Fridericia bulbosa ( Rosa, 1887 )
|
publication ID |
https://doi.org/ 10.1080/00222933.2015.1009514 |
|
DOI |
https://doi.org/10.5281/zenodo.4330409 |
|
persistent identifier |
https://treatment.plazi.org/id/039AAD67-FFFE-4513-FE2B-FC37FC420A5E |
|
treatment provided by |
Carolina |
|
scientific name |
Fridericia bulbosa ( Rosa, 1887 ) |
| status |
sensu stricto |
Fridericia bulbosa ( Rosa, 1887) sensu stricto
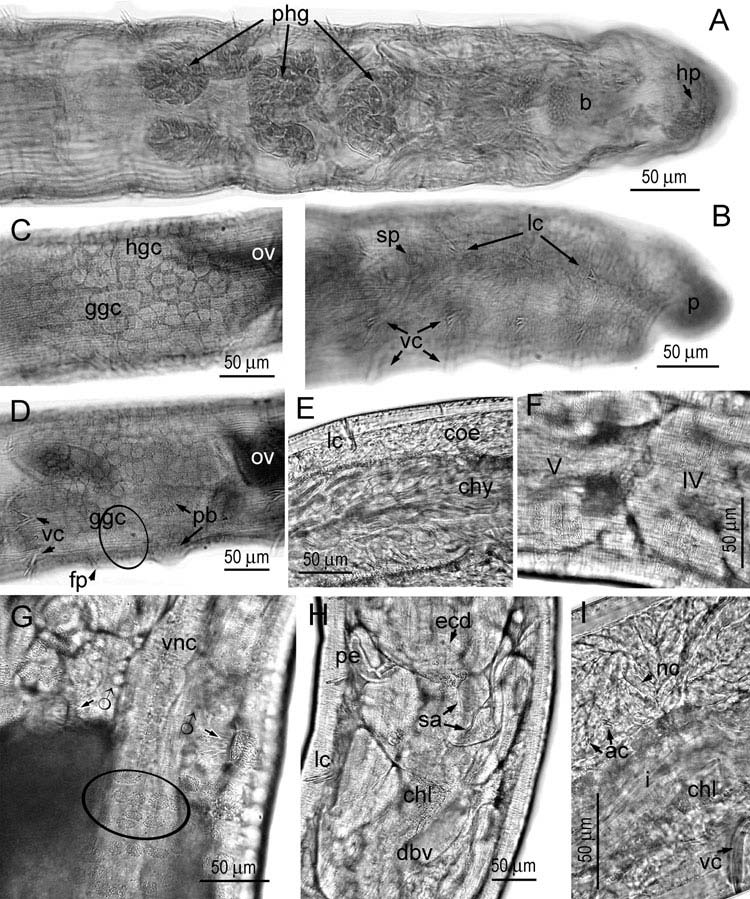
( Figure 8 View Figure 8 ; Table 2)
Neoenchytraeus bulbosus Rosa, 1887, p. 2 View in CoL .
Fridericia bulbosa ( Rosa, 1887) sensu stricto, Rota 1995, pp. 204–205; Rota et al. 2013, table 1 and figure 3 (species ‘s18’); Rota et al. 2014, tables 1, 2 and Suppl. 1.
Material examined
Published material. Specimens from Tuscany, Calabria and Sicily ( Rota 1995).
New material (in the author’ s collection). Some 50 specimens from Italy, Tuscany (Tu-7), plot S1 , 24.04.2009 and 05.11.2009 , and a total of about 400 specimens from Tuscany (Tu-5), 24.04.2009 and 05.11.2009 . About 75 specimens from Italy, Campania (Ca-2), plots 1 and 3, 13.05.2009 and 26.10.2009 .
Augmented diagnosis
Small worms (5–7 mm long, 0.18 mm wide at V, 0.22–0.26 mm wide at XII), transparent, slow moving. Segments 24–42 (investigation of urban populations has widened the variation range towards small values; see Table 2). Prostomium dorsally depressed in front of head pore ( Figure 8A, B View Figure 8 ). Epidermal gland cells large, rectangular, in 3–5 rows, only some complete ( Figure 8F View Figure 8 ). Clitellar gland cells ( Figure 8C, D, G View Figure 8 ) arranged in a chequered pattern (in fixed specimens the hyaline cells dominate in size and number) except in some ventral areas: between and before male pores, where glands are completely absent, and in posterior ventral part of XII where a field of granular cells occurs. Subneural glands on nerve cord absent. Male slits longitudinal ( Figure 8G View Figure 8 ).
Chaetal formula 2÷4 – 2: 2÷4 – (4,3),2. Behind the clitellum, lateral bundles are generally all bisetose, whereas ventral bundles become bisetose starting from XV– XVII. Size of chaetae in preclitellar lateral bundles one fourth smaller than in ventral ones ( Figure 8B View Figure 8 ) (e.g. in urban populations in Siena and Naples, measuring 24–32 μm in lateral bundles and 29–42 μm in ventral bundles, thickness 2–2.5 μm). Postclitellar chaetae, both lateral and ventral, beginning with minimal sizes in segments XIII– XVII (in urban populations: 26–30 μm long, 2.5 μm thick), then gradually increasing to reach an identical maximum in caudal segments (in urban populations: 40–42 μm long, 3–3.5 μm thick).
Peptonephridia short, unbranched ( Figure 8H View Figure 8 ). Pharyngeal glands ( Figure 8A View Figure 8 ) dorsally merging at 4/5 (widely) and 5/6 (narrowly), dorsally free at 6/7; ventral lobes small in IV, middle-sized in V, as large as dorsal lobes in VI. Five pairs of preclitellar nephridia (6/7–10/11), efferent ducts originating posteroventrally.
Coelomocytes ( Figure 8I View Figure 8 ): nucleated cells hyaline (type a), 32–40 μm long, anucleated small (4–9 μm). Chloragogen cells yellow-gold or yellow-green. Chylus cells in XIII–XIV, XIII–XV or XIV–XV with long intracellular canals ( Figure 8E View Figure 8 ). Ventral intestinal ridge poorly discerned, in one specimen seen in XXII–XXVII. Dorsal blood vessel from XVI–XVII, with four paired lateral commissures to ventral vessel, first two pairs (in III) rising from a common root.
Sperm funnels barrel-shaped, l:w = 2.5:1, in live urban specimens: 75–100 μm long and 43–50 μm wide, collar 2/3 as wide as funnel, covered with long spermatozoa (heads 75 μm long, tails about 100 μm in Naples worms), vas deferens 5–7.5 μm thick (narrower in distal course). Penial bulb oval, soft, 50–80 μm long after fixation. Ovary bush-like ( Figure 8C View Figure 8 ). One egg mature at a time. Spermathecal ectal ducts ( Figure 8H View Figure 8 ) very long (175–225 μm, 15 μm thick in vivo), each with an inconspicuous to small (13–23 μm long), sessile ectal gland; ampullae small and bulb-shaped, 30–32 μm across, containing a circle of sperm, attached close or jointly to one side of gut ( Figure 8H View Figure 8 ).
Remarks
Rosa (1887) described his Neoenchytraeus bulbosus from woodland soil near Turin, in the north of Italy, providing few details but still a precise combination of characters. The new species could be recognized by (i) its relatively small size (4–8 mm, segments 32–42); (ii) chessboard-like clitellum, made of large, irregularly squared, granular cells (‘areole’) on a smooth background (i.e. against the hyaline cells), the latter, however, becoming dominant by size after fixation; (iii) chaetae in bundles of four or three in the anterior half of the body, in couples in the posterior region, the ventral chaetae being longer than the dorsal; (iv) peptonephridia elongate, thin-walled, poorly branched, generally just bifurcated, likened for their simplicity to those illustrated by Vejdovský for F. leydigii ; (v) dorsal vessel originating in XVI–XVIII, with the first two lateral commissures originating from a common root; (vi) spermathecal ampullae bulb-shaped, adiverticulate, with ental portion elongate and firm, and ectal duct long and devoid of glands; (vii) nephridia with subterminal efferent ducts; (viii) coelomocytes medium-sized.
Unfortunately, for long time F. bulbosa has been diagnosed in the absence of an adequate correspondence with Rosa’ s description as detailed above, the species identification being rather based mostly on the possession of adiverticulate, bulbshaped spermathecal ampullae. This resulted in an accumulation of records worldwide and an overrating of intraspecific variation (in 1929 Ude allowed F. bulbosa up to six chaetae per bundle and as many as 70 segments). Nielsen and Christensen (1959) were the first to suspect that the species might have lost its genuine identity, but were inclined to believe that Rosa’ s account concerned a mixture of species or that some characters varied geographically, rather than to accept that Rosa’ s (1887) original account, taken in its entirety, unequivocally matched a taxon occurring in Italy.
In 1995, based on material collected in central and southern Italy, I rectified the morphological diagnosis of F. bulbosa sensu stricto in adhering to the original description ( Rota 1995). Thanks to a recent high-intensity sampling ( Rota et al. 2013, 2014), I could go more in depth and examine the intra- and interpopulation variation on a regional scale (see Table 2), so as to expand the list of consistent features characterizing the Italian taxon. F. bulbosa s.s. now appears to be morphologically fairly homogeneous. The description of the present new material collected in a central and a southern region of Italy agrees with the account of Rosa from northern Italy in virtually all points.
The same account is sufficiently detailed to exclude all the European adiverticulate species that (while yet undescribed) at one stage or another may have been included in F. bulbosa : species with fewer or missing chaetae (e.g. F. benti , F. composti , F. cusanica , F. semisetosa ), as well as those with more than four chaetae or larger body sizes (e.g. F. caprensis , F. tuberosa , F. striata , F. ilvana ), species possessing peptonephridia of type b (e.g. F. bulboides ), and species with large spermathecal glands (e.g. F. bretscheri ). Indeed, at the current level of taxonomic resolution, each of the above-mentioned species differs not in one, but in several critical traits from F. bulbosa in its strict original acception. Thus, Nielsen and Christensen (1959) and other authors’ statements that a clear conception of its morphology has never existed appears incorrect, and Schmelz’ s (2003) proposal to consider F. bulbosa a nomen dubium is not acceptable.
As concerns the presence of spermathecal ectal glands and the shape of peptonephridia, conflicting interpretations can be reconciled if early descriptions are understood in the appropriate historical context: in 1887, still few enchytraeid species were known to possess dorsal pores and multiple chaetae growing pairwise in a bundle (that group would be allocated by Michaelsen to Fridericia , in 1889). The sole other species known in that group to possess adiverticulate spermathecae ( F. striata ) was a very different worm (much bigger, with more chaetae, peptonephridia much branched, with two large spermathecal ectal glands; Levinsen 1884). Spermathecal ectal glands among the earliest known Fridericia spp. ( F. bisetosa , F. striata or F. hegemon ) were always conspicuous, while in F. bulbosa s.s. these glands range from small to a simple thickening of the distal end of the spermathecal duct, and may have been easily overlooked. Likewise, Vejdovsky’ s (1879b) drawing of a peptonephridium in F. leydigii showed the simplest shape of that organ in the group. Inferring from that comparison an elongate branching of the peptonephridia for F. bulbosa (e.g. Schmelz 2003) is questionable. In fact, it all depends on the length of branches. The same Fridericia species with short peptonephridia can vary between having peptonephridia unbranched or with few stump-like terminal branches. Among species of similar body size to F. bulbosa s.str., one finds F. benti having these organs either unbranched, or occasionally with 2–3 terminal stump-like branches ( Schmelz 2003). In F. bretscheri peptonephridia are generally unbranched, but Schmelz (2003) detected a terminal bifurcation in an Irish specimen. In F. paroniana , the short peptonephridia are either unbranched or shortly bifurcated distally. (For the authorities of species mentioned in the above remarks, see Rota 1995; Schmelz 2003).
Distribution
The occurrence of F. bulbosa s.s. outside Italy remains to be ascertained. In any case, the species appears very selective in its habitat, being exclusive of oak woods and meadows on limestone ( Rota 1995). During my survey of urban holm oak stands in Siena and Naples ( Rota et al. 2013, 2014), it showed peaks of abundance in samples from Villa Patrizia (plot S1) and Belcaro, where the soil was characterized by heavy carbonate precipitation (either as a horizontal accumulation layer or as nodular concretions). In Naples it was found in Capodimonte (plots N1 and N3), whereas it appeared absent from Astroni (N4), a site with subacidic soils and poor in calcium ( Rota et al. 2013).
| V |
Royal British Columbia Museum - Herbarium |
| VI |
Mykotektet, National Veterinary Institute |
No known copyright restrictions apply. See Agosti, D., Egloff, W., 2009. Taxonomic information exchange and copyright: the Plazi approach. BMC Research Notes 2009, 2:53 for further explanation.