality. A small fishless, recently (2004) excavated pool in the valley Český příkop (protected landscape area Kokořínsko, Czech Republic); N 50°28'54", E 14°41'10", alt. 289 m above sea level. The pool is 7 m long and 3 m wide with maximal depth ca. 2 m, situated in a deep, shaded valley with a cold microclimate. The type series was collected on 5 November 2007 by P. J. Juračka.
Holotype. Adult parthenogenetic female (total body length 1.7 mm) mounted in Canada balsam and stained with a mixture of lignin pink and chlorazol black E; Natural History Museum, London (NHM 2010.39).
Allotype. Adult male (body length without shell spine 1.0 mm) mounted and stained as above (NHM 2010.40).
Paratypes. Males and females (45 specimens), preserved in 96% ethanol and a small amount of glycerol (NHM 2010.53-62). Additional specimens from the type series are deposited in the collection of the National Museum, Prague (P6E3005).
Ephippial female (total body length 1.5 mm) stained and mounted as above (NHM 2010.41).
Dissected parthenogenetic female treated with hot 10% potassium hydroxide and mounted as above (NHM 2010.42).
Females and males (13 specimens) stained and mounted as above (NHM 2010.43-52).
Diagnosis. Parthenogenetic female with median keel on head shield, some populations with induced necktooth on its posterior margin. Similar neckteeth may be present in juveniles and males. Antennule completely reduced, median mound strongly vaulted with reticulated apex. Ocellus pigmented. Shallow cervical depression. Shell spine short or absent. Gnathobase of second thoracic limb extended distally into angular projection. Postabdominal claw with second (middle) pecten of spinules or teeth of variable size and shape: either spinules slightly longer than those in proximal pecten, or large teeth longer than width of claw.
Ephippium saddle-shaped, dorsal ridge smooth (without spinules), only reticulated; posterior carapace margin included into ephippium. Ephippial surface ultrastructure with many minute pits surrounded by fine lamellae.
Male with medium-sized rostrum hardly covering antennular socket. Antennule short, two to three times longer than wide. One of the three terminal setae on 2nd endopodite bent and heavily setulated. Pre-anal margin of postabdomen weakly depressed, anal margin convex.
Size. Total body length (without shell spine): parthenogenetic female 1.0– 1.7 mm; ephippial female 1.2–2.0 mm; male 0.9–1.2 mm.
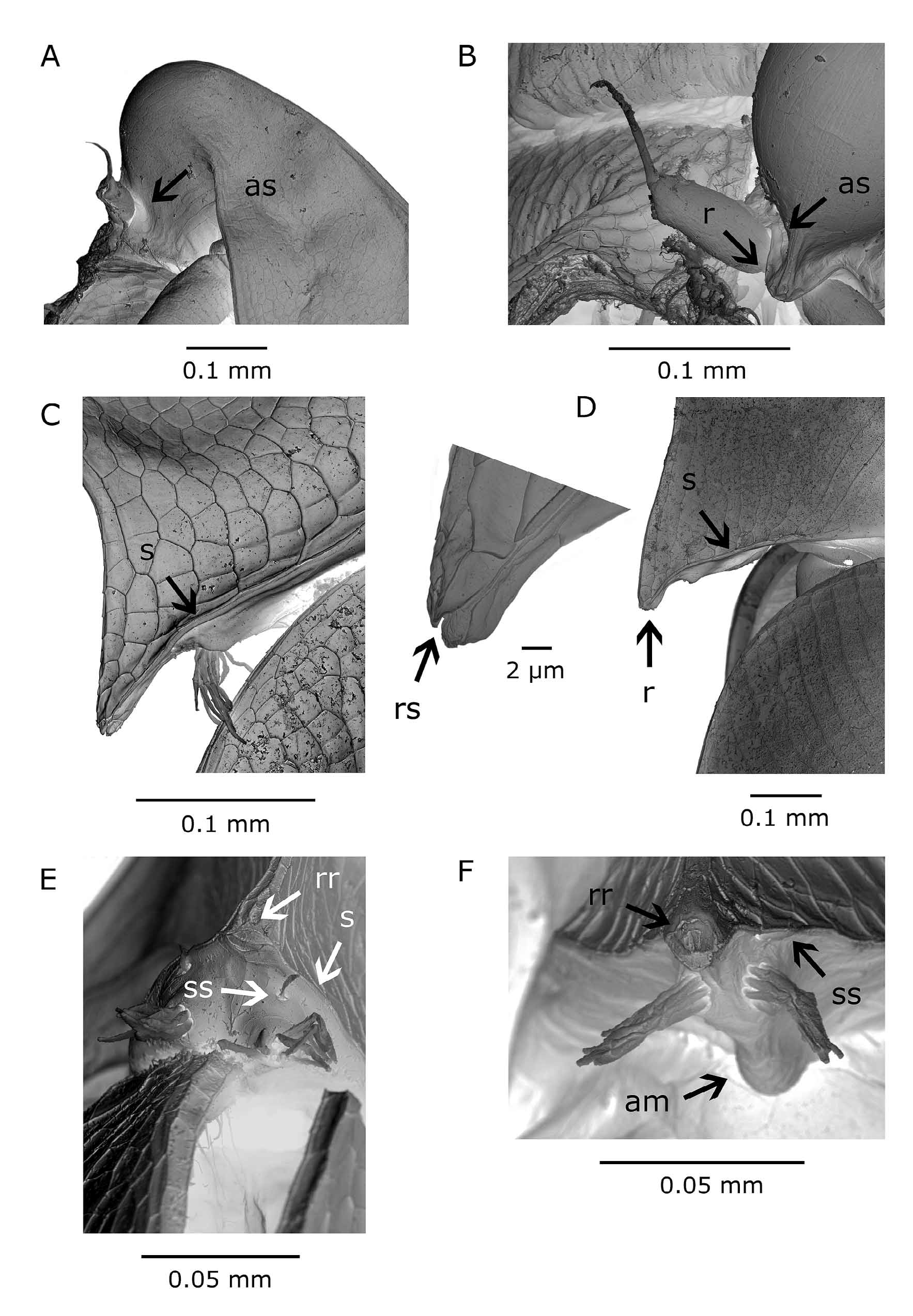
Description. Parthenogenetic female. Head: high, strongly vaulted apical part with median keel increasing in width dorsally. Keel extremely developed in some individuals; forming hump-shaped structure (Figs. 1C, E; 2B, F). Neckteeth rarely present in adult females (Figs. 1E; 2B, E). Dorsal margin with shallow cervical depression (Fig. 1B). Frontal contour of head concave above rostrum. Rostrum not prominent, its tip bent ventrally in some specimens. Tip of rostrum obtusely rounded and split into two lobes by suture or line between head shield and ventral side of head in lateral aspect ( Fig. 4 View FIGURE 4 C, E). Mid-antennular mound well developed, markedly reticulated on apex. Optic vesicle contiguous with frontal part of head. Ocellus pigmented. Fornix rounded at base of second antenna.
Antennule: not protruding, its body reduced, seen as lateral areole on median mound with 9 sensory setae; single lateral seta anterior to areole ( Fig. 4 View FIGURE 4 C, E).
Antenna: Setal formula of natatory setae: 0-0-1-3/1-1-3. Presumably sensorial setae and spinules: two setae on concertina-like basal joint, one apical spine-like on its outer side, one seta on inner side between both branches, one apical spinule on dorsal margin of second segment 4-segmented branch. Dark rings at base of distal part of swimming setae may be present in some individuals or populations. Surface of all segments covered with transversal groups of small teeth.
First maxilla: carrying three robust, curved and heavily setulated setae and one short stump-like distal seta.
Carapace: approximately sub-ovoid, length of posterior spine variable, forming up to 15% of body length (without shell spine) or completely reduced. Spinules on ventral margin cover 1/3 to 2/3 of its length, spinulation on dorsal margin developed only in posterior 1/4 of margin or only near posterior spine. Spinulation of dorsal margin completely missing in some individuals. Fringe of sub-marginal setae absent.
Thoracic limbs: agree with the re-description of Daphnia curvirostris in Ishida et al. (2006) with the exception of 2nd limb gnathobase, which extends in front of longest clearing seta into noticeable rectangular corner or small lobe (Fig. 5E, F).
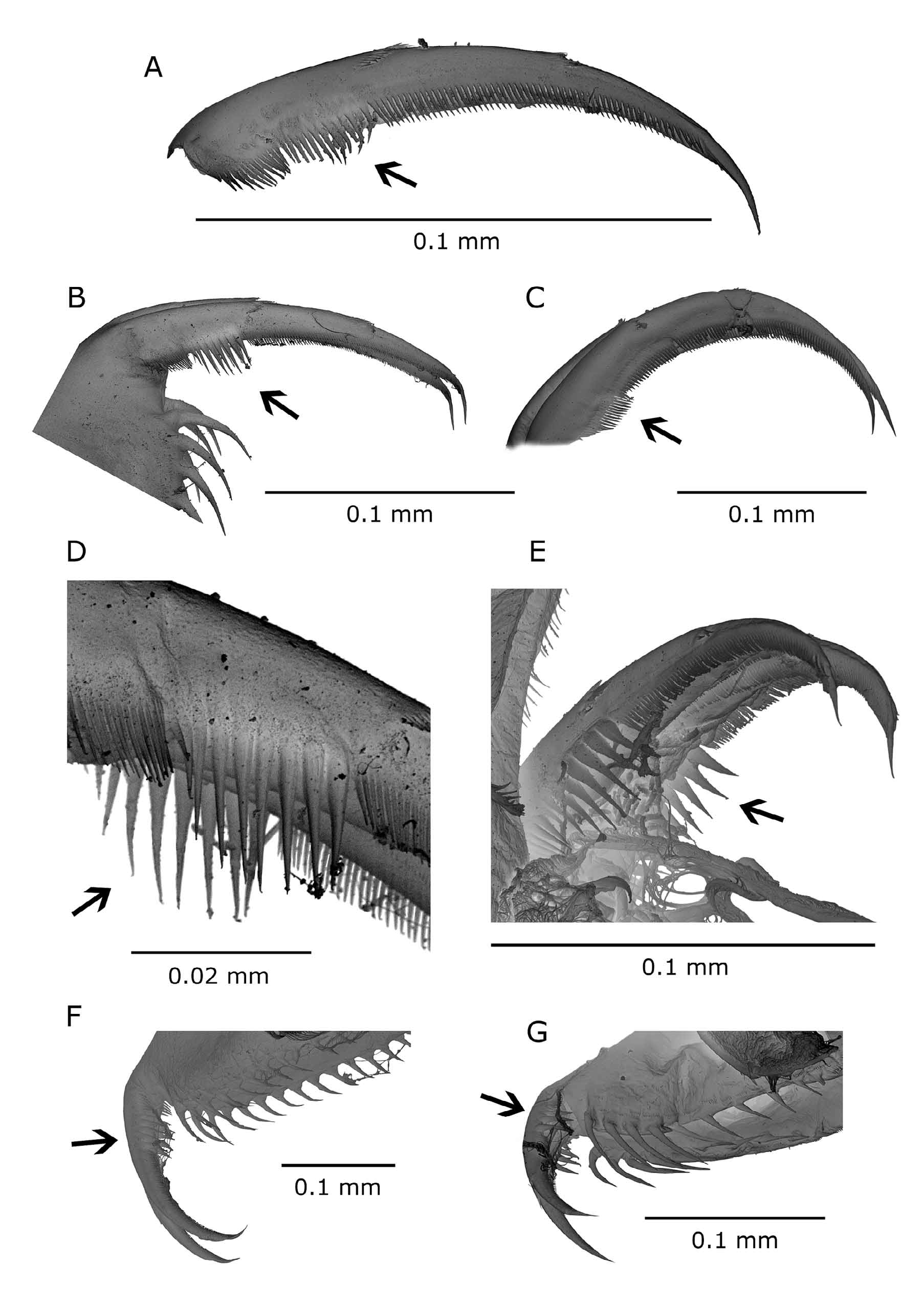
Postabdomen: elongated, tapering distally, pre-anal face even, covered with scattered groups of fine spinules, anal margin slightly convex, fringed with up to 15 strong teeth that increase in length distally. Distal portion of postabdominal setae slightly shorter than proximal one. Abdominal processes gradually diminishing distally, first twice as long as second, third reduced to 1/3 up to 1/2 of second one in specimens preserved in formalin. Terminal claw long, with three groups (pectens) of teeth and spinules. Proximal one of 13–19 minute spinules, middle pecten variable in size: either 8–9 large teeth markedly longer than width of claw or 11–13 spinules that only slightly exceed in length those of other two pectens. Distal row of about 60 fine spinules, not reaching tip of claw. Differences in size and length of claw spinules were observed among samples collected in different times of season, and between individuals from the wild and those cultured in laboratory ( Fig. 3 View FIGURE 3 A–D).
FIGURE 1. Daphnia hrbaceki . A. Adult male (K). B. Adult parthenogenetic female (K). C. Adult ephippial female (K). D. Adult male (RB) with necktooth indicated by arrow. E. Adult parthenogenetic female (RB) with morphology presumably induced by invertebrate predators; arrow indicates a hump-shaped dorsal outline of the carapace. F. Adult male (RB), hook-like apical seta (2nd limb) indicated by arrow. G. Adult male (RB), postabdomen (contrast increased at gonopore area); arrows indicate gonopore (g) and middle pecten on postabdominal claw (p).
FIGURE 5. Daphnia hrbaceki and Daphnia curvirostris . A. D. hrbaceki , adult male (RB); antennules (A1) and rostrum (r), arrow (fr) indicates valves fringed with row of long, sub-marginal feathered setae. B. D. curvirostris , adult male, arrows as in Fig. 5 A (LL). C. D. hrbaceki , adult male (K); antennule (indicated by arrow). D. D. curvirostris , adult male (KP); antennule (indicated by arrow). E, F. D. hrbaceki , adult females (RB); 2nd thoracic limb, gnathobase, arrows indicate gnathobase extending distally into angular projection.
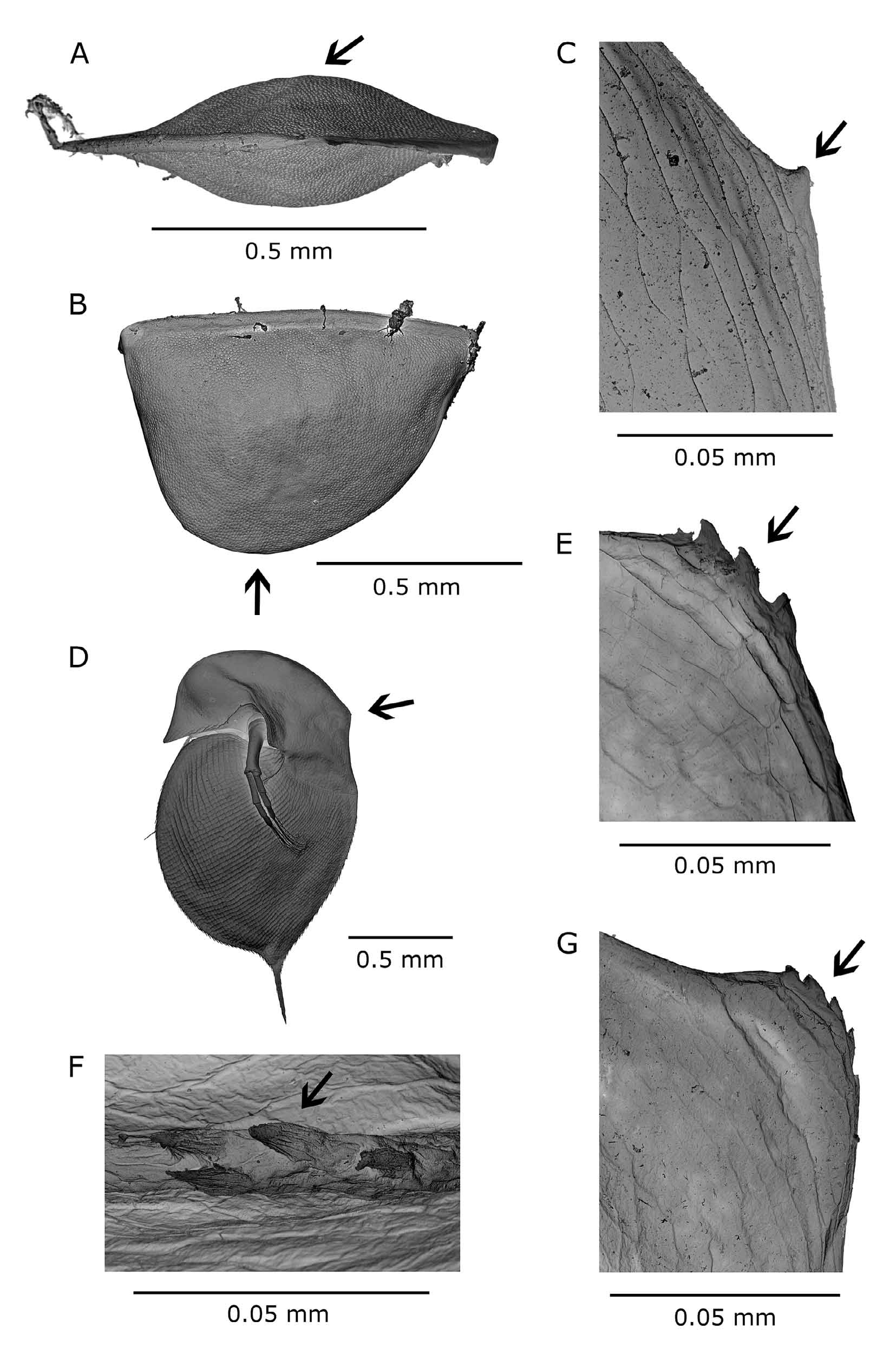
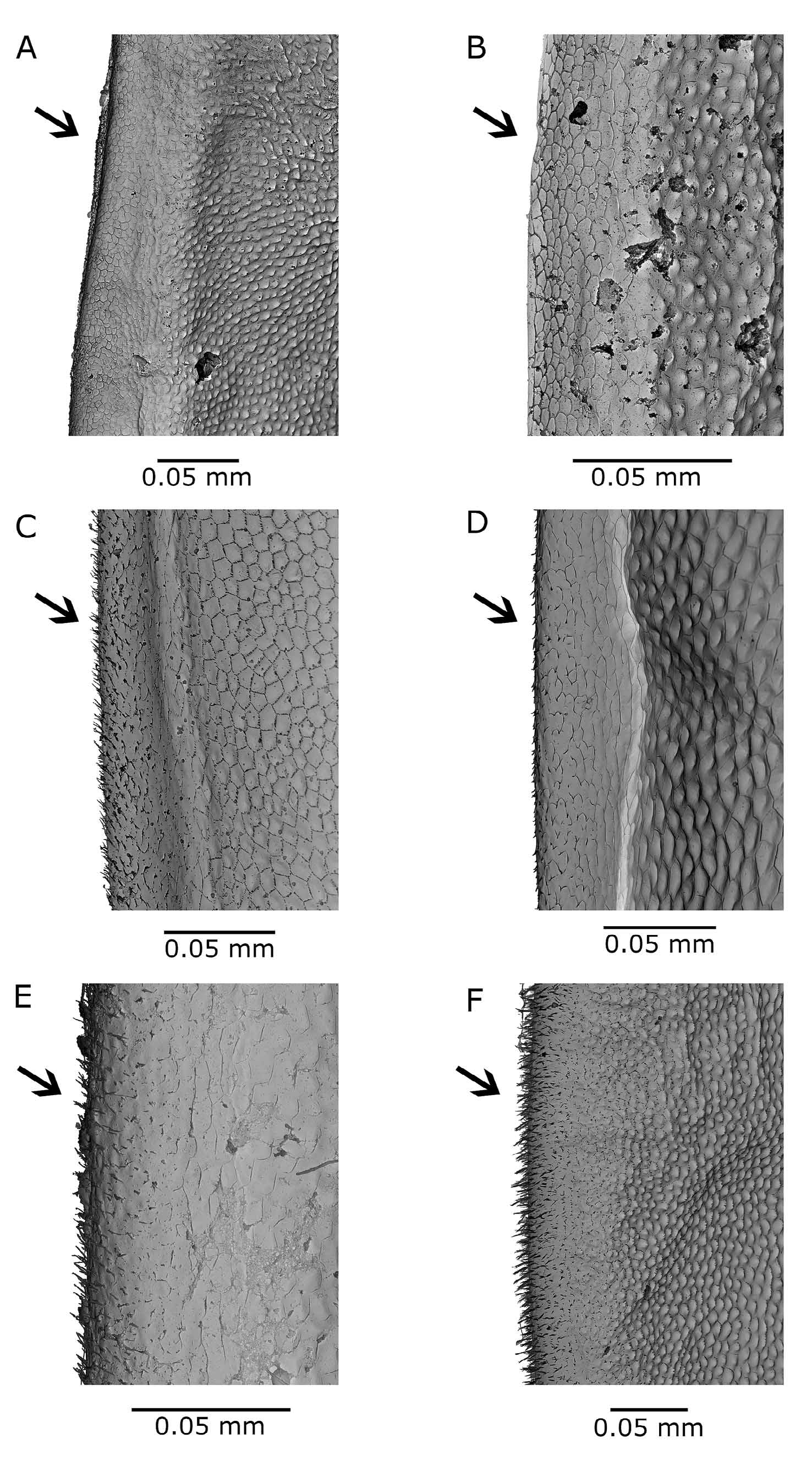
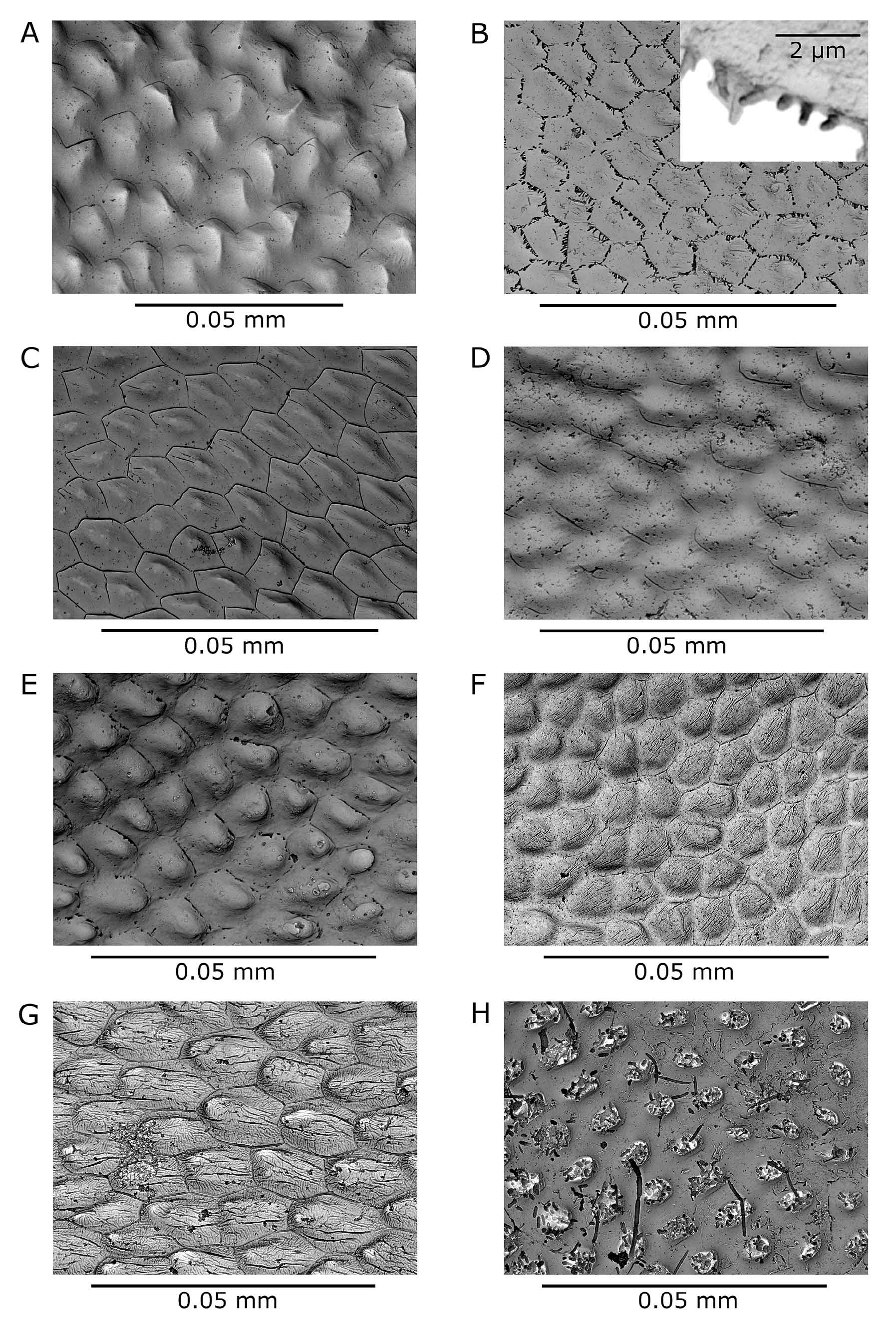
Ephippial female. Dorso-posterior part of head shield swollen, forming bulge over dorsal suture between carapace and head shield (Figs. 1C, 2G). Ephippial surface covered with sclerotized pneumatic cells reaching up to postero-dorsal angle of shell without any gap. Two resting eggs perpendicular to dorsal margin, egg chambers well separated from each other. Free post-molting ephippium ( Fig. 6 View FIGURE 6 A, B) asymmetrically saddle-shaped, with maximal width between centre and proximal third of its length. Dorsal ridge without any spinescence, only reticulated ( Fig. 7 View FIGURE 7 A, B). Postero-dorsal corner includes part of vaulted posterior margin and remnants of short shell spine, which is lost in older, freely floating ephippia. Surface ultrastructure with many minute pits surrounded by fine lamellae ( Fig. 8 View FIGURE 8 A).
Male. Head: rounded in frontal part around optic vesicle, apical contour only feebly convex, gradually descending dorsally to level of attachment of posterior antennal muscle or to necktooth (if present) (Figs. 1A, 2A, 4A). Compound eye large, filling half of frontal portion of head shield, ocellus pigmented. Obtuse rostrum short, covering only antennular socket. Antennular (ventral) part of head extends ventrally forming posterior wall of antennular sockets ( Fig. 4 View FIGURE 4 A, B).
Antennule: in adult males directed towards compound eye, its segment short, two to three times longer than wide, reaching hardly to pigmented part of compound eye. Flagellum inserted on conical butt elevated over shallow socket for sensory papillae. Dorsal seta inserted distally at about four fifths of antennular length ( Figs. 4 View FIGURE 4 B, 5C).
Antenna: surface sculpture of all segments weaker than in female.
Carapace: ventral aspect: wide anterior gap between valves fringed with row of long, sub-marginal feathered setae. Setae most densely spaced along anterior fold of valves, gradually shortened to mid carapace margin (Fig. 5A). No gap or sub-marginal setae at distal part of ventral margin, only small marginal spines and groups of submarginal setules present. Dorsal margin feebly convex.
Thoracic limbs conform with the description of Daphnia curvirostris male in Ishida et al. (2006). Hook-like seta of 2nd limb is shown in Fig. 1F.
Postabdomen: all abdominal processes reduced, proximal one very small, others mostly missing. Pre-anal part with shallow depression, anal margin convex, fringed with up to 12 lateral spines (Fig. 1D). Gonopores open ventrally of last three largest marginal spines (Fig. 1G). Distal part of postabdominal setae slightly shorter than their proximal part. Middle pecten on terminal claw with either 6–7 spines or 10–12 spinules ( Fig. 3 View FIGURE 3 E).
Differential diagnosis. The new species has to be differentiated from several other taxa present in the region of its occurrence: Daphnia curvirostris , members of the Daphnia pulex group, and Daphnia longispina (O. F. Müller, 1776) , as well as related taxa in Asia and two taxa showing some similarities in North America. The main differential characters are listed in Tab. 2 View TABLE 2 . Among locally occurring species, females in the D. pulex group are clearly distinguished by well developed antennules protruding from the antennular mound which contrast with the reduced, non-protruding antennule of Daphnia hrbaceki . Daphnia longispina has a flat, reduced inter-antennular mound, but parthenogenetic females in some of the populations are difficult to distinguish from those of D. hrbaceki that do not have enlarged middle pecten of the postabdominal claw. The Daphnia longispina ephippium is also widest in the anterior third of its length, its dorsal ridge covered with spinules and a shell spine always part of the free ephippium compared with the smooth dorsal ridge of the Daphnia hrbaceki ephippium whose greatest width is about mid-length. The apical stiff seta on the male second endopodite of Daphnia longispina is S-shaped, armed with a row of robust teeth or thorns; that of D. hrbaceki is hook-like (Fig. 1F), its distal part fringed on both margins with dense rows of spinules. The Daphnia curvirostris ephippium is asymmetrically saddle-shaped and widest at the proximal third of its length, with the dorsal ridge covered densely with minute spinules ( Fig. 8 View FIGURE 8 B). Ephippial surface covered with small pits framed with rows of small, blunt spinules ( Fig. 8 View FIGURE 8 B). D. curvirostris males have a longer basal segment of the antennule reaching nearly to the anterior margin of the pigmented part of the compound eye (Fig. 5D), whereas this reaches only the posterior contour of the eye in D. hrbaceki (Fig. 5C).
Two other related species have been described from eastern Asia ( Japan and the Russian Far East): Daphnia tanakai and Daphnia sinevi . The ephippium of Daphnia tanakai does not include the carapace posterior margin; the postero-dorsal corner of the ephippium is obtusely rounded. The ephippial dorsal ridge is covered with sparsely distributed fine spinules ( Fig. 7 View FIGURE 7 D). A wide gap is present between ephippial surfaces (covered with large sclerotized cells) and the posterior margin of carapace, separated by ecdysial suture. Ephippial surfaces are covered with shallow dimples and a pattern of hexagonal fine lamellae ( Fig. 8 View FIGURE 8 C). Males have a reduced rostrum and a long antennule. Daphnia sinevi has a robust inter-antennular mound with slightly protruding tips of antennules. The from small, fishless habitats), Daphnia minnehaha and Daphnia sp. (morphotype FLO9, denoted as D. arenata in some studies).
reaching over up to insertion of insertion of insertion of postabdominal postabdominal setae postabdomin setae al setae
ephippium is saddle-shaped, widest at the posterior third of its length, its dorsal ridge with fine spinules. The postero-dorsal corner of the ephippium is horn-shaped, not rounded. Male has long, slender antennule.
The body shape of some individuals of D. hrbaceki may superficially resemble Daphnia minnehaha ( Fig. 6 View FIGURE 6 E) and Daphnia sp. (morphotype FLO9, denoted as D. arenata in some studies) occurring on the North American continent. Both species have antennular tips partly protruding from the base of the head shield, and individuals of the FLO9 morphotype carry a row of sub-marginal plumose setae similar to those in the D. obtusa complex or in the subgenus Ctenodaphnia (this characteristic of the American taxon was omitted in Hebert 1995); such a row of plumose setae is not observed in Daphnia hrbaceki and D. curvirostris . Dorsal ridges of ephippia of both American species are covered with spinules ( Fig. 7 View FIGURE 7 E, F) in contrast to the smooth reticulated dorsal ridge of D. hrbaceki . Males of American species have a deep depression in the pre-anal part of the postabdomen contrasting with the even or slightly convex anal region in D. hrbaceki .
Other material examined. Daphnia hrbaceki : Czech Republic, Kokořínsko, small pool (N 50°29'11", E 14°41'24"), 13 July 2006, P. J. Juračka legit. Daphnia cf. hrbaceki : Slovakia, Rimavská Baňa, (48.5° N, 19.9° E) fluvial pool, 27 April 1951, O. Albertová legit. The first author sampled most pools in the vicinity of Rimavská Baňa village recently (three times in 2006–7) but without success. In the original sample from the mid 20th century, no other Daphnia species was present.
Distribution. So far, Daphnia hrbaceki has only been found in two isolated pools in Central Bohemia and at another locality in south-eastern Slovakia (for the Slovak sample, no DNA data is available). Apart from the type locality, the species was found in a similar pool created in 1999, located about 500 m away. Cladoceran fauna of the region where D. hrbaceki was discovered had been studied for at least one century. The species is thus certainly very rare and it is difficult to judge the area of its distribution. However, other populations may have escaped detection (being confused with D. curvirostris or other species) if individuals did not exhibit the characteristic hump-shaped body profile.
Ecology. The species was sampled in the summer zooplankton and survived up to the beginning of winter. It was outcompeted in spring by co-occurring Daphnia obtusa . Both species coexisted in summer. Summer water conditions: conductivity fluctuated within the range 39–768 μS.cm-1; pH 5.7–7.8; temperature up to 17.4 °C; dissolved oxygen 1.8–10.4 mg.l -1. The species was successfully cultivated in the laboratory on a diet of green algae (mostly Scenedesmus ).
Genetic analyses. All analysed mitochondrial genes of the analysed Czech Daphnia clearly showed a considerable divergence from all other so-far genetically characterised species in the genus: the genetically most similar species, Daphnia curvirostris , diverged by 13% at 12S, 23% at COI, and 41% at ND2 (all Kimura 2- parameter distances); other analyzed species, including all other known members of the D. curvirostris complex, diverged substantially more (over 46.8% at ND2; Fig. 9 View FIGURE 9 ). The divergence of the syntopically occurring D. obtusa (belonging to the D. pulex group) from D. hrbaceki exceeded 63% at ND2. No variation in sequences of any of the three mitochondrial genes was observed in several analysed individuals of D. hrbaceki .
The GTR+I+G model of nucleotide substitution consistently performed best among the different approaches to model selection, based on the 932 bp long alignment of ND2 sequences. All applied methods of phylogenetic reconstructions supported the sister relationship between the new species and D. curvirostris despite their relatively high divergence. The support for monophyly of the D. curvirostris complex was weaker but the whole complex was unambiguously assigned as a sister taxon of the D. longispina complex ( Fig. 9 View FIGURE 9 ).
Taxonomic and nomenclatural comments. Daphnia hrbaceki could be characterized both morphologically and genetically. Its morphological peculiarities have been known for more than fifty years, but difficult to evaluate as there was only a single sample from Slovakia available. The recent discovery of populations in Central Bohemia allowed DNA analyses and a comparison of both morphology and genetics with recently described East Asian taxa. The morphological diagnosis of the species and its membership within the D. curvirostris complex were thus substantiated.
Comparison with other taxa described over century ago from Japan ( Daphnia whitmani Ishikawa, 1895 and Daphnia morsei Ishikawa, 1895 ) is difficult as the original drawings are inadequate and the descriptions do not mention some important characters. For instance, the ephippium of D. whitmani is traced as not reaching to the posterior margin of carapace in Fig. 4 View FIGURE 4 in Ishikawa (1895), but clearly incorporating it in Fig. 4 View FIGURE 4 b in the same work. In general, D. whitmani seems to be similar to the recently described Daphnia sinevi . The male of D. morsei has a remarkably deep depression of the pre-anal or anal part of the postabdomen. A genetically clearly divergent Daphnia population found recently in Japan may have belonged to this taxon (Kotov et al. 2006). Recent genetic analysis ( Kotov & Taylor 2010) nevertheless suggested that the above-mentioned taxa described by Ishikawa likely belong to the D. pulex group and are therefore unrelated to the D. curvirostris complex.
Both American species mentioned in the differential diagnosis are in great need of re-description. Hebert (1995) documented some of their morphology on his CD-ROM on North American Daphnia fauna. While Daphnia minnehaha was described by Herrick (1884) according to the rules applied in the time of publication and the use of this name is not in doubt, the description of Daphnia arenata is lacking some of the attributes required by the International Code of Zoological Nomenclature. No types were designated, the description contained neither a short diagnosis nor differential diagnosis, and the text of the description itself was substituted by a set of microphotographs illustrating selected morphological characters. Coastal pond #9 at Florence (Oregon) was designated the type locality. The name Daphnia arenata has already been used in other regular publications (e.g., Colbourne et al. 1997; Benzie 2005; Mergeay et al. 2008). This situation clearly suggests that the name has to be considered a nomen nudum. The problem with the nomenclature of several North American taxa first named in Hebert (1995) is discussed in details in Benzie (2005). Therefore we prefer to label our comparative material as Daphnia sp. (morphotype FLO9).
TABLE 2. Main differential morphological characters among Daphnia hrbaceki, Daphnia curvirostris, Daphnia tanakai, Daphnia sinevi, Daphnia longispina (populations
| Character | D. hrbaceki sp. nov. | D. curvirostris | D. tanakai | D. sinevi | D. longispina | D. pulex | D. minnehaha | morphotype FLO9 |
|---|---|---|---|---|---|---|---|---|
| Female head documented ability to produce neckteeth (Fig. 6) | yes | yes(rarely) | no | yes | yes | yes | yes | yes |
| antennule body (Fig. 4) carapace | reduced | reduced | reduced | reduced | reduced | prominent | slightly prominent | slightly prominent |
| sub-marginal row of long | absent | absent | absent | absent | absent | absent | absent | present |
| setae thoracic limbs 2 nd gnathobase-posterior margin (Fig. 5) | extended, keel-like | rounded | rounded | rounded | rounded | rounded | rounded | rounded |
| postabdomen - terminal claw middle pecten (Fig. 3) ephippium postero-dorsal portion of carapace incorporated in ephippium | large teeth or fine spinules yes | large teeth yes | large teeth of fine spinules not | large teeth yes | fine setules yes | large teeth yes | large teeth yes | large teeth yes |
| dorsal spinescence (Fig. 7) surface ultrastructure (Fig. 8) | absent minute pits surrounded by fine lamellae | present minute pits surrounded by rows of blunt spinules | present shallow dimples with remnants of longitudinal striation | present not studied | present variable | present dimples surrounded with well developed lamellae | present shallow pits surrounded by toothed lamellae | present shallow dimples with remnants of surrounding lamellae |
| Male 2 nd limb, terminal seta on last endite distal portion postabdomen dorsal margin, anal region 1st postabdominal processes | bent setulated even reduced | bent setulated even reduced | bent,but variable setulated slightly convex reduced | strongly bent setulated slightly convex reduced | not bent with strong teeth even reduced | bent setulated shallow depression long, | bent setulated deep depression short, reaching | robust. slightly bent setulated deep depression short, reaching up to |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.